Hearing - Middle Ear Development
| Embryology - 9 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
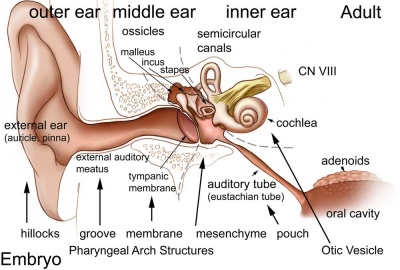
The embryology of the middle ear requires many separate components from different embryonic origins. The tympanic membrane separates the outer ear from the middle ear and is formed initially from the first pharyngeal arch membrane. The middle ear bones (ossicles) are derived from separate origins in the first and second arch mesenchyme. The space in which these bones sit (tympanic cavity) is derived from the first pharyngeal pouch and is connected directly to the oral cavity by a hollow tube (auditory tube). In addition there are two muscles (tensor tympani and stapedius) formed from arch mesenchyme. (More? pharyngeal arch, bone, muscle).
Note that in other species, such as the guinea pig, the malleus and incus are normally found as a single complex.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Middle Ear Development | Stapes Development | Malleus Development | Incus Development | Tensor Tympani Development | Stapedius Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
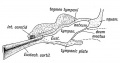
Middle Ear Origins
- neural crest from the first and second pharyngeal arches forms the cartilage origin of the middle ear bones (ossicles).
- first arch cartilage - (Meckel's) malleus and incus
- second arch cartilage - (Reichart's) stapes
- first pharyngeal pouch endoderm forms the auditory tube that enlarges to incorporate the tympanic cavity surrounding the ossicles.
- endoderm forms the lining epithelium and glands.
- mesoderm from the first and second arch forms the middle ear muscles
- first arch - tensor tympani muscle
- second arch - stapedius muscle
Middle Ear Genes - gooscoid, RARs, Prx1, Otx2, Hoxa1, Hoxb1, endothelian related molecules
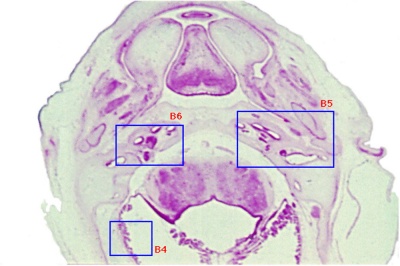
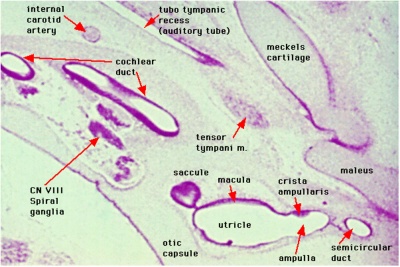
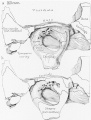
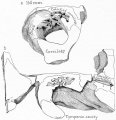
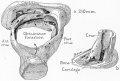
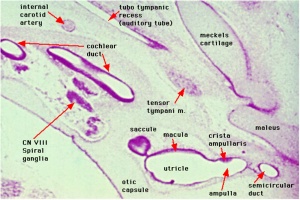
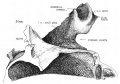
Week 8
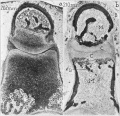
Cross-section of human embryo Carnegie stage 22 during Week 8.
Middle ear structure visible are the primordial maleus, the developing tensor tympani muscle, the developing auditory tube (tubo tympanic recess) extending from the oral cavity towards the middle ear region. Note the ossicle is still embedded, and surrounded by, the mesenchyme of the head.
Ossicles
|
|
|
Middle ear development begins closely associated with head formation and involves both the foregut tube (pharynx) and the pharyngeal arches. Pharyngeal arches form the main anatomical structures of the head and neck, including all components of the middle and outer ear. The three middle ear bones or auditory ossicles (malleus, incus, stapes) are formed from the cartilage template found within pharyngeal arch 1 and 2.
