Gastrointestinal Tract - Intestine Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
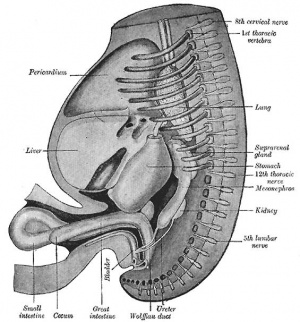
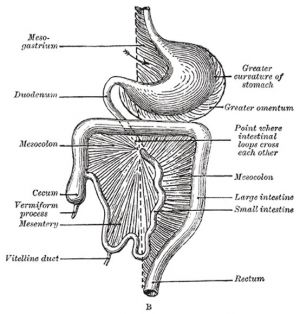
The part of the gastrointestinal tract (GIT) lying between the stomach and anus, is described as the intestine or bowel. This region is further divided anatomically and functionally into the small intestine or bowel (duodenum, Template:Jejunum and Template:Ileum) and large intestine or bowel (cecum and colon). Initially development concerns the midgut region, connected to the yolk sac, and the hindgut region, ending at the cloacal membrane. This is followed by two mechanical processes of elongation and rotation. Elongation, growth in length, leaves the midgut "herniated" at the umbilicus and external to the abdomen. Rotation, around a mesentery axis, establishes the anatomical position of the large intestine within the peritoneal space.
Migration of neural crest cells into the wall establishes the enteric nervous system, which has a role in peristalsis and secretion. Prenatally, secretions also accumulate in this region and are the first postnatal bowel movement, the meconium.
The small intestine grows in length rapidly in the last trimester, at birth it is about half the eventual adult length (More? Small Intestine Length). Like most of the gut, this region is not "functional" until after birth, when development continues by populating the large intestine with commensal bacteria and the establishment of the immune structure in the wall.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Intestine Embryology | Intestine Development | Small Intestine Development | Large Intestine Development |Duodenum Development | Jejunum Development | Ileum Development | Appendix Development | Colon Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Small Intestine
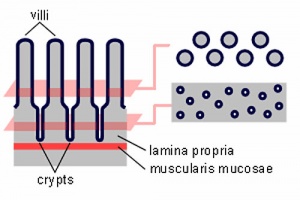
The small intestine (or bowel) consists of 3 main anatomical regions. A notable feature of this epithelium is the continuous replacement throughout life, from a stem cell population residing in the crypts, migrating towards the tips of the villi.
Adult Length
- Duodenum (25 cm)
- Jejunum (1.4 m)
- Ileum (3.5 m)
Duodenum
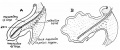
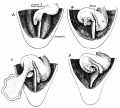
The duodenum develops from the part of the tube immediately after the stomach and uniquely forms the junction between foregut (upper) and midgut (lower). Unlike the remainder of the small intestine it grows significantly less in overall length. Initially suspended by a mesentery, and projects forward in the form of a loop, both of which rotate with the fusion of the two pancreatic buds, and subsequently displaced by the transverse colon. The right surface of the duodenal mesentery is directed backward to parietal peritoneum and becomes retroperitoneal. Both the biliary and the pancreatic ducts empty into the duodenum.
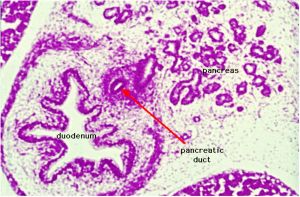
|
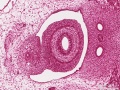
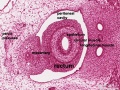
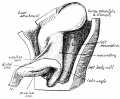
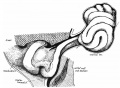
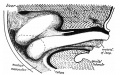
Note the distinctive "star-shaped" lumen of duodenum and the developing muscular wall.
