Endocrine - Pancreas Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
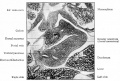
The pancreas is a two-headed organ, not only in origin but also in function. In origin, the pancreas develops from two separate primordia. In function, the organ has both endocrine function in relation to regulating blood glucose (and also other hormone secretions) and gastrointestinal function as an exocrine (digestive) organ, see exocrine pancreas.
In recent years there has been much research due to the increasing incidence of diabetes in humans and the potential for stem cell therapeutics. Much is now known about the epithelial/mesenchymal and molecular regulation of pancres development.
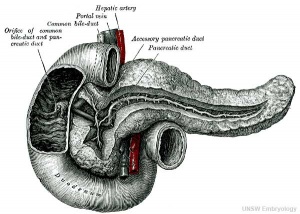
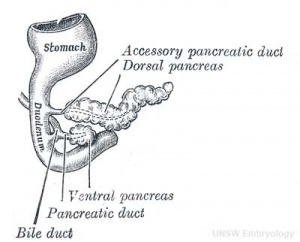
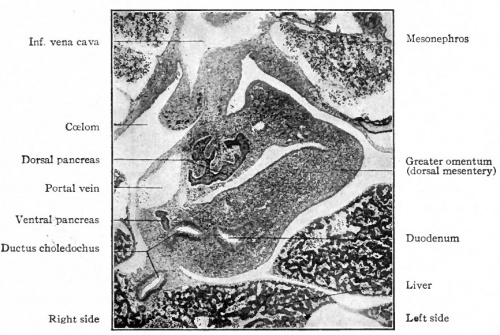
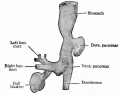
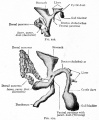
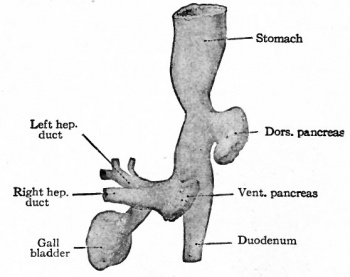
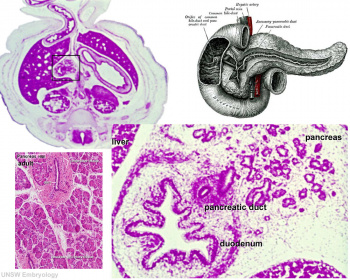
At the foregut/midgut junction the septum transversum generates 2 pancreatic buds (dorsal and ventral endoderm) which will fuse to form the pancreas. The dorsal bud arises first and generates most of the pancreas. The ventral bud arises beside the bile duct and forms only part of the head and uncinate process of the pancreas.
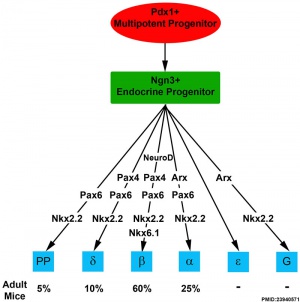
In the fetal period islet cell clusters (icc) differentiate from pancreatic bud endoderm. These cell clusters form acini and ducts (exocrine). On the edge of these cell clusters pancreatic islets (endocrine) also form. Pancreatic hormonal function is mainly to secrete insulin and glucagon that together regulate blood glucose levels. Pancreatic progenitor cells give rise to five endocrine cell types secreting: insulin, glucagon, somatostatin, pancreatic polypeptide and ghrelin. Furthermore gastrin, secreted by stomach G-cells, is also embryonically expressed in the pancreas but disappears after birth.[2]
The pancreas exocrine function begins after birth, while the endocrine function (hormone release) can be measured from 10 to 15 weeks onward. At this stage, it is not clear what the exact roles of these hormones are in regulating fetal growth.
- Functions - exocrine (amylase, alpha-fetoprotein), 99% by volume; endocrine (pancreatic islets) 1% by volume about 1 million islets
- Exocrine function - begins after birth
- Endocrine function - from 10 to 15 weeks onward hormone release
- exact roles of hormones in regulating fetal growth?