These bones are commonly named the "hammer" (malleus), "anvil" (incus) and "stirrup" (stapes), and the cartilage bands are historically named after two German anatomists and are called Meckel’s cartilage (first pharyngeal arch; named after Johann Friedrich Meckel, 1781–1833) and Reichert’s cartilage (second pharyngeal arch; named after Karl Bogislaus Reichert, 1811–1883). There are several theories as to how each arch cartilage contributes individual components of the middle ear ossicles. For example, a recent study suggests a mesenchymal origin for the stapes rather than directly from Reichert's cartilage.[10] Meckel’s cartilage first appears histologically at Carnegie stage 16 and Reichert’s cartilage slightly later.
The early stages of auditory ossicle development all occur within the solid mesenchyme of the pharyngeal arches until the eighth month of development, then within a fluid-filled space for the final month, and finally only postnatal in the neonate in the air-filled tympanic cavity. This transition in auditory ossicle environment means that the middle ear does not function correctly until after birth, and any prenatal conduction to the cochlea must be mediated through bone conduction.
During development of the tympanic cavity, the auditory ossicles are held in their correct anatomic positions by supporting ligaments. Arch cartilages ossify by the process of endochondral ossification, where a pre-existing cartilage template is first formed and later replaced by bone. Endochondral ossification is the main process of bone formation throughout the entire skeleton, except for the cranial vault and the clavicle that ossify by a process of intramembranous ossification.
Initially, the malleus and incus form as a single structure, and it is only later that they separate to form two separate bones. Ossification continues through the entire fetal period, and the newly formed bones also have a transient bone marrow cavity. The marrow cavity is still present at birth, in both the malleus and the incus, and with continued ossification is lost during the first two years after birth. Postnatally, first the malleus and then the incus lose their marrow spaces.
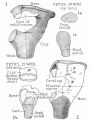
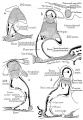
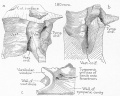
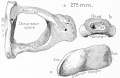
Malleus
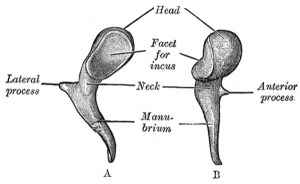
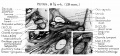
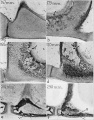
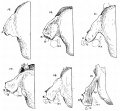
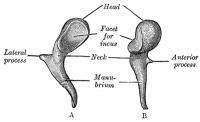
Malleus (left) A. From behind. B. From within. |
The malleus develops from the first pharyngeal arch cartilage (Meckel's cartilage) and was named from its resemblance to a hammer. The structure of the adult bone can be divided into a head, neck, and three processes (manubrium, anterior and lateral processes). In the fetus, of the three processes the anterior process is the longest and is in direct continuity with Meckel's cartilage. Malleus ossification is initiated in the fetal period.[11] [12] The newborn and infant malleus head normally contains bone marrow, that is eventually replaced by bone.[13] Malleus Development (timing from[12])
|
| Component | Latin | Description | ||
| Head | capitulum mallei | large upper extremity of the bone, oval in shape | articulates posteriorly with the incus, being free in the rest of its extent | facet for articulation with the incus is constricted near the middle (consists of an upper larger and lower smaller part nearly a right angle with each other) opposite the constriction (lower margin of the facet projects in the form of a process, cog-tooth or malleus spur) |
| Neck | collum mallei | narrow contracted part, just beneath the head | below it is a prominence | various processes are attached to the prominence |
| Handle | manubrium mallei | connected by its lateral margin with the tympanic membrane | directed downward, medialward, and backward | decreases in size toward its free end, which is curved slightly forward, and flattened transversely. Medial side, near its upper end, is a slight projection, into which the tendon of the tensor tympani is inserted |
| Anterior Process | processus anterior (Folii), processus gracilis | delicate spicule from the eminence below the neck | directed forward to the petrotympanic fissure | to which it is connected by ligamentous fibers |
| Lateral Process | processus lateralis, processus brevis | a slight conical projection from the root of the manubrium | directed laterally, and is attached to the upper part of the tympanic membrane | attached by means of the anterior and posterior malleolar folds, to the extremities of the notch of Rivinus. |
Abnormalities
A series of different abnormalities of the anterior process and manubrium mallei (malleus handle) have been described.[14]
- thick bony bar - extending from the neck of the malleus fused with the posterior bony wall or the tympanic bone.