The pancreas and its duct can also be seen on the right of image. |
- Search PubMed: Duodenum Development
Jejunum
The part of the small intestine between the duodenum and ileum. A recent study has shown that the postnatal jejunal lumen contains a distinctive bacterial population (facultative anaerobes and oxygen-tolerant obligate anaerobes) similar to those found in the oral cavity though with additional enterobacteriaceae (Gram-negative bacteria).[7]
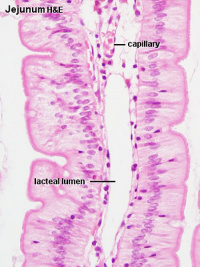
Adult jejunum histology
Search PubMed: Jejunum Development
Ileum
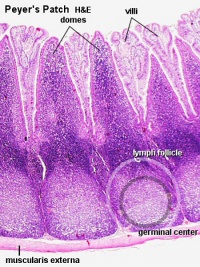
The adult ileum contains specialised aggregated lymphoid nodules known as Peyer's patches.
Adult ileum Peyer's patches
See small intestine or bowel length (see also Fetal Intestine Length and Small Intestine Length)
Search PubMed: Ileum Development
Large Intestine
Large intestine or bowel is where water is absorbed from the indigestible food residue and where the majority of the enteric microbiota reside. Before birth the intestine is sterile, after birth the microbiota is established from the mother. Later postnatally by diet, lifestyle and antibiotic exposure. The large intestine bacteria is mainly anaerobic, compared to the aerobic found in other regions.
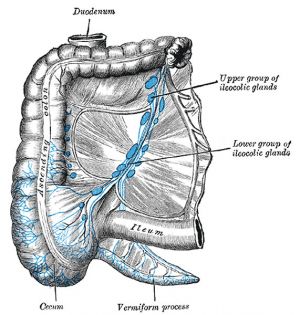
Cecum
(Latin, caecum) forms the beginning of the large intestine.
Vermiform Appendix
("appendix", adult 2 to 20 cm length) The vermiform appendix is a highly immune structure and can act as a "microbiota reservoir" during antibiotic and other treatment.
Colon
Anatomical Parts
- Ascending colon (adult 25 cm length)
- Transverse colon
- Descending colon
- Sigmoid colon
- Search PubMed: Large Intestine Development | Cecum Intestine Development | Vermiform appendix Intestine Development | Colon Development |
Intestinal Functions
Small Intestine
- absorption of nutrients and minerals found in food
- Duodenum - principal site for iron absorption
Cecum
- connects the ileum with the ascending colon
- separated by the ileocecal valve (ICV, Bauhin's valve)
- connected to the vermiform appendix ("appendix")
Colon
- absorbs fluid, water and salts, from solid wastes
- site of commensal bacteria (flora) fermentation of unabsorbed material
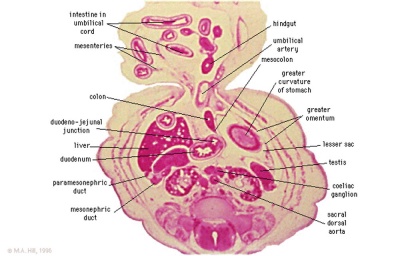
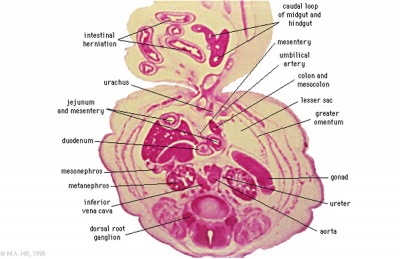
Embryonic Development
Week 4
|
Colour code:
|
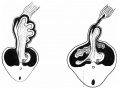
Week 7
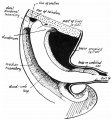
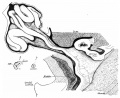
Human embryo small intestine secondary loops (week 7 to 8).[8]
Week 8
|
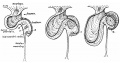
Late embryonic small intestine commencing at the duodenum, continuing as ventrally herniated and returning to join the colon.