- Links: Endocrine Pancreas | Exocrine Pancreas
See also: Lecture - Gastrointestinal Development | Maternal Diabetes
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Pancreas Embryology | Pancreas Development | Endocrine Pancreas Development | Exocrine Pancreas Development | pancreatic islet |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Pancreas Development
- Pancreatic buds - duodenal level endoderm, splanchnic mesoderm forms dorsal and ventral mesentery, dorsal bud (larger, first), ventral bud (smaller, later)
- Pancreas Endoderm - pancreas may be opposite of liver
- Heart cells promote/notochord prevents liver formation
- Notochord may promote pancreas formation
- Heart may block pancreas formation
- Duodenum growth/rotation - brings ventral and dorsal buds together, fusion of buds
- Pancreatic duct - ventral bud duct and distal part of dorsal bud, exocrine function
- Islet cells - cords of endodermal cells form ducts, from which cells bud off to form islets[9]
Human Pancreas Timeline
| Carnegie Stage | Days | Event |
|---|---|---|
| 10 | 25-27 | distal endoderm foregut |
| 12 | 29-31 | pancreatic duodenal endoderm
extra-hepatic billiard duct |
| 13 | 30-33 | pancreatic bud |
| 19 | 47 | "trunk" progenitor
"tip" progenitor |
| 23 | 8 weeks + | fetal beta cell
ductal cell |
| fetal | 14 weeks | acinar cell |
| Table data[10] Links: pancreas | exocrine pancreas | pancreas molecular timeline | timeline | ||
|
|
| Human (week 4) pancreatic buds | Human (week 8, Stage 22) pancreas |
- Week 7 to 20 - pancreatic hormones secretion increases, small amount maternal insulin
- Week 10 - glucagon (alpha) differentiate first, somatostatin (delta), insulin (beta) cells differentiate, insulin secretion begins
- Week 15 - glucagon detectable in fetal plasma
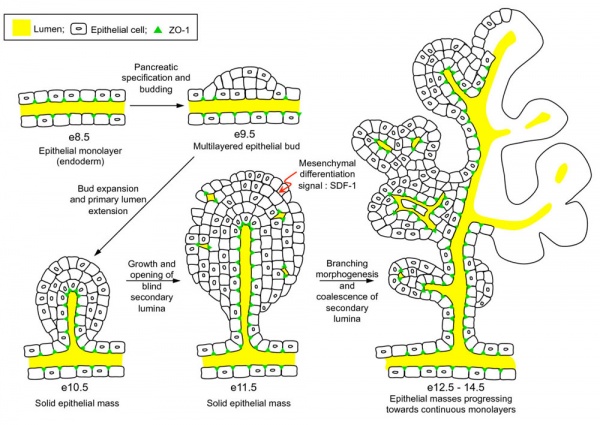
Mouse pancreas duct development cartoon
|
|
|
|
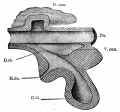
Pig embryo (14 mm CRL) (ventral and dorsal)
Fetal Pancreas
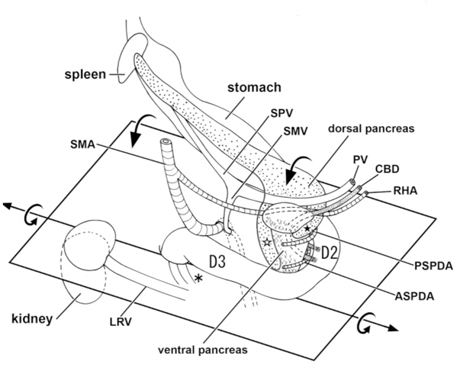
Fetal topographical anatomy of the pancreatic head and duodenum with special reference to courses of the pancreaticoduodenal arteries.[11]
A diagram showing joining processes between the dorsal and ventral primordia of the pancreas as well as the hypothetical rotation of the duodenum along a left-right axis. Viewed from the posterosuperior side of the body. A horizontal plane including most parts of the duodenum is shown to emphasize, in contrast to adults, the course of the second portion (D2) directing posteriorly rather than inferiorly.