- thick bony bar and a cartilaginous malleus handle - cartilage appears attached to the anterior part of the bony bar.
- no bony bar - a V-shaped ossicle one end connected to the malleus head by fibrous tissue.
Historic Embryology
Hanson JR. and Anson BJ. Development of the malleus of the human ear; Illustrated in atlas series. (1962) Q Bull Northwest Univ Med Sch. 36(2): 119–137. PMID: 13904457.
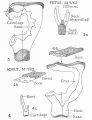
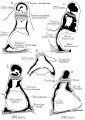
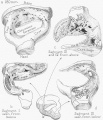
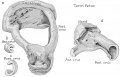
Incus
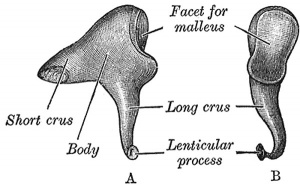
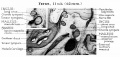
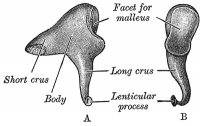
Incus (left) A. From within. B. From the front |
The incus develops from the first pharyngeal arch cartilage (Meckel's cartilage) and named from the resemblance to an anvil, onsists of a body and two crura.
The two crura diverge from one another nearly at right angles. Gray suggested the incus has more like a "premolar tooth" appearance, with two roots, which differ in length, and are widely separated from each other. Incus ossification is initiated in the fetal period.[11] Incus Development (timing from[12])
The newborn and infant incus body normally contains bone marrow, that is eventually replaced by bone.[13] |
From recent study of histological sections from 55 human embryos and fetuses at 6 to 13 weeks of development.[5]
6 weeks
- (Carnegie Stage 16) - incus anlage was found at the cranial end of the first pharyngeal arch. At this stage, each of the three anlagen of the ossicles in the middle ear were independent in different locations.
- (Carnegie Stage 16) - a homogeneous interzone clearly defined the incus and malleus anlagen. The cranial end of the incus was located very close to the otic capsule.
- 7 and 8 weeks - the short limb of the incus connecting with the otic capsule.
- 9 weeks - an initial disconnection of the incus from the otic capsule.
- 13 weeks - a cavity appeared between the otic capsule and incus.
| Component | Latin | Description | ||
| Body | corpus incudis | somewhat cubical but compressed transversely | anterior surface is a deeply concavo-convex facet | facet articulates with malleus head |
| Short Crus | crus breve (short process) | somewhat conical in shape | projects almost horizontally backward | attached to fossa incudis, in lower and back part of epitympanic recess |
| Long Crus | crus longum (long process) | descends nearly vertically behind and parallel to manubrium of malleus | bending medialward, ends in a rounded projection, the lenticular process | lenticular process is tipped with cartilage, and articulates with stapes head |
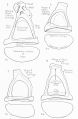
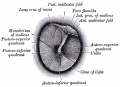
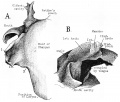
Stapes
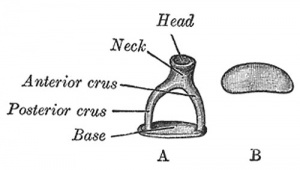
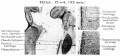
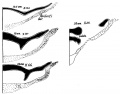
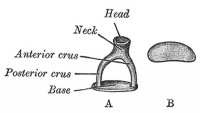
A. Left stapes. B. Base of stapes, medial surface. |
The stapes develops mainly from the second pharyngeal arch neural crest cartilage (Reichardt's cartilage). The stapedial footplate like the nearby annular ligament and the connected otic capsule around the oval window is of mesoderm origin.[15]
|
The adult ossicle is by its resemblance to a stirrup, structurally consisting of a head, neck, two crura, and a base.
| Component | Latin | Description | |
| Head | capitulum stapedis | presents a depression covered by cartilage | articulates with lenticular process of incus |
| Neck | constricted part of the bone, succeeding the head | gives insertion to tendon of stapedius muscle | |
| Two crura | crus anterius and crus posterius | diverge from the neck , connected at ends by a flattened oval plate | anterior is shorter and less curved than the posterior |
| Base | basis stapedis | forms the foot-plate of the stirrup | fixed to margin of fenestra vestibuli by a ring of ligamentous fibers |
Historic Embryology
For historical development and adult anatomy of the stapes see the series of papers by Cauldwell and Anson from the 1930's to 1940's.