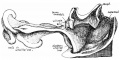
Small intestine tertiary loops week 8.[8]
- Links: Carnegie stage 22 | Week 8
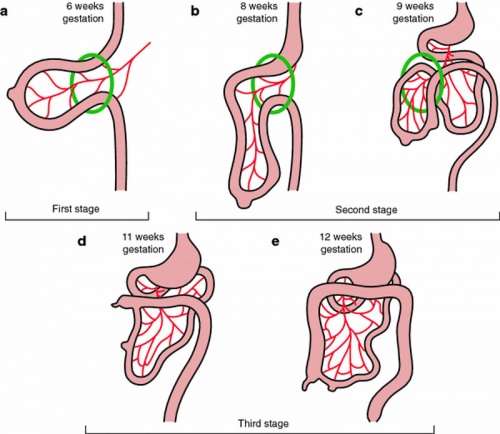
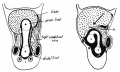
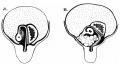
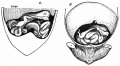
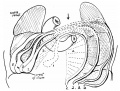
Rotation
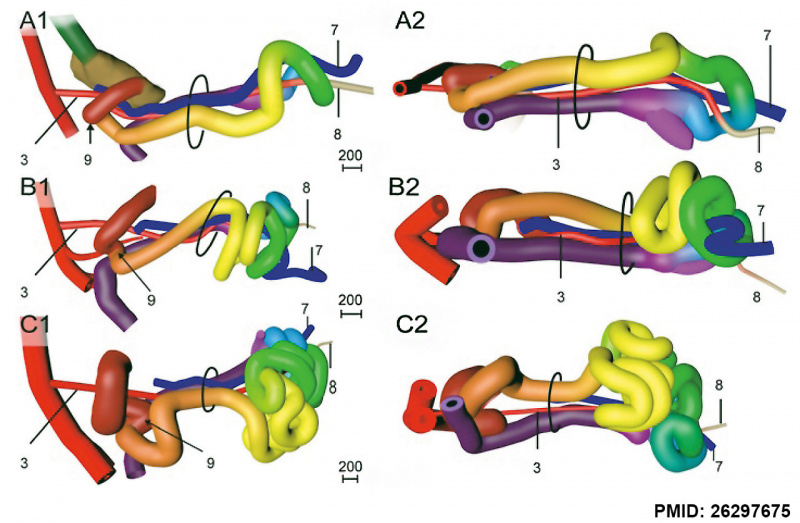
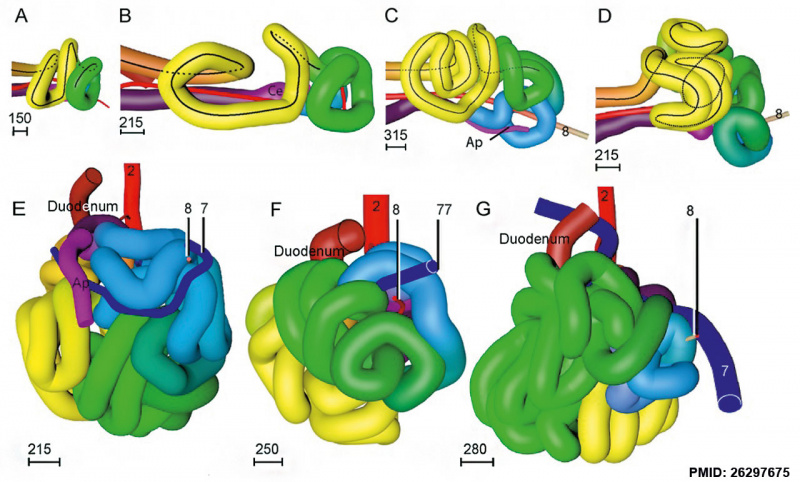
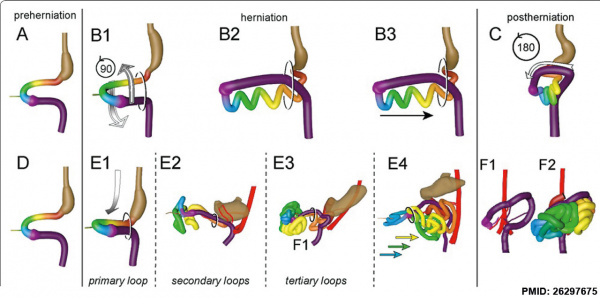
A recent 3 dimensional study[8] has suggested a modified “en-bloc rotation” of the small intestine, compared the the earlier simplified description of 270 degree rotation (below).