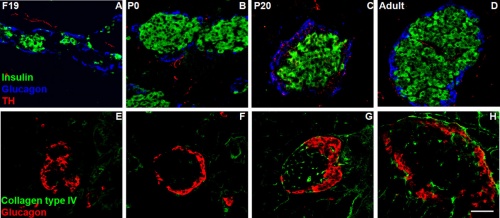
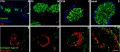
Developing Pancreatic Islets
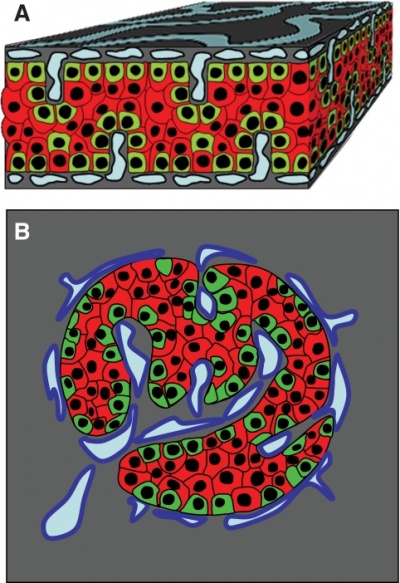
| Model of endocrine cell and vessel organization in human islets[12] | A α-Cells (green) and β-cells (red) are organized into a thick folded plate lined at both sides with vessels (blue).
|
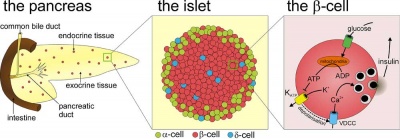
Adult Pancreatic Islets
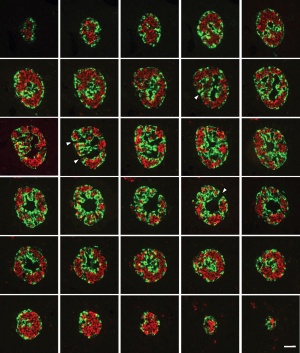
The adult pancreatic islets (Islets of Langerhans) contain four distinct endocrine cell types.
Alpha Cells
- glucagon, mobilizes lipid
Beta Cells
- insulin, increase glucose uptake
- stimulate fetal growth, continue to proliferate to postnatal, in infancy most abundant
- amylin - a peptide hormone that is cosecreted with insulin.[13]
- maternal amylin levels appear to be elevated during pregnancy relative to insulin.
Molecular - Nkx6.1 - NK2 Homeobox 6.1
- homeobox (Hox) containing transcription factor contain a 60-amino acid evolutionarily conserved DNA-binding homeodomain.
- required for beta cells development and is completely conserved between rat, mouse, and human.
- Links: Maternal Diabetes | Nkx6.1 OMIM 602563
Delta Cells
- somatostatin, inhibits glucagon, insulin secretion
F-cells
- pancreatic polypeptide
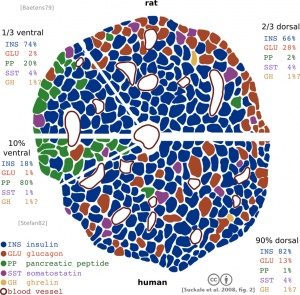
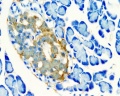
Rat - pancreatic islet development[14]
Animal Models
The following species comparison table has been slightly modified from Table 1 data from a review paper.[15]
- Islet size is described as an effective diameter of a circle, which depicts the same area as a measured islet area.
- β-cell ratio is the area ratio of β-cells in an islet.
