Neural Crest System - Abnormalities
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
While the cells originate from the ectoderm layer, unlike the neural tube which "pinches off" from the surface ectoderm, the neural crest cells head off on migrations throughout the embryo forming a diverse range of cell types and contributions to many different tissues.
This behaviour also means that failure of correct migration or differentiation can lead to a number of different abnormalities.
| Neural Crest Links: neural crest | Lecture - Early Neural | Lecture - Neural Crest Development | Lecture Movie | Schwann cell | adrenal | melanocyte | peripheral nervous system | enteric nervous system | cornea | cranial nerve neural crest | head | skull | cardiac neural crest | Nicole Le Douarin | Neural Crest Movies | neural crest abnormalities | Category:Neural Crest | |||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Neural Crest Abnormalities
|
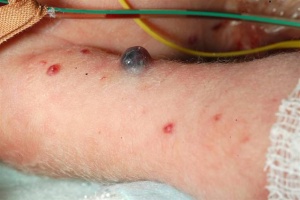
Neuroblastoma
This childhood cancer can arise initially from the adrenal or other tissues. A smaller recent infant cancer study (Tiwan, Medical Center Cancer Registry, 1995 - 2001) of 82 infants (40 males and 42 females, 12 neonates) showed neuroblastoma as the third most common infant cancer (12 infants 14.6%). The disease development is classified by the International Neuroblastoma Staging System.[6]
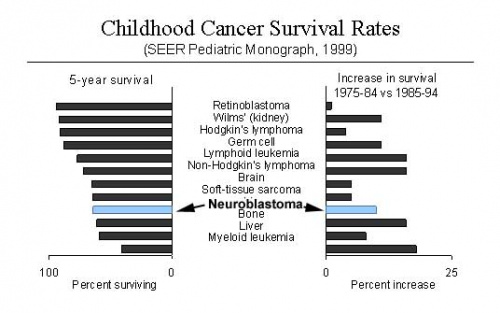
Changes in Childhood Survival Rates
| Cancer | Infant number | percentage |
|---|---|---|
| acute leukemia | 21 infants (25.6%; acute myeloid leukemia in 12, and acute lymphoblastic leukemia in 9) | |
| retinoblastoma | 14 | 17.1% |
| neuroblastoma | 12 | 14.6% |
| brain tumor | 9 | 11.0% |
| germ cell tumor | 8 | 9.8% |
| hepatoblastoma | 5 | 6.1% |
| soft tissue sarcoma | 5 (rhabdomyosarcoma 1, fibrosarcoma 3, other sarcoma 1) | |
| Table data modified from: Childhood Cancer Survivor Study Links: neural crest abnormalities | ||
See also data from[7] and another Italian study[8] showed: "Age of less than 1 year at time of diagnosis was a favorable prognostic factor for neuroblastoma and ganglioneuroblastoma. The extent of disease at diagnosis was related to prognosis for neuroblastoma and ganglioneuroblastoma and other selected solid tumors."
Pubmed Search: OMIM- Neuroblastoma (2006 - 242 search results)
Links: OMIM - Neuroblastoma
Neurofibromatosis
Neurofibromatosis type 1 (NF1, von Recklinghausen's disease) is an autosomal dominant disease caused by a germ-line-inactivating mutation in the NF1 gene on chromosome 17.
Links: StatPearls
DiGeorge syndrome
DiGeorge syndrome (velocardiofacial syndrome, third and fourth pharyngeal pouch syndrome, Hypoplasia of thymus and parathyroids) is the most frequent microdeletion syndrome in humans caused by a hemizygous deletion (1.5 to 3.0-Mb) of chromosome 22q11.2.[9]
Molecularly, the transcription factor Tbx1 and its downstream targets SDF1/CXCR4 appear critical for events of neural crest cell migration and survival.[10] While generally described as a neural crest abnormality, there are indications of pharyngeal endoderm and mesoderm involvement through other signaling pathways (Wnt11r and Fgf8a).[11] A mouse study[12] has shown that vitamin B12 treatment can enhances Tbx1 gene expression and improve outcomes.
| Table - Human Tbx Family | ||||
| Approved Symbol |
Approved Name | Previous Symbols | Synonyms | Chromosome |
|---|---|---|---|---|
| TBX1 | T-box 1 | VCF | CATCH22 | 22q11.21 |
| Human TBX Family | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abnormalities: cardiovascular, thymic and parathyroid, craniofacial anomalies, renal anomalies, hypocalcemia and immunodeficiency.