Human intestine “en-bloc rotation” model.[8]
- "If one insists on using the term rotation for this movement, it would be largely around a craniocaudal axis (in the transverse plane) rather than a dorsoventral axis (frontal plane). In view of the brief time window and orientation of the apparent rotational axis, we conclude that the distal ileum and cecum “slide” rather than “rotate” as from the umbilical orifice to the lower-right abdominal cavity."
Normal intestinal rotation[9]
Fetal Intestine Length
|
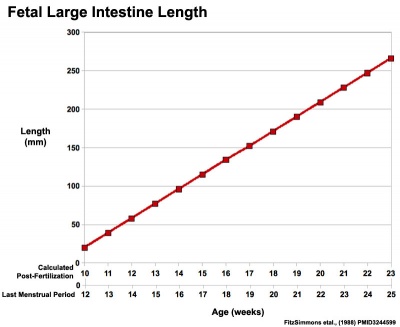
|
| Fetal small Intestine length growth | Fetal Large Intestine length growth |
Data from[3]
Small Intestine Length
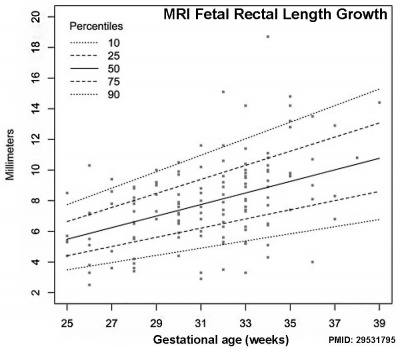
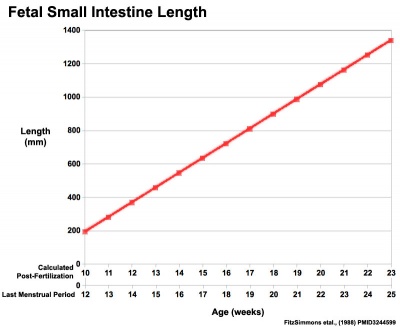
Small intestine growth in length is initially linear (first half pregnancy to 32 cm CRL), followed by rapid growth in the last 15 weeks doubling the overall length. Growth continues postnatally but after 1 year slows again to a linear increase to adulthood.[12]
| Small Intestine Length | |
|---|---|
| Age (weeks gestational age) | Average Length (cm) |
| 20 | 125 |
| 30 | 200 |
| term | 275 |
| 1 year postnatal | 380 |
| 5 years | 450 |
| 10 years | 500 |
| 20 years | 575 |
| Table data from 8 published reports of necropsy measurement of 1010 guts.[12] | |
Appendix
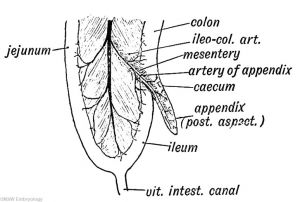
The appendix (vermiform appendix, vermix) is a finger-like diverticulum located anatomically at the cecum, the junction between the small and large intestines (colon). The length (2.5-13 cm) is longer in both infants and children and also has more abundant lymphatic tissue in early life. The wall structure is similar to the small intestine (though with no villi), nor plicae circularis. Its immune function is associated with the many lymph nodules surrounding the lumen that extend from the mucosa into the submucosa. It has also been suggested as a repository for beneficial intestinal microflora. See also the review comparing the appendix in different species.[14]
There appears to be a developmental anatomical differences in the fetal position of the appendix in males and females.[15] In the fetus, lymphocyte aggregates first appear in this region during the second trimester, week 15 (GA week 17).[16]
Historically, Berengario da Carpi (1460-c.1530) in Commentaria cum amplissimis additionibus super Anatomia Mundini (1521) was the first to describe the human appendix.