- Both data sets are expressed as the mean value with its standard deviation.
| Pancreas Islet Species Comparison | |||
|---|---|---|---|
| Species | Age | Islet size (μm) | β-cell ratio |
| Human | 39 years (adult) | 50 ± 29 | 0.64 ± 0.21 |
| Monkey | 1 year | 67 ± 38* | 0.79 ± 0.14* |
| Pig | 6 month | 49 ± 15a | 0.89 ± 0.11* |
| Rabbit | 6 month | 64 ± 28* | 0.79 ± 0.17* |
| Bird | 40 day | 24 ± 6* | 0.46 ± 0.24* |
| Wild-type mouse | 6 month | 116 ± 80* | 0.85 ± 0.14* |
| Pregnant mouse | 3 month | 112 ± 94* | 0.84 ± 0.22* |
| ob/ob mouse | 15 week | 86 ± 76* | 0.92 ± 0.11* |
| db/db mouse | 15 week | 47 ± 24b | 0.53 ± 0.24c |
|
*p < 0.0001 ap = 0.65 bp = 0.42 cp = 0.0004 compared with human.
| |||
| Reference: [15] | |||
Mouse
|
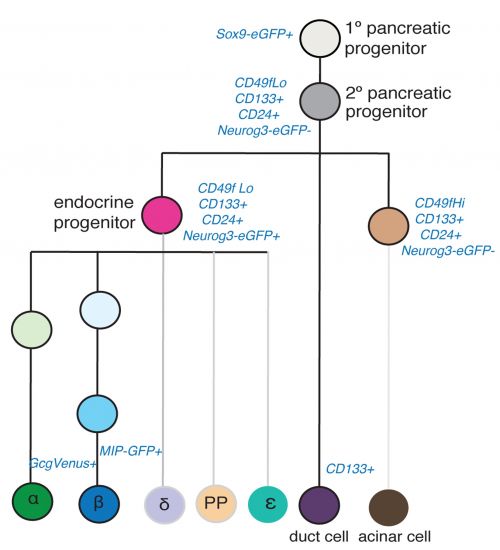
The following cell types were collected: E11 and E15 pancreatic progenitors, E15 acinar cells, E15 endocrine progenitors (EP), E15, E17, P1, P15, 8–12 week beta cells, P1 and 8–12 week alpha cells, and adult duct cells.[16]
|
Hormones
Insulin
- Source - synthesized by the beta cells of the islets of Langerhans.
- Protein
- 2 dissimilar polypeptide chains, A and B, which are linked by 2 disulphide bonds.
- both chains are derived from a 1-chain precursor, proinsulin.
- proinsulin - converted to insulin by the enzymatic removal of a segment that connects the amino end of the A chain to the carboxyl end of the B chain.
- Links: OMIM
Glucagon
- Source - synthesized by the alpha cells of the islets of Langerhans.
- Protein
- 29-amino acid hormone
- human, rabbit, rat, pig, and cow proteins are identical.
- member of a multigene family that includes - secretin, vasoactive intestinal peptide, gastric inhibitory peptide, glicentin, and others.
- Function
- counteracts the glucose-lowering action of insulin
- stimulates glycogenolysis and gluconeogenesis.
- Links: OMIM
Molecular
| Human Pancreas Molecular Timeline | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
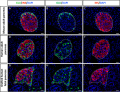
| Mouse Pancreas Cell Lineage
In this study[16] mouse cell types were collected at different ages E11 and E15 pancreatic progenitors, E15 acinar cells, E15 endocrine progenitors (EP), E15, E17, P1, P15, 8–12 week beta cells, P1 and 8–12 week alpha cells, and adult duct cells. The following markers were used in determining the lineages, not both endocrine and exocrine cells derive from a common precursor.