1938 Stapes - 7 to 21 weeks[16]
1938 Stapes - Term to Adult[17]
1940 Adult form of the human stapes in the light of its development[18]
- 1940 Historic Stapes
1942 Stapes Embryo 6.7 to 50 mm[19]
1943 Stapes Fetus 75 to 150 mm[20]
1948 Stapes Fetus 160 mm to Term[21]
- 1948 Historic Stapes
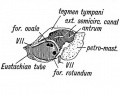
Tympanic Membrane
The tympanic membrane (membrana tympani) or "ear drum", separates the external acoustic meatus from the tympanic cavity. In the adult, this thin membrane is nearly oval in shape about 9 to 10 mm in diameter. The circumference is slightly thickened to form a fibrocartilaginous ring that is attached to the tympanic sulcus at the inner end of the meatus. The malleus manubrium is attached to the medial surface of the membrane and is the mechanism for transmitting vibration of the tympanic membrane to the other middle ear ossicles. See also recent review.[22]
Middle Ear Muscles
The middle ear also contains the two smallest muscles of the body, the stapedius and tensor tympani muscles, which both differentiate from arch mesenchyme. These muscles form and differentiate in a similar fashion to other developing skeletal muscle. Initially myoblasts proliferate under the influence of growth factors in the region of where the muscle will form. Myoblasts are the embryonic undifferentiated single cells of all skeletal muscles.
Tensor Tympani
Adult tensor tympani is classed as a mixed muscle containing slow (type 1) and fast (type 2A, and probably 2X) muscle fibers.
Stapedius
Adult mammalian stapedius muscle contains mainly (77%) fast oxidative glycolytic type muscle fibers and the avian muscle only contains fast fibers.
A recent study[23] of the stapedius region (see table below) and muscle development in 50 human embryos and fetuses between 38 days to 17 weeks post-conception identified 2 origins:
- tendon - derives from the internal segment of the interhyale
- belly - located in the second pharyngeal arch, medially to the facial nerve and near the interhyale
interhyale - term describing the internal part of the second pharyngeal arch that forms the tendon of the stapedius muscle
| Carnegie Stage |
CRL (mm) | Description |
|---|---|---|
| 13 | 6 | Presumptive stapedial area |
| 14 | 7 | Appearance of the stapedial anlage |
| 16 | 9 | Relationship between the stapedial artery and the stapedial anlage. Appearance of the interhyale |
| 17 | 12 | Delimitation of the parts of the stapedial anlage |
| 18 | 16 | Chondrogenesis phase. Start of involution of the stapedial artery |
| 20 | 18.5 | Delimitation of the ossicular anlages. Cartilaginous phase. Disappearance of the stapedial artery |
| 22 | 26 | Delimitation of the interhyale |
| 23 | 28 | Anlage of the stapedial muscle tendon |
| Data from Table 1[23] Links: middle ear | ||
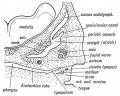
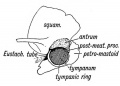
Tympanic Cavity
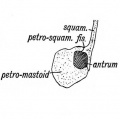
The tympanic cavity (cavum tympani) extends from the endoderm of the first pharyngeal arch pouch to surround the middle ear ossicles. This small air-filled space is connected to the oral cavity by the narrow auditory tube. The lining epithelium has been suggested as having dual origins, but a recent study has confirmed the endodermal origin of this epithelium.[2] The adult tympanic cavity anatomy is a single space constricted slightly into an upper (attic) and a lower (atrium) chamber.
