Endocrine System - Abnormalities
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The endocrine system has an ongoing important role in embryonic, fetal and postnatal development as well as maintainance of homeostasis and reproductive function. There exists a complex interaction between the maternal and fetal endocrine system during development and failure for fetal endocrine development has a cascading effect on many other developing systems. There are additional pages covering abnormalities of specific endocrine organs.
The endocrine system resides within specific endocrine organs and both organs and tissues with other specific functions. Epithelia (ectoderm and endoderm) form the majority of the “ductless” endocrine glands like gastrointestinal and skin associated “ducted” glands. Differentiation of several also organs involves a epithelial/mesenchye interaction, seen in repeated in many differentiation of many different tissues. The endocrine glands produce hormones, which are distributed by the vascular system to the many body tissues, subsequently these organs are richly vascularized.
Hormones are recognised by either cell surface receptors (modified amino acids, peptides, proteins) or cytoplasmic/nuclear receptors (steroids). Hormones “orchestrate” responses in other tissues, including other endocrine organs, and these overall effects can be similar or different in different tissues. In addition, these hormone effects (like music) can be rapid, slow, brief, diurnal, or long-term. Hormone effects can be mimicked, stimulated, and blocked by therapeutic drugs, nutritional and environmental chemicals.
The human fetus is dependent upon endocrine development for hormones, which support normal development. Peripheral endocrine glands (thyroid, pancreas, adrenals, gonads) form early in the second month from epithelial/mesenchye interactions and differentiate into the third month. The fetus also has a unique hormonal system that combines not only its own developing endocrine system, but also that of the placenta and maternal hormones.
Abnormal endocrine development/function can impact on many different systems. For example, insufficient maternal dietary iodine impacts on fetal thyroid gland thyroid hormone production, which in turn can lead to abnormal neural development. Alternatively, we now know many environmental and therapeutic chemicals have a wide range of effects on the endocrine system.
Sex hormones from the gonads also have significant effects prenatally and postnatally, specifically at puberty with a role to play in male/female biological maturity and have wide actions throughout the body. Finally, each endocrine organ page listed below has additional abnormalities information specific to that organ.
| System Abnormalities | ||||
|---|---|---|---|---|
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Endocrine Congenital Abnormalities | Endocrine Developmental Abnormalities | Endocrine Congenital Disruptors |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Pineal
- Hypoplasia - associated with retinal disease.
- Tumours - in children are associated with abnormal puberty development.
Pituitary
- craniopharyngeal canal - Rathke's pouch abnormality, from the anterior part of the fossa hypophyseos of the sphenoid bone to the under surface of the skull.
- pituitary tumours (adenomas) - several abnormalities associated with abnormal levels of the hormonal output of the pituitary.
- Growth hormone (GH) adenomas - benign pituitary tumors lead to chronic high GH output levels, that may lead to acromegaly.
- Cushing's disease - caused either by a pituitary adenoma produces excess adrenocorticotropic hormone (ACTH, corticotropin) or due to ectopic tumors secreting ACTH or corticotropin-releasing hormone (CRH).
- Links: pituitary
Thyroid
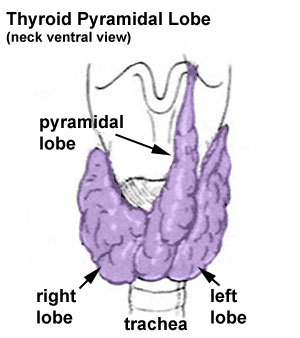
- Pyramidal lobe - from isthmus (50% of people) attached to hyoid bone distal end of thryoglossal duct.
- Congenital hypothyroidism - approximately 1 in 3000 births, associated with neurological abnormalities.
- Lingual thyroid gland - failure of thyroid descent.
- Thyroglossal cyst - persistance of thyroglossal duct. Image - thyroglossal duct
- Thyroglossal fistula - partial degeneration of the thyroglossal duct.
- Abnormal development of the thyroid - incomplete or excessive descent.
- Childhood hypothyroidism delays ossification and bone mineralization.