|
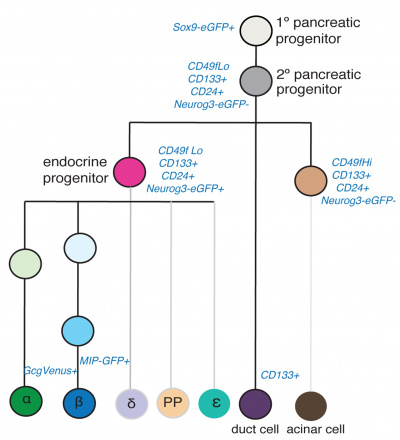
Identification of pancreas cell lineages[16] |
| Developmental Factors | |
|---|---|
|
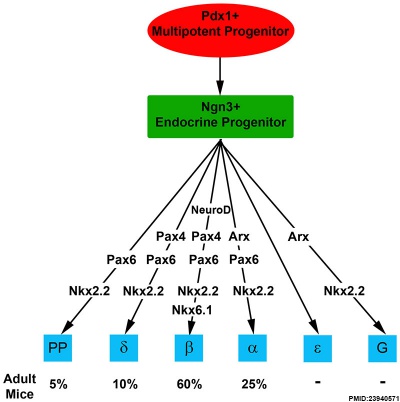
Molecular Development of Endocrine Pancreas Cells[2] |
- Links: Molecular Development
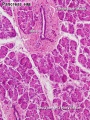
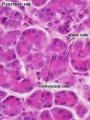
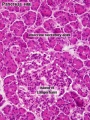
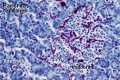
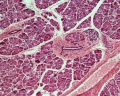
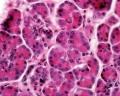
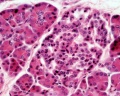
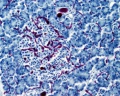
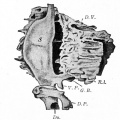
Pancreas Histology
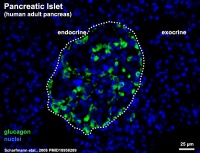
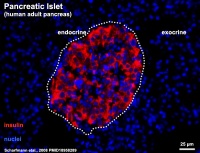
- Pancreas Histology Links: overview (label) | exocrine (label) | endocrine (label) | blood vessels (label) | insulin (label) | overview | exocrine | endocrine | blood vessels | insulin | Islet labeled for insulin and Glucagon | Insulin (Fl) | Glucagon (Fl) | GIT Histology
Diabetes
Diabetes is a condition where pancreatic insulin is no longer produced in sufficient required amounts (or at all) meaning that glucose cannot be converted into energy, resulting in health issues related to blood sugar levels. There are two main types:
- Type 1 diabetes - (10% of all cases) most common chronic childhood condition. An auto-immune condition, where the immune system is activated to destroy the beta cells in the pancreas which produce insulin. Type 1 diabetes is not linked to modifiable lifestyle factors.
- Type 2 diabetes - (85–90% of all cases) most common in adults. A progressive condition in which the body becomes resistant to the normal effects of insulin and/or gradually loses the capacity to produce enough insulin in the pancreas. Type 2 diabetes is associated with modifiable lifestyle risk factors and has strong genetic and family related risk factors.
Secondary Health Issues:
- Diabetic retinopathy - is a leading cause of preventable blindness.
- Diabetic ketoacidosis (DKA) occurs among children and young people with type 1 diabetes and is 1.4 times higher in females.
- Links: Maternal Diabetes | External Links
Abnormalities
Listed below are a number of pancreatic developmental abnormalities, see also the 2003 article "Lifetime consequences of abnormal fetal pancreatic development".[17]
Accessory Pancreatic Tissue
Pancreatic tissue located in associated gastrointestinal tract tissues/organs such as the wall of the stomach, duodenum, jejunum or Meckel's diverticulum.
Annular Pancreas
Incidence (1 in 7,000 people) pancreas forms as a "ring" of tissue surrounding the duodenum which is subsequently narrowed.
Diabetes Mellitus
Maternal diabetes (and hyperglycaemia) have been shown to lead to increased fetal islet hyperplasia of the insulin producing beta cells and insulin secretion.
Intrauterine growth restriction
Can lead to a delayed development of the insulin producing beta cells and low insulin secretion.
Tumours
Serous Cystadenoma (endocrine tumour), Somatostatinoma (tumour of delta cell origin), intraductal papillary-mucinous neoplasm
Diabetic ketoacidosis
(DKA) occurs among children and young people with type 1 diabetes and is 1.4 times higher in females.