Auditory Tube
The auditory tube or eustachian tube (named after Bartolomeo Eustachi, 1500–1574) (or otopharyngeal or pharyngotympanic tube) develops from the first pharyngeal pouch and is lined with endoderm. This narrow cavity links the pharynx to the middle ear and is continuous with the tympanic cavity. The auditory tube has two main functions: ventilation, to allow the equalization of pressure in the middle ear, and clearance, to allow the middle ear fluid continuously produced by the epithelial lining to drain from the middle ear.
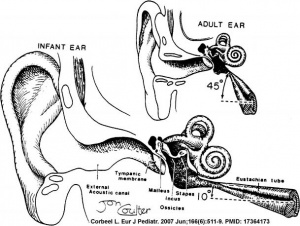
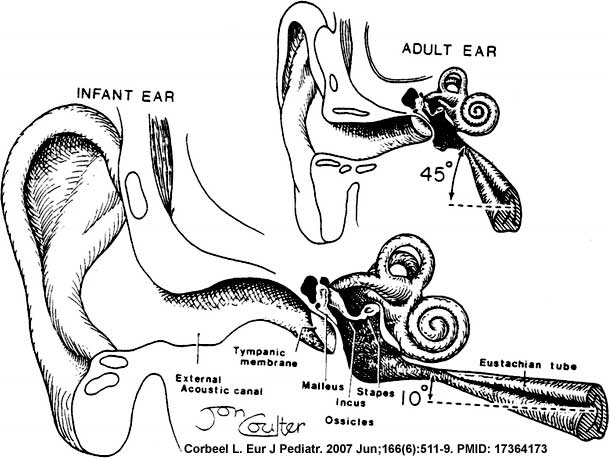
In normal human development, the auditory tube has an almost straight posterolateral to anteromedial pathway. The main growth of the auditory tube occurs in extension and lumen of the cartilaginous portion in the fetal period between weeks 16 to 28.
At birth, and in the young child, the tube is both shorter (8-9 mm) compared to the adult length (17-18 mm), runs almost horizontal and is narrower in diameter. Head growth in the child to adult size results in a longer wider tube that runs at approximately 45 degrees to the horizontal. The auditory tube is also normally closed and is opened by muscles—in the infant this is only a single muscle, the tensor palati muscle. In the adult the auditory tube is now opened by two separate muscles, the tensor palati and levator palati muscles.
The middle ear cavity or tympanic cavity is formed by an expansion of the pharynx. The initial early cavity lining is formed by the pharyngeal endoderm epithelium. The epithelium will then continue to expand, to eventually also line the entire mastoid antrum.
- derived from first pharyngeal pouch
- extends as tubotympanic recess - during week 5 recess contacts outer ear canal
- mesoderm between 2 canals forms tympanic membrane
- expands to form tympanic recess
- stalk of recess forms auditory tube(eustachian tube, pharyngotympanic tube)
Skeletal elements surrounding the auditory tube have 2 parts:
- bony lateral portion - arises from the anterior wall of the auditory bulla.
- cartilage portion - covers the dorsal region along the length of the tube.
Mouse models have shown that the cartilage is mostly mesoderm in origin with a small part formed also from neural crest.
Muscles
The auditory tube is surrounded by four muscles, two are attached to the muscles insert into the palate and key in opening the tube:
- tensor veli palatini - first pharyngeal arch
- levator veli palatini - fourth pharyngeal arch
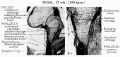
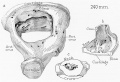
Auditory Tube Postnatal Changes
|
Adult
|
Adult Middle Ear
The adult middle ear, like the inner ear, eventually will lie within the petrous portion of temporal bone. Initially, both the middle and inner ear form within mesenchyme, embryonic connective tissue, forming the otic capsule, and this will also form the base of the skull. The mesenchyme differentiates first to form cartilage, forming a structure known as the chondrocranium. This initial cartilage is gradually replaced by bone forming at a number of sites within the cartilage, ossification centers. The initial bone that is formed also contains marrow spaces that disappear with ongoing ossification (Yokoyama et al., 1999). Between the weeks 16 to 24, centers of ossification appear in the remaining cartilage of the otic capsule, and these continue to ossify to eventually form mastoid process of temporal bone.