Iodine Deficiency
- A teaspoon of iodine, total lifetime requirement, cannot be stored for long periods by our body, tiny amounts are needed regularly
- Areas of endemic iodine deficiency, where soil and therefore crops and grazing animals do not provide sufficient dietary iodine to the populace
- food fortification and supplementation - Iodized salt programs and iodized oil supplements are the most common tools in fight against IDD
Thyroid Disruptors
| Groups of chemicals | Level of Acton | 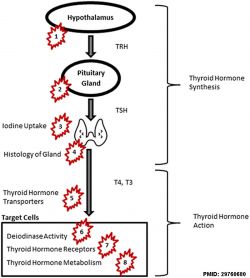
|
|---|---|---|
| polychlorinated biphenyl (PCB) and polychlorinated dibenzodioxins (PCDD) | 5, 7 | |
| polybrominated diphenyl ethers (PBDEs) | 5, 6, 7, 8 | |
| pesticides | 4, 5, 7 | |
| perfluoroalkyl substances (PFASs) | 5, 6; NIS: 3 | |
| bisphenol A (BPA) | 2, 7 | |
| phthalates | 1, 2, 5, 8. | |
| Table Data[6] Links: thyroid | chemicals | endocrine abnormalities | ||
- Links: thyroid
Familial Goitrous Cretinism
Synthesis of thyroid hormones is disrupted due to an enzymatic defect. Features do not appear at birth, though if not treated, these features appear early in the neonatal period.
- Low levels of thyroid hormones
- Cause increased release of TSH by the pituitary
- Causes in turn enlargement of the thyroid gland
Parathyroid
- Usually four glands are present (2 on each side), but three to six glands have been found in human.
- Lower parathyroid glands arise from the third pharyngeal pouch and descend with the thymus. Variable descent can lead to a range of adult locations, from just beneath the mandible to the anterior mediastinum.
- Links: parathyroid
Thymus
Ectopic thymus - rare anomaly where the thymus does not completely descend can cause
- tracheal or esophageal compression
- Horner's syndrome (oculosympathetic paresis)[7]
- Links: thymus
Pancreas
- Type 1 Diabetes - juvenile onset diabetes, more severe form of illness, increases risk of blindness, heart disease, kidney failure, neurological disease, T-lymphocyte-dependent autoimmune disease, infiltration and destruction of the islets of Langerhans, Approx 16 million Americans
- Type 2 Diabetes - loosely defined as "adult onset" diabetes, becoming more common cases of type 2 diabetes seen in younger people
- Risk of developing diabetes - environmental factors (food intake and exercise play an important role, either overweight or obese), Inherited factors (genes involved remain poorly defined)
- Persistent hyperinsulinemic hypoglycemia of infancy - (PHHI, nesidioblastosis, congenital hyperinsulinism of infancy) a rare condition associated with persistently elevated levels of circulating insulin caused by hypersecretion by pancreatic β-cells and an absence of ketone production.
- Hyperinsulinemic hypoglycemia - (HH) is the unregulated secretion of insulin from the pancreatic β-cells in the presence of low blood glucose levels. Genetic abnormalities in nine genes (ABCC8, KCNJ11, GCK, SCHAD, GLUD1, SLC16A1, HNF1A, HNF4A, and UCP2) have been identified.[8]
- Links: pancreas | maternal diabetes
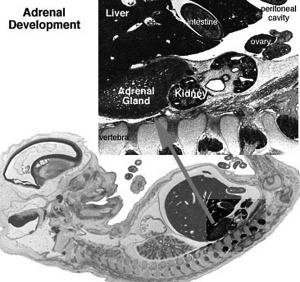
Adrenal
- Congenital Adrenal Hyperplasia (CAH) - family of inherited disorders of adrenal steroidogenesis enzymes which impairs cortisol production by the adrenal cortex. Androgen excess leads newborn females with external genital ambiguity and postnatal progressive virilization in both sexes.
- Enzymes most commonly affected: 21-hydroxylase (21-OH), 11beta-hydroxylase, 3beta-hydroxysteroid dehydrogenase.
- Enzymes less commonly affected: 17alpha-hydroxylase/17,20-lyase and cholesterol desmolase.
- Pheochromocytomas (PCC) - Catecholamine-producing (neuro)endocrine tumor located in the adrenal medulla. Similar catecholamine-producing tumors outside the adrenal gland are called paragangliomas (PGL).
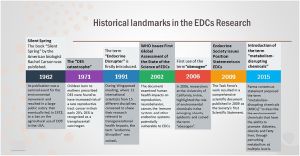
Endocrine Disruptors
Exogenous chemicals that interfere with the function of hormones are described as endocrine disruptors. There are 3 main mechanisms: mimic, block or interfere.