Search PubMed: Digeorge Syndrome | Takao syndrome
Links: TBX | Neural Crest Development | Head Development
- OMIM: Digeorge Syndrome | T-BOX 1 | CXCR4
Neurofibromatosis Type 1 (NF1)
- Neurofibromatosis Type 1 (von Recklinghausen) occurs in 1 in 3,000 to 4,000 people with characteristic skin blemishes forming in early childhood.[13]
- Multiple café-au-lait spots (flat skin patches darker than the surrounding area) appear in early childhood which increase in both size and number with age.
- tumors can develop along nerves in the skin, brain, and other parts of the body. In the iris of the eye, Lisch nodules (benign growths) also appear
- (French, café-au-lait = coffee with milk)
Links: OMIM - Neurofibromatosis Type 1 | Genetics Home Reference - Neurofibromatosis Type 1 | Nemours Foundation - Neurofibromatosis | Neurofibromatosis, Inc. (USA) | Atlas of Genetics and Cytogenetics in Oncology- Neurofibroma
Intestinal Aganglionosis
Intestinal Aganglionosis, also known as Hirschsprung's Disease or Megacolon, is a lack of enteric nervous system (neural ganglia) in the intestinal tract responsible for gastric motility (peristalsis).
In general, its severity is dependent upon the amount of the GIT that lacks intrinsic ganglia, due to developmental lack of neural crest migration into those segments. (More? Gastrointestinal Tract - Abnormalities)
- Intestinal Aganglionosis, Hirschsprung's Disease or Megacolon
- lack of enteric nervous system (neural ganglia) in the intestinal tract responsible for gastric motility (peristalsis).
- severity is dependent upon the amount of the GIT that lacks intrinsic ganglia, due to developmental lack of neural crest migration into those segments.
- first indication in newborns is an absence of the first bowel movement, other symptoms include throwing up and intestinal infections.
- Clinically this is detected by one or more tests (barium enema and x ray, manometry or biopsy) and can currently only be treated by surgery. A temoporary ostomy (Colostomy or Ileostomy) with a stoma is carried out prior to a more permanent pull-through surgery.
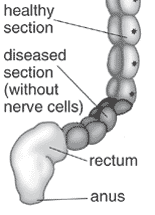
|
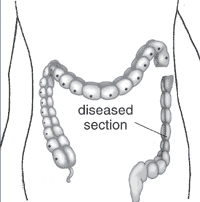
|
| Short section of the colon without smooth muscle neural ganglia | Longer section without ganglia |
The first indication in newborns is an absence of the first bowel movement, other symptoms include throwing up and intestinal infections. Clinically this is detected by one or more tests (barium enema and x ray, manometry or biopsy) and can currently only be treated by surgery. A temoporary ostomy (Colostomy or Ileostomy) with a stoma is carried out prior to a more permanent pull-through surgery.
Congenital failure of autonomic control describes the occasional association with congenital central hypoventilation syndrome (CCHS, Ondine's curse or Haddad Syndrome) perhaps correlating with a failure of neural crest associated with the respiratory system. This condition is a decrease in respiration (hypoventilation) usually during sleep and an insensitivity to stimuli which should increase respiration (hypercarbia and hypoxia).[14] Recent CCHS research has also identified Paired-like Homeobox 2B (PHOX2B) gene mutations associated with this disorder.
OMIM: Hirschsprung's Disease | Congenital failure of autonomic control | PHOX2B)
Search PubMed: hirschprung's+disease
Links:NIH - NIDDK - Hirschsprungs | MedlinePlus - Hirschsprung's disease
Tetralogy of Fallot
Cardiac abnormality possibly stemming from abnormal neural crest migration. Named after Etienne-Louis Arthur Fallot (1888) who described it as "la maladie blue".
Links: Cardiovascular System Development | Cardiac Tutorial | Lecture - Heart | Cardiovascular System - Abnormalities
Treacher Collins syndrome
(TCS) A genetic developmental abnormality results from autosomal dominant mutations of the gene TCOF1 encoding the protein Treacle, identified in 2006. The syndrome is characterized by hypoplasia of the facial bones, cleft palate, and middle and external ear defects. These defects may relate to the effects on neural crest migration.
Links: Neural Crest Development | OMIM - TCOF1 | PMID: 8563749)
Charcot-Marie-Tooth disease
Charcot-Marie-Tooth (CMT) disease is a hereditary demyelinating disease of the peripheral nervous system.
Subtype 1A has duplication mutation of peripheral myelin protein 22 gene on chromosome {{Chr17)).
Melanoma
In Australia each year 8,800 people are diagnosed with melanoma, and almost 1000 people die (Data, Cancer Council Australia).
Two different findings on the reprogramming of melanoma cells, which have a neural crest origin, when transplanted between species into embryos.
Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, Hendrix MJ. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A. 2006 Feb 27; [Epub ahead of print]
Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005 Aug;233(4):1560-70.
Links: Medline Plus - Melanoma | Cancer Council Australia - Skin cancer in Australia | American Academy of Dermatology - Melanoma: What It Looks Like | New Zealand Dermatological Society - Melanoma
Self Assessment Questions
OMIM Database
Online Mendelian Inheritence in Man Database. OMIM
Internet Search OMIM database with the keyword "neural crest" or the above abnormality names.
OMIM Sample Entries
Neural Crest disease entries from 74 entries found (1999 search), using "neural crest"
*193500 WAARDENBURG SYNDROME, TYPE I; WS1
*162200 NEUROFIBROMATOSIS, TYPE I; NF1
*188400 DIGEORGE SYNDROME; DGS
#171400 MULTIPLE ENDOCRINE NEOPLASIA, TYPE II; MEN2#277580 WAARDENBURG-SHAH SYNDROME600501 ABCD SYNDROME*256700 NEUROBLASTOMA#142623 HIRSCHSPRUNG DISEASE#162300 MULTIPLE ENDOCRINE NEOPLASIA, TYPE IIB; MEN2B#130650 BECKWITH-WIEDEMANN SYNDROME; BWS164210 OCULOAURICULOVERTEBRAL DYSPLASIA#171300 PHEOCHROMOCYTOMA#176270 PRADER-WILLI SYNDROME; PWS#193510 WAARDENBURG SYNDROME, TYPE IIA; WS2A214800 CHOANAL ATRESIA, POSTERIOR; PCA*600594 DIGEORGE CRITICAL REGION GENE 2249400 MELANOSIS, NEUROCUTANEOUS*155735 MELANOMA ADHESION MOLECULE; MCAM217100 CONSTRICTING BANDS, CONGENITAL#146150 HYPOMELANOSIS OF ITO; HMI#137600 IRIDOGONIODYSGENESIS, TYPE 2; IRID2#188550 THYROID CARCINOMA, PAPILLARY*601499 RIEGER SYNDROME, TYPE 2; RIEG2#601631 IRIDOGONIODYSGENESIS, TYPE 1; IRID1*601654 EYES ABSENT 2; EYA2#118200 CHARCOT-MARIE-TOOTH DISEASE, TYPE 1B; CMT1B#106200 ANIRIDIA; AN1*602942 NEUROBLASTOMA STAGE 4S GENE603807 PETERS ANOMALY WITH CATARACT
References
- ↑ Wang XJ & Camilleri M. (2019). Hirschsprung disease: Insights on genes, penetrance, and prenatal diagnosis. Neurogastroenterol. Motil. , 31, e13732. PMID: 31609069 DOI.
- ↑ LeBel DP, Wolff DJ, Batalis NI, Ellingham T, Matics N, Patwardhan SC, Znoyko IY & Schandl CA. (2017). First Report of Prenatal Ascertainment of a Fetus With Homozygous Loss of the SOX10 Gene and Phenotypic Correlation by Autopsy Examination. Pediatr. Dev. Pathol. , , 1093526617744714. PMID: 29216801 DOI.
- ↑ Ngan ES, Garcia-Barceló MM, Yip BH, Poon HC, Lau ST, Kwok CK, Sat E, Sham MH, Wong KK, Wainwright BJ, Cherny SS, Hui CC, Sham PC, Lui VC & Tam PK. (2011). Hedgehog/Notch-induced premature gliogenesis represents a new disease mechanism for Hirschsprung disease in mice and humans. J. Clin. Invest. , 121, 3467-78. PMID: 21841314 DOI.
- ↑ High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS & Epstein JA. (2007). An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J. Clin. Invest. , 117, 353-63. PMID: 17273555 DOI.
- ↑ Hornyak TJ. (2006). The developmental biology of melanocytes and its application to understanding human congenital disorders of pigmentation. Adv Dermatol , 22, 201-18. PMID: 17249303
- ↑ Colon NC & Chung DH. (2011). Neuroblastoma. Adv Pediatr , 58, 297-311. PMID: 21736987 DOI.
- ↑ Dama E, Pastore G, Mosso ML, Maule MM, Zuccolo L, Magnani C & Merletti F. (2006). Time trends and prognostic factors for survival from childhood cancer: a report from the Childhood Cancer Registry of Piedmont (Italy). Eur. J. Pediatr. , 165, 240-9. PMID: 16411094 DOI.
- ↑ Yang CP, Hung IJ, Jaing TH, Shih LY & Chang WH. (2005). Cancer in infants: a review of 82 cases. Pediatr Hematol Oncol , 22, 463-81. PMID: 16169813 DOI.
