Developmental Signals - Tbx
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
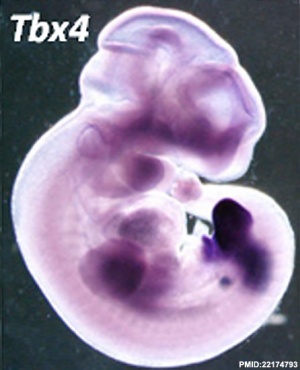
Brachyury was the first of this transcription factor family to be identified in mouse.[2] Genes in the TBX gene family provide instructions for making proteins called T-box proteins that play critical roles during embryonic development. These proteins are especially important for normal development of the arms, hands, and heart. T-box proteins regulate the activity of other genes by attaching (binding) to specific regions of DNA. On the basis of this action, T-box proteins are called transcription factors. Genes in the T-box family are grouped together because the proteins produced from these genes share a similar segment called a T box. The T box is the part of the protein that binds to DNA. T-box proteins often interact with one another or with other transcription factors that regulate gene activity.
Researchers have identified at least 17 genes in the T-box gene family. Mutations in these genes lead to disorders that involve the abnormal development of tissues in which a particular T-box gene is active (expressed). Many genetic disorders caused by T-box gene mutations are characterized by heart problems and/or skeletal abnormalities of the hands and arms.
(text from Genetics Home Reference http://ghr.nlm.nih.gov/geneFamily/tbx)
| Factor Links: AMH | hCG | BMP | sonic hedgehog | bHLH | HOX | FGF | FOX | Hippo | LIM | Nanog | NGF | Nodal | Notch | PAX | retinoic acid | SIX | Slit2/Robo1 | SOX | TBX | TGF-beta | VEGF | WNT | Category:Molecular |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Tbx Embryology | Tbx Development | Tbx Gastrointestinal | Tbx Heart | Tbx Limb | Tbx Renal | Tbx Respiratory |
|
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Human TBX Family
| Table - Human Tbx Family | ||||
| Approved Symbol |
Approved Name | Previous Symbols | Synonyms | Chromosome |
|---|---|---|---|---|
| TBX1 | T-box 1 | VCF | CATCH22 | 22q11.21 |
| TBX2 | T-box 2 | 17q23.2 | ||
| TBX3 | T-box 3 | UMS | "TBX3-ISO, XHL" | 12q24.21 |
| TBX4 | T-box 4 | 17q23.2 | ||
| TBX5 | T-box 5 | HOS | 12q24.21 | |
| TBX6 | T-box 6 | 16p11.2 | ||
| TBX10 | T-box 10 | TBX7 | TBX13 | 11q13.2 |
| TBX15 | T-box 15 | TBX14 | 1p12 | |
| TBX18 | T-box 18 | 6q14.3 | ||
| TBX19 | T-box 19 | "dj747L4.1, TPIT" | 1q24.2 | |
| TBX20 | T-box 20 | 7p14.2 | ||
| TBX21 | T-box 21 | "TBLYM, T-bet" | 17q21.32 | |