Other Species
Avian - the columella is a single ossicle, it has a shaft and footplate inserted into the oval window.
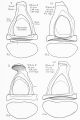
Additional Images
Historic
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Keith A. Human Embryology and Morphology. (1902) London: Edward Arnold.
Frazer JE. The early formations of the middle ear and eustachian tube - a criticism. (1922) J Anat. 57(1): 18-30. PMID 17103958
- “Middle
References
- ↑ Elias TGA & Santos F. (2022). Developmental Disruptions of the Human Stapes. Otol Neurotol , 43, e461-e466. PMID: 35120079 DOI.
- ↑ 2.0 2.1 van Waegeningh HF, Ebbens FA, van Spronsen E & Oostra RJ. (2019). Single origin of the epithelium of the human middle ear. Mech. Dev. , , 103556. PMID: 31121244 DOI.
- ↑ Ankamreddy H, Min H, Kim JY, Yang X, Cho ES, Kim UK & Bok J. (2019). Region-specific endodermal signals direct neural crest cells to form the three middle ear ossicles. Development , 146, . PMID: 30630826 DOI.
- ↑ Yang F & Liu Y. (2018). Reporting and Description for Congenital Middle Ear Malformations to Facilitate Surgical Management. Ann Otol Rhinol Laryngol , 127, 717-725. PMID: 30091369 DOI.
- ↑ 5.0 5.1 Rodríguez-Vázquez JF, Yamamoto M, Abe S, Katori Y & Murakami G. (2018). Development of the Human Incus With Special Reference to the Detachment From the Chondrocranium to be Transferred into the Middle Ear. Anat Rec (Hoboken) , , . PMID: 29669196 DOI.
- ↑ Urban DJ, Anthwal N, Luo ZX, Maier JA, Sadier A, Tucker AS & Sears KE. (2017). A new developmental mechanism for the separation of the mammalian middle ear ossicles from the jaw. Proc. Biol. Sci. , 284, . PMID: 28179517 DOI.
- ↑ Burford CM & Mason MJ. (2016). Early development of the malleus and incus in humans. J. Anat. , 229, 857-870. PMID: 27456698 DOI.
- ↑ Takanashi Y, Shibata S, Katori Y, Murakami G, Abe S, Rodríguez-Vázquez JF & Kawase T. (2013). Fetal development of the elastic-fiber-mediated enthesis in the human middle ear. Ann. Anat. , 195, 441-8. PMID: 23706648 DOI.
- ↑ Richter CA, Amin S, Linden J, Dixon J, Dixon MJ & Tucker AS. (2010). Defects in middle ear cavitation cause conductive hearing loss in the Tcof1 mutant mouse. Hum. Mol. Genet. , 19, 1551-60. PMID: 20106873 DOI.
- ↑ Rodríguez-Vázquez JF. (2005). Development of the stapes and associated structures in human embryos. J. Anat. , 207, 165-73. PMID: 16050903 DOI.
- ↑ 11.0 11.1 Sánchez-Fernández JM, Saint-Gerons S & Sánchez del Rey A. (1992). A microanalytical study on human auditory ossicles in normal and pathological conditions. Acta Otolaryngol. , 112, 317-21. PMID: 1604999
- ↑ 12.0 12.1 12.2 12.3 Whyte J, Cisneros A, Yus C, Obón J, Whyte A, Serrano P, Pérez-Castejón C & Vera A. (2008). Development of the dynamic structure (force lines) of the middle ear ossicles in human foetuses. Histol. Histopathol. , 23, 1049-60. PMID: 18581276 DOI.