Hyperinsulinemic hypoglycemia
Hyperinsulinemic hypoglycemia (HH) is the unregulated secretion of insulin from the pancreatic β-cells in the presence of low blood glucose levels. Genetic abnormalities in nine genes (ABCC8, KCNJ11, GCK, SCHAD, GLUD1, SLC16A1, HNF1A, HNF4A, and UCP2) have been identified.[18]
References
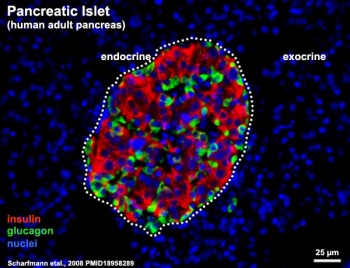
- ↑ Scharfmann R, Xiao X, Heimberg H, Mallet J & Ravassard P. (2008). Beta cells within single human islets originate from multiple progenitors. PLoS ONE , 3, e3559. PMID: 18958289 DOI.
- ↑ 2.0 2.1 2.2 2.3 Suissa Y, Magenheim J, Stolovich-Rain M, Hija A, Collombat P, Mansouri A, Sussel L, Sosa-Pineda B, McCracken K, Wells JM, Heller RS, Dor Y & Glaser B. (2013). Gastrin: a distinct fate of neurogenin3 positive progenitor cells in the embryonic pancreas. PLoS ONE , 8, e70397. PMID: 23940571 DOI.
- ↑ Ryu GR, Lee E, Kim JJ, Moon SD, Ko SH, Ahn YB & Song KH. (2018). Comparison of enteroendocrine cells and pancreatic β-cells using gene expression profiling and insulin gene methylation. PLoS ONE , 13, e0206401. PMID: 30379923 DOI.
- ↑ Scavuzzo MA, Hill MC, Chmielowiec J, Yang D, Teaw J, Sheng K, Kong Y, Bettini M, Zong C, Martin JF & Borowiak M. (2018). Endocrine lineage biases arise in temporally distinct endocrine progenitors during pancreatic morphogenesis. Nat Commun , 9, 3356. PMID: 30135482 DOI.
- ↑ Vieira A, Vergoni B, Courtney M, Druelle N, Gjernes E, Hadzic B, Avolio F, Napolitano T, Navarro Sanz S, Mansouri A & Collombat P. (2018). Neurog3 misexpression unravels mouse pancreatic ductal cell plasticity. PLoS ONE , 13, e0201536. PMID: 30092080 DOI.
- ↑ Rovira M, Huang W, Yusuff S, Shim JS, Ferrante AA, Liu JO & Parsons MJ. (2011). Chemical screen identifies FDA-approved drugs and target pathways that induce precocious pancreatic endocrine differentiation. Proc. Natl. Acad. Sci. U.S.A. , 108, 19264-9. PMID: 22084084 DOI.
- ↑ Landsman L, Nijagal A, Whitchurch TJ, Vanderlaan RL, Zimmer WE, Mackenzie TC & Hebrok M. (2011). Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS Biol. , 9, e1001143. PMID: 21909240 DOI.
- ↑ Dames P, Puff R, Weise M, Parhofer KG, Göke B, Götz M, Graw J, Favor J & Lechner A. (2010). Relative roles of the different Pax6 domains for pancreatic alpha cell development. BMC Dev. Biol. , 10, 39. PMID: 20377917 DOI.
- ↑ Suckale J & Solimena M. (2008). Pancreas islets in metabolic signaling--focus on the beta-cell. Front. Biosci. , 13, 7156-71. PMID: 18508724
- ↑ 10.0 10.1 Jennings RE, Berry AA, Kirkwood-Wilson R, Roberts NA, Hearn T, Salisbury RJ, Blaylock J, Piper Hanley K & Hanley NA. (2013). Development of the human pancreas from foregut to endocrine commitment. Diabetes , 62, 3514-22. PMID: 23630303 DOI.
- ↑ Jin ZW, Yu HC, Cho BH, Kim HT, Kimura W, Fujimiya M & Murakami G. (2010). Fetal topographical anatomy of the pancreatic head and duodenum with special reference to courses of the pancreaticoduodenal arteries. Yonsei Med. J. , 51, 398-406. PMID: 20376893 DOI.