- ↑ Wurdak H, Ittner LM & Sommer L. (2006). DiGeorge syndrome and pharyngeal apparatus development. Bioessays , 28, 1078-86. PMID: 17041894 DOI.
- ↑ Duband JL, Escot S & Fournier-Thibault C. (2016). SDF1-CXCR4 signaling: A new player involved in DiGeorge/22q11-deletion syndrome. Rare Dis , 4, e1195050. PMID: 27500073 DOI.
- ↑ Choe CP & Crump JG. (2014). Tbx1 controls the morphogenesis of pharyngeal pouch epithelia through mesodermal Wnt11r and Fgf8a. Development , 141, 3583-93. PMID: 25142463 DOI.
- ↑ Lania G, Bresciani A, Bisbocci M, Francone A, Colonna V, Altamura S & Baldini A. (2017). Vitamin B12 ameliorates the phenotype of a mouse model of DiGeorge syndrome. Hum. Mol. Genet. , 26, 4540. PMID: 29036321 DOI.
- ↑ De Schepper S, Boucneau J, Lambert J, Messiaen L & Naeyaert JM. (2005). Pigment cell-related manifestations in neurofibromatosis type 1: an overview. Pigment Cell Res. , 18, 13-24. PMID: 15649148 DOI.
- ↑ Croaker GD, Shi E, Simpson E, Cartmill T & Cass DT. (1998). Congenital central hypoventilation syndrome and Hirschsprung's disease. Arch. Dis. Child. , 78, 316-22. PMID: 9623393
Reviews
Vega-Lopez GA, Cerrizuela S, Tribulo C & Aybar MJ. (2018). Neurocristopathies: New insights 150 years after the neural crest discovery. Dev. Biol. , , . PMID: 29802835 DOI.
Cordero DR, Brugmann S, Chu Y, Bajpai R, Jame M & Helms JA. (2011). Cranial neural crest cells on the move: their roles in craniofacial development. Am. J. Med. Genet. A , 155A, 270-9. PMID: 21271641 DOI.
Trainor PA. (2010). Craniofacial birth defects: The role of neural crest cells in the etiology and pathogenesis of Treacher Collins syndrome and the potential for prevention. Am. J. Med. Genet. A , 152A, 2984-94. PMID: 20734335 DOI.
Kim S & Chung DH. (2006). Pediatric solid malignancies: neuroblastoma and Wilms' tumor. Surg. Clin. North Am. , 86, 469-87, xi. PMID: 16580935 DOI.
Yang CP, Hung IJ, Jaing TH, Shih LY & Chang WH. (2005). Cancer in infants: a review of 82 cases. Pediatr Hematol Oncol , 22, 463-81. PMID: 16169813 DOI.
Croaker GD, Shi E, Simpson E, Cartmill T & Cass DT. (1998). Congenital central hypoventilation syndrome and Hirschsprung's disease. Arch. Dis. Child. , 78, 316-22. PMID: 9623393
Articles
Fernández RM, Bleda M, Luzón-Toro B, García-Alonso L, Arnold S, Sribudiani Y, Besmond C, Lantieri F, Doan B, Ceccherini I, Lyonnet S, Hofstra RM, Chakravarti A, Antiñolo G, Dopazo J & Borrego S. (2013). Pathways systematically associated to Hirschsprung's disease. Orphanet J Rare Dis , 8, 187. PMID: 24289864 DOI.
Dama E, Pastore G, Mosso ML, Maule MM, Zuccolo L, Magnani C & Merletti F. (2006). Time trends and prognostic factors for survival from childhood cancer: a report from the Childhood Cancer Registry of Piedmont (Italy). Eur. J. Pediatr. , 165, 240-9. PMID: 16411094 DOI.
Bajaj R, Smith J, Trochet D, Pitkin J, Ouvrier R, Graf N, Sillence D & Kluckow M. (2005). Congenital central hypoventilation syndrome and Hirschsprung's disease in an extremely preterm infant. Pediatrics , 115, e737-8. PMID: 15930201 DOI.
Search PubMed
Search April 2010 "Neural Crest Development" - All (4354) Review (843) Free Full Text (1621)
Search Pubmed: neural crest abnormalities | neuroblastoma | Neurofibromatosis type 1
Additional Images
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Children's Neuroblastoma Cancer Foundation CNCF
- Childhood Cancer Survivor Study CCSS
- AIHW Cancer compendium: information and trends by cancer type
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Neural Crest System - Abnormalities. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_Crest_System_-_Abnormalities
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G