| TBX22 | T-box 22 | "CPX, CLPA" | Xq21.1 | |
| TBX23P | T-box 23, pseudogene | TBX23 | 1q25 | |
| TBR1 | T-box, brain 1 | 2q24.2 | ||
| EOMES | eomesodermin | TBR2 | 3p24.1 | |
| MGA | MGA, MAX dimerization protein | "KIAA0518, MAD5, MXD5, FLJ12634" | 15q15 | |
| TBXT | T-box transcription factor T | T | 6q27 | |
| Links: Developmental Signals - Tbx | OMIM Tbx3 | HGNC | Bmp Family | Sox Family | Tbx Family | ||||
| Human TBX Family | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Limb Development
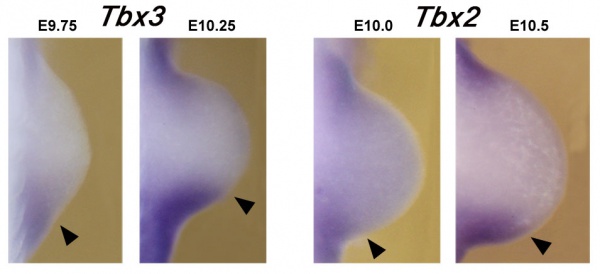
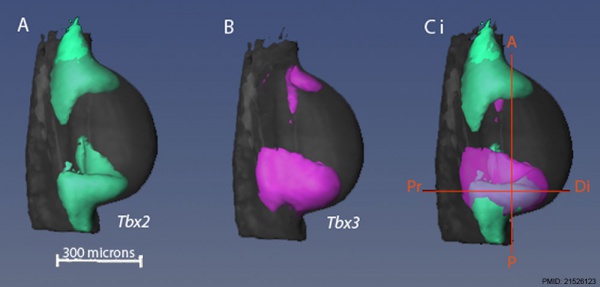
Mouse forelimb bud Tbx3 and Tbx2 expression[8]
Chicken stage 21 wing bud[9]
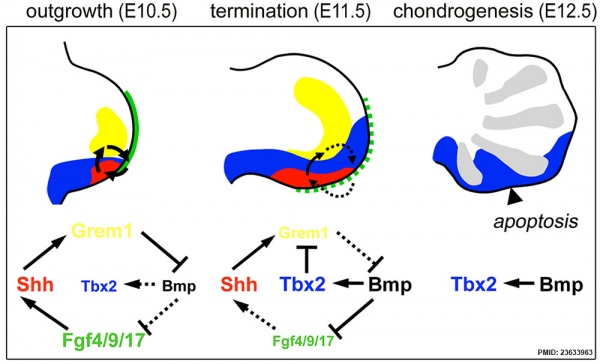
Hindlimb Tbx2 model[10]
- Links: Limb Development
Respiratory Development
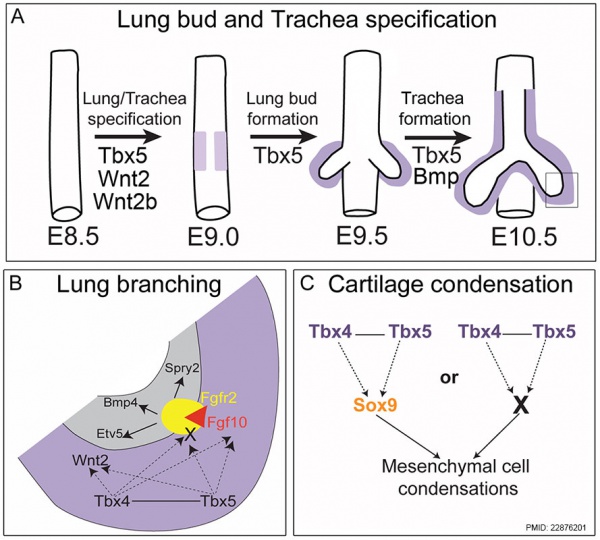
|
Mouse respiratory Tbx4 and Tbx5 model[11]
(Text from figure legend) |
Heart Development
Tbx3 and Tbx18 are both involved with sinoatrial node development.
Cardiac conduction system[12] "Here, we assessed the genome-wide occupation of conduction system-regulating transcription factors TBX3, NKX2-5, and GATA4 and of enhancer-associated coactivator p300 in the mouse heart, uncovering cardiac enhancers throughout the genome. Many of the enhancers colocalized with ion channel genes repressed by TBX3, including the clustered sodium channel genes Scn5a, essential for cardiac function, and Scn10a. We identified 2 enhancers in the Scn5a/Scn10a locus, which were regulated by TBX3 and its family member and activator, TBX5, and are functionally conserved in humans. We also provided evidence that a SNP in the SCN10A enhancer associated with alterations in cardiac conduction patterns in humans disrupts TBX3/TBX5 binding and reduces the cardiac activity of the enhancer in vivo."
Gastrointestinal Development
Tbx1 and Tbx2 genes regulate foregut and pharyngeal development. Mutations in Tbx1 gene are associated with the foregut abnormality of oesophageal atresia with tracheo-oesophageal fistula (Q39.1).[13]
- Links: Gastrointestinal Tract Development | Gastrointestinal_Tract_-_Oesophagus_Development#Abnormalities Oesophagus Abnormalities | OMIM - TBX1
Renal Development
Tbx18, Tbx1, Tbx2, Tbx3, and Tbx20 have various roles in renal development, see this recent review.[14]
- TBX18 mutations associated with kidney and urinary tract anomalies - hydroureter (ureter dilation) and ureteropelvic junction obstruction.