- ↑ 12.0 12.1 Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G & Berney T. (2010). Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes , 59, 1202-10. PMID: 20185817 DOI.
- ↑ Boyle CN & Le Foll C. (2019). Amylin and Leptin interaction: Role During Pregnancy, Lactation and Neonatal Development. Neuroscience , , . PMID: 31846753 DOI.
- ↑ Cabrera-Vásquez S, Navarro-Tableros V, Sánchez-Soto C, Gutiérrez-Ospina G & Hiriart M. (2009). Remodelling sympathetic innervation in rat pancreatic islets ontogeny. BMC Dev. Biol. , 9, 34. PMID: 19534767 DOI.
- ↑ 15.0 15.1 Kim A, Miller K, Jo J, Kilimnik G, Wojcik P & Hara M. (2009). Islet architecture: A comparative study. Islets , 1, 129-36. PMID: 20606719 DOI.
- ↑ 16.0 16.1 16.2 Benitez CM, Qu K, Sugiyama T, Pauerstein PT, Liu Y, Tsai J, Gu X, Ghodasara A, Arda HE, Zhang J, Dekker JD, Tucker HO, Chang HY & Kim SK. (2014). An integrated cell purification and genomics strategy reveals multiple regulators of pancreas development. PLoS Genet. , 10, e1004645. PMID: 25330008 DOI.
- ↑ Holemans K, Aerts L & Van Assche FA. (2003). Lifetime consequences of abnormal fetal pancreatic development. J. Physiol. (Lond.) , 547, 11-20. PMID: 12562919 DOI.
- ↑ Nessa A, Rahman SA & Hussain K. (2016). Hyperinsulinemic Hypoglycemia - The Molecular Mechanisms. Front Endocrinol (Lausanne) , 7, 29. PMID: 27065949 DOI.
Journals
- Pancreas The official journal of the American Pancreatic Association and the Japan Pancreas Society | PubMed
- Pancreatology Official Journal of the International Association of Pancreatology (IAP); European Pancreatic Club (EPC)and 16 other societies and study groups.
- Journal of the Pancreas electronic journal of pancreatology
- Diabetologia | PubMed
Online Textbooks
Endocrinology: An Integrated Approach Nussey, S.S. and Whitehead, S.A. Oxford, UK: BIOS Scientific Publishers, Ltd; 2001. table of Contents
NIH Genes & Disease Chapter 41 - Endocrine
Pathophysiology of the Endocrine System The Endocrine Pancreas
Developmental Biology (6th ed) Gilbert, Scott F. Sunderland (MA): Sinauer Associates, Inc.; c2000.
Molecular Biology of the Cell (4th Edn) Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter. New York: Garland Publishing; 2002. table 15-1. Some Hormone-induced Cell Responses Mediated by Cyclic AMP
Health Services/Technology Assessment Text (HSTAT) Bethesda (MD): National Library of Medicine (US), 2003 Oct.
Search NLM Online Textbooks- "pancreas development" : Endocrinology | Molecular Biology of the Cell | The Cell- A molecular Approach
Search Bookshelf Pancreas Development
Reviews
Honoré C, Rescan C, Hald J, McGrath PS, Petersen MB, Hansson M, Klein T, Østergaard S, Wells JM & Madsen OD. (2016). Revisiting the immunocytochemical detection of Neurogenin 3 expression in mouse and man. Diabetes Obes Metab , 18 Suppl 1, 10-22. PMID: 27615127 DOI.
Ranjan AK, Joglekar MV & Hardikar AA. (2009). Endothelial cells in pancreatic islet development and function. Islets , 1, 2-9. PMID: 21084843 DOI.
Kinkel MD & Prince VE. (2009). On the diabetic menu: zebrafish as a model for pancreas development and function. Bioessays , 31, 139-52. PMID: 19204986 DOI.
Gittes GK. (2009). Developmental biology of the pancreas: a comprehensive review. Dev. Biol. , 326, 4-35. PMID: 19013144 DOI.