See also notes on appendix duplication.
Hindgut
Anatomically the distal third of the transverse colon and the splenic flexure, the descending colon, sigmoid colon and rectum. The developmental timing of the anus and rectum formation[17] in human embryos of the Carnegie Collection has been previously carried out (1974). A more recent study[18] has also been made of the Kyoto Collection embryos.
There has been some recent controversy over the "anal membrane" formation.
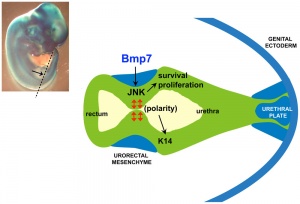
A recent study hindgut and anorectum development in human embryos shows that WNT5a is active in this region prior to anus formation, when it is down-regulated.[19]
Other studies - [20][21][22][23][24] (rat)
Intestinal Motility
The enteric nervous system neural crest-derived neurons interacts with the circular and longitudinal smooth muscle layers and the interstitial cells of Cajal to generate motility. The developmental timing data shown below is from a recent review.[25]
Neural Crest
week 5 - migrating neural crest cells reach the midgut
week 7 - neural crest cells have colonized the entire gut
- colonization occurs in a rostro-caudal sequence
| Myenteric plexus | Submucosal plexus |
|---|---|
| Auerbach's plexus | Meissner's plexus |
| Leopold Auerbach (1828–1897) a German anatomist and neuropathologist. | Georg Meissner (1829–1905) a German anatomist and physiologist. |
|
|
| Links: enteric nervous system | intestine | neural crest | PMID 25428846 |
Smooth Muscle
week 8 - esophagus circular muscle
week 11 - hindgut circular muscle
week 14 - hindgut concentric muscularis mucosae, circular muscle, and longitudinal muscle
Interstitial Cells of Cajal
Interstitial cells of Cajal (ICC) are electrical pacemaker cells within the gastrointestinal tract smooth muscle. They create the basal (slow waves) rhythm required for contraction and peristalsis. They are mesodermal in origin.
weeks 7-9 - cells initially appear
week 11 - distinct clusters
week 12-14 - clustered around myenteric ganglia along the entire gut
- Links: Neural Crest Development
Abnormalities
- Abnormality Links: Gastrointestinal Tract - Abnormalities | Intestine Development | Gastrointestinal Tract
- Lumen Abnormalities: Image - Duplication sites | Pyloric atresia | Jejunal atresia
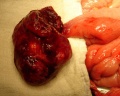
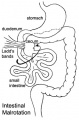
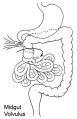
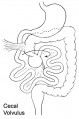
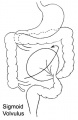
- Rotation: Image - Midgut volvulus | Image - Intestinal malrotation | Image - Cecal volvulus | Image - Sigmoid volvulus | Ladd's band
- Meckel's Diverticulum: Meckel's Image 1 | Meckel's Image 2 | Meckel's Image 3 |
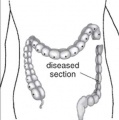
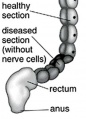
- Intestinal Aganglionosis: Image - Ostomy | Image - Stoma | Surgery 1 | Surgery 2 | Surgery 3
Appendix Duplication
Appendix duplication is an extremely rare congenital anomaly (0.004% to 0.009% of appendectomy specimens) first classified according to their anatomic location by Cave in 1936[26] and a later modified by Wallbridge in 1963[27], subsequently two more types of appendix abnormalities have been identified.[28][29]
Modified Cave-Wallbridge Classification (table from[30])
| Classification of types of appendix duplication |
Features |
| A | Single cecum with various degrees of incomplete duplication |
| B1 (bird type) | Two appendixes symmetrically placed on either side of the ileocecal valve |
| B2 (tenia coli type) | ne appendix arises from the cecum at the usual site, and the second
appendix branches from the cecum along the lines of the tenia at various distances from the first |
| B3 | One appendix arises from the usual site, and the second appendix arises from
the hepatic flexura |
| B4 | One appendix arises from the usual site, and the second appendix arises from
the splenic flexura |
| C | Double cecum, each with an appendix |
| Horseshoe appendix | One appendix has two openings into a common cecum |
| Triple appendix | One appendix arises from the cecum at the usual site, and two additional appendixes arise from the colon |
Short Bowel Syndrome
Short bowel syndrome (SBS) results typically due to developmental abnormalities, extensive intestinal resection during the neonatal period, or necrotising enterolitis.[31]
- reduces gut function for digestion and absorption of nutrients (intestinal failure).