Mimic
Replicate the effects of natural hormones by binding receptors.
| Diethylstilbestrol | |
|---|---|
|
(DES or diethylstilbetrol) a drug prescribed to women from 1938-1971 to prevent miscarriage in high-risk pregnancies. Acts as a potent estrogen (mimics natural hormone) and therefore a potential endocrine disruptor. Banned by the USA FDA in 1979 as a teratogen, previously used as livestock growth promoter.
|
Block
Inhibit the binding of a hormone to receptor or hormone synthesis.
| Finasteride | Vinclozolin |
|---|---|

|
|
| Chemical used to prevent male pattern baldness and enlargement of prostate glands. An anti-androgen (blocks synthesis of dihydrotestosterone) and therefore a potential endocrine disruptor, exposed pregnant women can impact on male fetus genetial development. | Dicarboximide fungicide, perinatal exposure in rats inhibits morphological sex differentiation. In adult rats, shown to cause gonad tumours (Leydig cell) and atrophy. Chemical has androgen-antagonist (antiandrogenic) activity, metabolies compete with natural androgen |
Interfere
Compromise with the hormone transport or elimination.
Polychlorinated biphenyl pollutants - (PCBs) Rats exposed to PCBs have low levels of thyroid hormone. Compete for binding sites of thyroid hormone transport protein. Without being bound to this protein, thyroid hormones are excreted from the body (McKinney et al. 1985; Morse et al. 1996)
| Groups of chemicals | Level of Acton | 
|
|---|---|---|
| polychlorinated biphenyl (PCB) and polychlorinated dibenzodioxins (PCDD) | 5, 7 | |
| polybrominated diphenyl ethers (PBDEs) | 5, 6, 7, 8 | |
| pesticides | 4, 5, 7 | |
| perfluoroalkyl substances (PFASs) | 5, 6; NIS: 3 | |
| bisphenol A (BPA) | 2, 7 | |
| phthalates | 1, 2, 5, 8. | |
| Table Data[6] Links: thyroid | chemicals | endocrine abnormalities | ||
Ziram - (zinc dimethyldithiocarbamate) agricultural fungicide acts as an endocrine disruptor and may cause birth abnormality of the male reproductive system.
International Classification of Diseases
Transitory endocrine and metabolic disorders specific to fetus and newborn (P70-P74)
Incl.: transitory endocrine and metabolic disturbances caused by the infant's response to maternal endocrine and metabolic factors, or its adjustment to extrauterine existence
P70 Transitory disorders of carbohydrate metabolism specific to fetus and newborn
- P70.0 Syndrome of infant of mother with gestational diabetes Fetus or newborn (with hypoglycaemia) affected by maternal gestational diabetes
- P70.1 Syndrome of infant of a diabetic mother Fetus or newborn (with hypoglycemia) affected by maternal diabetes mellitus (pre-existing)
- P70.2 Neonatal diabetes mellitus
- P70.3 Iatrogenic neonatal hypoglycaemia
- P70.4 Other neonatal hypoglycaemia Transitory neonatal hypoglycaemia
- P70.8 Other transitory disorders of carbohydrate metabolism of fetus and newborn
- P70.9 Transitory disorder of carbohydrate metabolism of fetus and newborn, unspecified
P71 Transitory neonatal disorders of calcium and magnesium metabolism
- P71.0 Cow's milk hypocalcaemia in newborn
- P71.1 Other neonatal hypocalcaemia Excl.: neonatal hypoparathyroidism (P71.4)
- P71.2 Neonatal hypomagnesaemia
- P71.3 Neonatal tetany without calcium or magnesium deficiency Neonatal tetany NOS
- P71.4 Transitory neonatal hypoparathyroidism
- P71.8 Other transitory neonatal disorders of calcium and magnesium metabolism
- P71.9 Transitory neonatal disorder of calcium and magnesium metabolism, unspecified
P72 Other transitory neonatal endocrine disorders Excl.: congenital hypothyroidism with or without goitre (E03.0-E03.1) dyshormogenetic goitre (E07.1) Pendred's syndrome (E07.1)
- P72.0 Neonatal goitre, not elsewhere classified Transitory congenital goitre with normal function
- P72.1 Transitory neonatal hyperthyroidism Neonatal thyrotoxicosis
- P72.2 Other transitory neonatal disorders of thyroid function, not elsewhere classified Transitory neonatal hypothyroidism
- P72.8 Other specified transitory neonatal endocrine disorders
- P72.9 Transitory neonatal endocrine disorder, unspecified
P74 Other transitory neonatal electrolyte and metabolic disturbances
- P74.0 Late metabolic acidosis of newborn
- P74.1 Dehydration of newborn
- P74.2 Disturbances of sodium balance of newborn
- P74.3 Disturbances of potassium balance of newborn
- P74.4 Other transitory electrolyte disturbances of newborn
- P74.5 Transitory tyrosinaemia of newborn
- P74.8 Other transitory metabolic disturbances of newborn
- P74.9 Transitory metabolic disturbance of newborn, unspecified
References
- ↑ 1.0 1.1 Papalou O, Kandaraki EA, Papadakis G & Diamanti-Kandarakis E. (2019). Endocrine Disrupting Chemicals: An Occult Mediator of Metabolic Disease. Front Endocrinol (Lausanne) , 10, 112. PMID: 30881345 DOI.