Bonal C & Herrera PL. (2008). Genes controlling pancreas ontogeny. Int. J. Dev. Biol. , 52, 823-35. PMID: 18956314 DOI.
Oliver-Krasinski JM & Stoffers DA. (2008). On the origin of the beta cell. Genes Dev. , 22, 1998-2021. PMID: 18676806 DOI.
Articles
Portha B, Chavey A & Movassat J. (2011). Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res , 2011, 105076. PMID: 22110471 DOI.
Riedel MJ, Asadi A, Wang R, Ao Z, Warnock GL & Kieffer TJ. (2012). Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia , 55, 372-81. PMID: 22038519 DOI.
Ma F, Haumaitre C, Chen F & Han Z. (2011). Comparison of murine embryonic pancreatic development in vitro and in vivo. Pancreas , 40, 1012-7. PMID: 21926540 DOI.
McDonald E, Li J, Krishnamurthy M, Fellows GF, Goodyer CG & Wang R. (2012). SOX9 regulates endocrine cell differentiation during human fetal pancreas development. Int. J. Biochem. Cell Biol. , 44, 72-83. PMID: 21983268 DOI.
Yang KM, Yong W, Li AD & Yang HJ. (2011). Insulin-producing cells are bi-potential and differentiatorsprior to proliferation in early human development. World J Diabetes , 2, 54-8. PMID: 21537461 DOI.
Meier JJ, Köhler CU, Alkhatib B, Sergi C, Junker T, Klein HH, Schmidt WE & Fritsch H. (2010). Beta-cell development and turnover during prenatal life in humans. Eur. J. Endocrinol. , 162, 559-68. PMID: 20022941 DOI.
Jeon J, Correa-Medina M, Ricordi C, Edlund H & Diez JA. (2009). Endocrine cell clustering during human pancreas development. J. Histochem. Cytochem. , 57, 811-24. PMID: 19365093 DOI.
Oliver-Krasinski JM, Kasner MT, Yang J, Crutchlow MF, Rustgi AK, Kaestner KH & Stoffers DA. (2009). The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice. J. Clin. Invest. , 119, 1888-98. PMID: 19487809 DOI.
Eberhard D, Tosh D & Slack JM. (2008). Origin of pancreatic endocrine cells from biliary duct epithelium. Cell. Mol. Life Sci. , 65, 3467-80. PMID: 18810318 DOI.
Scharfmann R, Xiao X, Heimberg H, Mallet J & Ravassard P. (2008). Beta cells within single human islets originate from multiple progenitors. PLoS ONE , 3, e3559. PMID: 18958289 DOI.
Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI & Hanley NA. (2004). Beta cell differentiation during early human pancreas development. J. Endocrinol. , 181, 11-23. PMID: 15072563
Search Pubmed
Search April 2010
- Endocrine Development - All (14277) Review (4620) Free Full Text (3140)
Search Pubmed: pancreas development
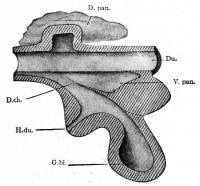
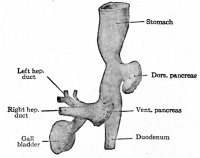
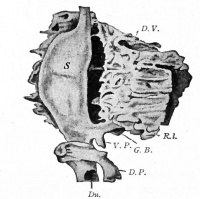
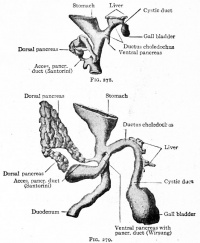
Additional Images
Historic Images
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- F1000 Reports - Recent advances in pancreas development
- Howard Hughes Medical Institute - Seung Kim Lab
- Australian Institute of Health and Welfare - [Factsheet - Diabetic ketoacidosis (DKA) among children and young people with type 1 diabetes]
- Diabetes Australia - Type 1 Diabetes | Type 2 Diabetes
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Endocrine - Pancreas Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Endocrine_-_Pancreas_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G