- Links: PubMed Health | Better Health
Molecular Factors
- Cdx (Caudal-type homeobox) group of ParaHox genes (mouse Cdx1, Cdx2 and Cdx4)[32]
- FGF9
References
- ↑ 1.0 1.1 Xu K, Wu X, Shapiro E, Huang H, Zhang L, Hickling D, Deng Y, Lee P, Li J, Lepor H & Grishina I. (2012). Bmp7 functions via a polarity mechanism to promote cloacal septation. PLoS ONE , 7, e29372. PMID: 22253716 DOI.
- ↑ Wang S, Cebrian C, Schnell S & Gumucio DL. (2018). Radial WNT5A-Guided Post-mitotic Filopodial Pathfinding Is Critical for Midgut Tube Elongation. Dev. Cell , 46, 173-188.e3. PMID: 30016620 DOI.
- ↑ 3.0 3.1 Ben-Nun MS, Ben-Shlush A & Raviv Zilka L. (2018). Growth of the colon and rectum throughout gestation: evaluation with fetal MRI. Acta Radiol Open , 7, 2058460118761206. PMID: 29531795 DOI.
- ↑ Ueno S, Yamada S, Uwabe C, Männer J, Shiraki N & Takakuwa T. (2016). The Digestive Tract and Derived Primordia Differentiate by Following a Precise Timeline in Human Embryos Between Carnegie Stages 11 and 13. Anat Rec (Hoboken) , 299, 439-49. PMID: 26995337 DOI.
- ↑ Wells JM & Spence JR. (2014). How to make an intestine. Development , 141, 752-60. PMID: 24496613 DOI.
- ↑ Geske MJ, Zhang X, Patel KK, Ornitz DM & Stappenbeck TS. (2008). Fgf9 signaling regulates small intestinal elongation and mesenchymal development. Development , 135, 2959-68. PMID: 18653563 DOI.
- ↑ Sundin OH, Mendoza-Ladd A, Zeng M, Diaz-Arévalo D, Morales E, Fagan BM, Ordoñez J, Velez P, Antony N & McCallum RW. (2017). The human jejunum has an endogenous microbiota that differs from those in the oral cavity and colon. BMC Microbiol. , 17, 160. PMID: 28716079 DOI.
- ↑ 8.0 8.1 8.2 8.3 Soffers JH, Hikspoors JP, Mekonen HK, Koehler SE & Lamers WH. (2015). The growth pattern of the human intestine and its mesentery. BMC Dev. Biol. , 15, 31. PMID: 26297675 DOI.
- ↑ Martin V & Shaw-Smith C. (2010). Review of genetic factors in intestinal malrotation. Pediatr. Surg. Int. , 26, 769-81. PMID: 20549505 DOI.
- ↑ FitzSimmons J, Chinn A & Shepard TH. (1988). Normal length of the human fetal gastrointestinal tract. Pediatr Pathol , 8, 633-41. PMID: 3244599
- ↑ Archie JG, Collins JS & Lebel RR. (2006). Quantitative standards for fetal and neonatal autopsy. Am. J. Clin. Pathol. , 126, 256-65. PMID: 16891202 DOI.