- ↑ Miranda A & Sousa N. (2018). Maternal hormonal milieu influence on fetal brain development. Brain Behav , 8, e00920. PMID: 29484271 DOI.
- ↑ Towers CV, Terry PD, Lewis D, Howard B, Chambers W, Armistead C, Weitz B, Porter S, Borman CJ, Kennedy RC & Chen J. (2015). Transplacental passage of antimicrobial paraben preservatives. J Expo Sci Environ Epidemiol , 25, 604-7. PMID: 25944699 DOI.
- ↑ Berger E, Potouridis T, Haeger A, Püttmann W & Wagner M. (2015). Effect-directed identification of endocrine disruptors in plastic baby teethers. J Appl Toxicol , 35, 1254-61. PMID: 25988240 DOI.
- ↑ Marcucci G, Cianferotti L, Beck-Peccoz P, Capezzone M, Cetani F, Colao A, Davì MV, degli Uberti E, Del Prato S, Elisei R, Faggiano A, Ferone D, Foresta C, Fugazzola L, Ghigo E, Giacchetti G, Giorgino F, Lenzi A, Malandrino P, Mannelli M, Marcocci C, Masi L, Pacini F, Opocher G, Radicioni A, Tonacchera M, Vigneri R, Zatelli MC & Brandi ML. (2015). Rare diseases in clinical endocrinology: a taxonomic classification system. J. Endocrinol. Invest. , 38, 193-259. PMID: 25376364 DOI.
- ↑ 6.0 6.1 Ghassabian A & Trasande L. (2018). Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Front Endocrinol (Lausanne) , 9, 204. PMID: 29760680 DOI.
- ↑ Berroth ML, Morozova LV & Pollock JM. (2020). Ectopic thymus as a cause of Horner's syndrome. Radiol Case Rep , 15, 23-25. PMID: 31737141 DOI.
- ↑ Nessa A, Rahman SA & Hussain K. (2016). Hyperinsulinemic Hypoglycemia - The Molecular Mechanisms. Front Endocrinol (Lausanne) , 7, 29. PMID: 27065949 DOI.
- ↑ Laronda MM, Unno K, Ishi K, Serna VA, Butler LM, Mills AA, Orvis GD, Behringer RR, Deng C, Sinha S & Kurita T. (2013). Diethylstilbestrol induces vaginal adenosis by disrupting SMAD/RUNX1-mediated cell fate decision in the Müllerian duct epithelium. Dev. Biol. , 381, 5-16. PMID: 23830984 DOI.
- ↑ Singh NP, Miranda K, Singh UP, Nagarkatti P & Nagarkatti M. (2018). Diethylstilbestrol (DES) induces autophagy in thymocytes by regulating Beclin-1 expression through epigenetic modulation. Toxicology , 410, 49-58. PMID: 30153466 DOI.
- NIH Genes & Disease Chapter 41 - Glands and Hormones
- Endocrinology: An Integrated Approach Nussey, S.S. and Whitehead, S.A. London:Taylor & Francis; c2001 Major hormone types
- Genes and Disease, Bethesda (MD): National Library of Medicine (US), NCBI Chapter 41 - Glands and Hormones
Reviews
Nowak K, Jabłońska E & Ratajczak-Wrona W. (2019). Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ Int , 125, 350-364. PMID: 30743143 DOI.
Search
- Bookshelf endocrine | pineal gland | hypothalamus | pituitary gland | thyroid gland | parathyroid gland | thymus gland | endocrine pancreas | adrenal gland
- Pubmed abnormal endocrine development
NCBI - Policies and Guidelines | PubMed | Help:Reference Tutorial
Terms
| Endocrine Terms (expand to view) |
|---|
|
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Endocrine System - Abnormalities. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Endocrine_System_-_Abnormalities
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G