- ↑ 13.0 13.1 Yokoyama T, Iino Y, Kakizaki K & Murakami Y. (1999). Human temporal bone study on the postnatal ossification process of auditory ossicles. Laryngoscope , 109, 927-30. PMID: 10369284
- ↑ Minatogawa T, Kanoh N, Kumoi T & Nishimura Y. (1994). Developmental anomaly of the process of Folius. Eur Arch Otorhinolaryngol , 251, 105-8. PMID: 8024756
- ↑ Thompson H, Ohazama A, Sharpe PT & Tucker AS. (2012). The origin of the stapes and relationship to the otic capsule and oval window. Dev. Dyn. , 241, 1396-404. PMID: 22778034 DOI.
- ↑ Anson BJ. Karabin JE. and Martin J. Stapes, fissula ante fenestram and associated structures in man: I. From embryo of seven weeks to that of twenty-one weeks (1938) Arch. Otolaryng. 28: 676-697.
- ↑ Anson BJ. Karabin JE. and Martin J. Stapes, fissula ante fenestram and associated structures in man: II. From Fetus at Term to Adult of Seventy (1938) Arch. Otolaryng. 28: 676-697.
- ↑ Beaton LE. and Anson BJ. Adult form of the human stapes in the light of its development (1940) Q Bull Northwest Univ Med Sch. 14(4): 258–269. PMC3802306
- ↑ Cauldwell EW. and Anson BJ. Stapes, fissula ante fenestram and associated structures in man III. from embryos 6.7 to 50 mm in length. (1942) Arch. Otolaryng. 36: 891-925.
- ↑ Anson BJ. and Cauldwell EW. Stapes, fissula ante fenestram and associated structures in man: IV. From fetuses 75 to 150 mm in length. (1943) Arch. Otolaryng. 37: 650-671.
- ↑ Anson BJ. and Cauldwell EW. Stapes, fissula ante fenestram and associated structures in man: V . From the fetus of 160 mm to term. (1948) 48(3): 263-300.
- ↑ Takechi M, Kitazawa T, Hirasawa T, Hirai T, Iseki S, Kurihara H & Kuratani S. (2016). Developmental mechanisms of the tympanic membrane in mammals and non-mammalian amniotes. Congenit Anom (Kyoto) , 56, 12-7. PMID: 26754466 DOI.
- ↑ 23.0 23.1 Rodríguez-Vázquez JF, Mérida-Velasco JR & Verdugo-López S. (2010). Development of the stapedius muscle and unilateral agenesia of the tendon of the stapedius muscle in a human fetus. Anat Rec (Hoboken) , 293, 25-31. PMID: 19899117 DOI.
Reviews
Tucker AS. (2017). Major evolutionary transitions and innovations: the tympanic middle ear. Philos. Trans. R. Soc. Lond., B, Biol. Sci. , 372, . PMID: 27994124 DOI.
Takechi M, Kitazawa T, Hirasawa T, Hirai T, Iseki S, Kurihara H & Kuratani S. (2016). Developmental mechanisms of the tympanic membrane in mammals and non-mammalian amniotes. Congenit Anom (Kyoto) , 56, 12-7. PMID: 26754466 DOI.
Chapman SC. (2011). Can you hear me now? Understanding vertebrate middle ear development. Front Biosci (Landmark Ed) , 16, 1675-92. PMID: 21196256
Takechi M & Kuratani S. (2010). History of studies on mammalian middle ear evolution: a comparative morphological and developmental biology perspective. J. Exp. Zool. B Mol. Dev. Evol. , 314, 417-33. PMID: 20700887 DOI.
Articles
Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y & Pettigrew MM. (2011). Microbial communities of the upper respiratory tract and otitis media in children. MBio , 2, e00245-10. PMID: 21285435 DOI.
Rodríguez-Vázquez JF. (2005). Development of the stapes and associated structures in human embryos. J. Anat. , 207, 165-73. PMID: 16050903 DOI.
Search PubMed
May 2010 "Middle Ear Development" All (2368) Review (226) Free Full Text (272)
Search Pubmed: Middle Ear Development | Ossicle Development | Malleus Development | Incus Development | Stapes Development | Tympanic Membrane Development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Neuroscience Neuroscience - The Middle Ear
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 9) Embryology Hearing - Middle Ear Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Hearing_-_Middle_Ear_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G