- ↑ 12.0 12.1 Weaver LT, Austin S & Cole TJ. (1991). Small intestinal length: a factor essential for gut adaptation. Gut , 32, 1321-3. PMID: 1752463
- ↑ Keith A. Human Embryology and Morphology. (1902) London: Edward Arnold.
- ↑ Fisher RE. (2000). The primate appendix: a reassessment. Anat. Rec. , 261, 228-36. PMID: 11135184
- ↑ Malas MA, Gökçimen A & Sulak O. (2001). Growing of caecum and vermiform appendix during the fetal period. Fetal. Diagn. Ther. , 16, 173-7. PMID: 11316934 DOI.
- ↑ Malas MA, Sulak O, Gökçimen A & Sari A. (2004). Development of the vermiform appendix during the fetal period. Surg Radiol Anat , 26, 202-7. PMID: 15173960 DOI.
- ↑ de Vries PA. and Friedland GW. The staged sequential development of the anus and rectum in human embryos and fetuses. (1974) J. Pediatr. Surg., 9(5): 755-69 PMID 4424274
- ↑ Hashimoto R. (2013). Development of the human tail bud and splanchnic mesenchyme. Congenit Anom (Kyoto) , 53, 27-33. PMID: 23480355 DOI.
- ↑ Li FF, Zhang T, Bai YZ, Yuan ZW & Wang WL. (2011). Spatiotemporal expression of Wnt5a during the development of the hindgut and anorectum in human embryos. Int J Colorectal Dis , 26, 983-8. PMID: 21431850 DOI.
- ↑ van der Putte SC. (2009). The development of the human anorectum. Anat Rec (Hoboken) , 292, 951-4. PMID: 19496155 DOI.
- ↑ Kromer P. (1999). Further study of the urorectal septum in staged human embryos. Folia Morphol. (Warsz) , 58, 53-63. PMID: 10504783
- ↑ Nievelstein RA, van der Werff JF, Verbeek FJ, Valk J & Vermeij-Keers C. (1998). Normal and abnormal embryonic development of the anorectum in human embryos. Teratology , 57, 70-8. PMID: 9562679 <70::AID-TERA5>3.0.CO;2-A DOI.
- ↑ Kromer P. (1996). Development of the urorectal septum and differentiation of the urogenital sinus in human embryos of stages 13 to 19. Folia Morphol. (Warsz) , 55, 362-3. PMID: 9243909
- ↑ Kluth D, Fiegel HC & Metzger R. (2011). Embryology of the hindgut. Semin. Pediatr. Surg. , 20, 152-60. PMID: 21708335 DOI.
- ↑ Burns AJ, Roberts RR, Bornstein JC & Young HM. (2009). Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin. Pediatr. Surg. , 18, 196-205. PMID: 19782301 DOI.
- ↑ Cave AJ. (1936). Appendix Vermiformis Duplex. J. Anat. , 70, 283-92. PMID: 17104589
- ↑ WALLBRIDGE PH. (1962). Double appendix. Br J Surg , 50, 346-7. PMID: 13998581
- ↑ Mesko TW, Lugo R & Breitholtz T. (1989). Horseshoe anomaly of the appendix: a previously undescribed entity. Surgery , 106, 563-6. PMID: 2772830
- ↑ Tinckler LF. (1968). Triple appendix vermiformis--a unique case. Br J Surg , 55, 79-81. PMID: 5635427
- ↑ Canbay E & Akman E. (2011). Appendix perforation in appendix duplication in a man: a case report. J Med Case Rep , 5, 162. PMID: 21513538 DOI.
- ↑ Davì G, Pinto A, Palumbo MG, Gallo V, Mazza A & Strano A. (1985). Dipyridamole and aspirin in arteriosclerosis obliterans of the lower limbs. Adv. Prostaglandin Thromboxane Leukot. Res. , 13, 271-5. PMID: 3159212
- ↑ Beck F & Stringer EJ. (2010). The role of Cdx genes in the gut and in axial development. Biochem. Soc. Trans. , 38, 353-7. PMID: 20298182 DOI.
Reviews
Kostouros A, Koliarakis I, Natsis K, Spandidos DA, Tsatsakis A & Tsiaoussis J. (2020). Large intestine embryogenesis: Molecular pathways and related disorders (Review). Int. J. Mol. Med. , , . PMID: 32319546 DOI.
Chin AM, Hill DR, Aurora M & Spence JR. (2017). Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. , 66, 81-93. PMID: 28161556 DOI.
Viotti M, Foley AC & Hadjantonakis AK. (2014). Gutsy moves in mice: cellular and molecular dynamics of endoderm morphogenesis. Philos. Trans. R. Soc. Lond., B, Biol. Sci. , 369, . PMID: 25349455 DOI.
Wells JM & Spence JR. (2014). How to make an intestine. Development , 141, 752-60. PMID: 24496613 DOI.
Shyer AE, Tallinen T, Nerurkar NL, Wei Z, Gil ES, Kaplan DL, Tabin CJ & Mahadevan L. (2013). Villification: how the gut gets its villi. Science , 342, 212-8. PMID: 23989955 DOI.
Noah TK, Donahue B & Shroyer NF. (2011). Intestinal development and differentiation. Exp. Cell Res. , 317, 2702-10. PMID: 21978911 DOI.
Burns AJ, Roberts RR, Bornstein JC & Young HM. (2009). Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin. Pediatr. Surg. , 18, 196-205. PMID: 19782301 DOI.
Articles
Cho BH, Kim JH, Jin ZW, Wilting J, Rodríguez-Vázquez JF & Murakami G. (2018). Topographical anatomy of the intestines during in utero physiological herniation. Clin Anat , 31, 583-592. PMID: 29044646 DOI.
Soffers JH, Hikspoors JP, Mekonen HK, Koehler SE & Lamers WH. (2015). The growth pattern of the human intestine and its mesentery. BMC Dev. Biol. , 15, 31. PMID: 26297675 DOI.
Ueda Y, Yamada S, Uwabe C, Kose K & Takakuwa T. (2016). Intestinal Rotation and Physiological Umbilical Herniation During the Embryonic Period. Anat Rec (Hoboken) , 299, 197-206. PMID: 26599074 DOI.
Kim TH, Kim BM, Mao J, Rowan S & Shivdasani RA. (2011). Endodermal Hedgehog signals modulate Notch pathway activity in the developing digestive tract mesenchyme. Development , 138, 3225-33. PMID: 21750033 DOI.
Kim WK, Kim H, Ahn DH, Kim MH & Park HW. (2003). Timetable for intestinal rotation in staged human embryos and fetuses. Birth Defects Res. Part A Clin. Mol. Teratol. , 67, 941-5. PMID: 14745932 DOI.
Search Pubmed
Search Bookshelf Intestine Development
Search Pubmed Now: Intestine Embryology | Intestine Development
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Tandler J. The development of the human duodenum in early embryonic stages. (Zur Entwicklungsgeschichte des menschlichen Duodenum in frṻhen Embryonalstadien) (1900) Morphol. Jahrb. 29:187-216.
Tandler (1900) was the first to recognize that the lumen of the duodenum in embryos between " 30 to 60 days," is normally "more or less completely" obliterated.
Frazer JE. and Robbins RH. On the factors concerned in causing rotation of the intestine in man. (1915) J Anat. 50(1): 75-110. PMID 17233053
- Intestine Development
Cave AJ. Appendix vermiformis duplex. (1936) J Anat. 70: 283-292. PMID 171045891
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- NIH PubMed Health Short Bowel Syndrome
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Gastrointestinal Tract - Intestine Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Gastrointestinal_Tract_-_Intestine_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G