- Links: renal | OMIM - TBX18]
Muscle Development
Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism[5]
- "Skeletal muscle is composed of both slow-twitch oxidative myofibers and fast-twitch glycolytic myofibers that differentially impact muscle metabolism, function and eventually whole-body physiology. Here we show that the mesodermal transcription factor T-box 15 (Tbx15) is highly and specifically expressed in glycolytic myofibers. Ablation of Tbx15 in vivo leads to a decrease in muscle size due to a decrease in the number of glycolytic fibres, associated with a small increase in the number of oxidative fibres. This shift in fibre composition results in muscles with slower myofiber contraction and relaxation, and also decreases whole-body oxygen consumption, reduces spontaneous activity, increases adiposity and glucose intolerance. Mechanistically, ablation of Tbx15 leads to activation of AMPK signalling and a decrease in Igf2 expression. Thus, Tbx15 is one of a limited number of transcription factors to be identified with a critical role in regulating glycolytic fibre identity and muscle metabolism."
- Links: muscle | OMIM - TBX15]
References
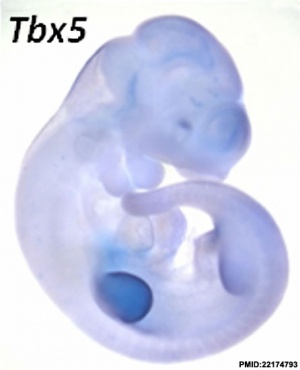
- ↑ 1.0 1.1 Taher L, Collette NM, Murugesh D, Maxwell E, Ovcharenko I & Loots GG. (2011). Global gene expression analysis of murine limb development. PLoS ONE , 6, e28358. PMID: 22174793 DOI.
- ↑ Korzh V & Grunwald D. (2001). Nadine Dobrovolskaïa-Zavadskaïa and the dawn of developmental genetics. Bioessays , 23, 365-71. PMID: 11268043 DOI.
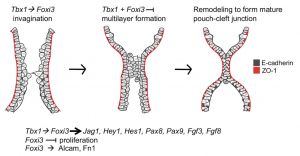
- ↑ 3.0 3.1 Hasten E & Morrow BE. (2019). Tbx1 and Foxi3 genetically interact in the pharyngeal pouch endoderm in a mouse model for 22q11.2 deletion syndrome. PLoS Genet. , 15, e1008301. PMID: 31412026 DOI.
- ↑ 4.0 4.1 Karolak JA, Vincent M, Deutsch G, Gambin T, Cogné B, Pichon O, Vetrini F, Mefford HC, Dines JN, Golden-Grant K, Dipple K, Freed AS, Leppig KA, Dishop M, Mowat D, Bennetts B, Gifford AJ, Weber MA, Lee AF, Boerkoel CF, Bartell TM, Ward-Melver C, Besnard T, Petit F, Bache I, Tümer Z, Denis-Musquer M, Joubert M, Martinovic J, Bénéteau C, Molin A, Carles D, André G, Bieth E, Chassaing N, Devisme L, Chalabreysse L, Pasquier L, Secq V, Don M, Orsaria M, Missirian C, Mortreux J, Sanlaville D, Pons L, Küry S, Bézieau S, Liet JM, Joram N, Bihouée T, Scott DA, Brown CW, Scaglia F, Tsai AC, Grange DK, Phillips JA, Pfotenhauer JP, Jhangiani SN, Gonzaga-Jauregui CG, Chung WK, Schauer GM, Lipson MH, Mercer CL, van Haeringen A, Liu Q, Popek E, Coban Akdemir ZH, Lupski JR, Szafranski P, Isidor B, Le Caignec C & Stankiewicz P. (2019). Complex Compound Inheritance of Lethal Lung Developmental Disorders due to Disruption of the TBX-FGF Pathway. Am. J. Hum. Genet. , , . PMID: 30639323 DOI.
- ↑ 5.0 5.1 Lee KY, Singh MK, Ussar S, Wetzel P, Hirshman MF, Goodyear LJ, Kispert A & Kahn CR. (2015). Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism. Nat Commun , 6, 8054. PMID: 26299309 DOI.
- ↑ Choe CP & Crump JG. (2014). Tbx1 controls the morphogenesis of pharyngeal pouch epithelia through mesodermal Wnt11r and Fgf8a. Development , 141, 3583-93. PMID: 25142463 DOI.
- ↑ Funato N, Nakamura M, Richardson JA, Srivastava D & Yanagisawa H. (2012). Tbx1 regulates oral epithelial adhesion and palatal development. Hum. Mol. Genet. , 21, 2524-37. PMID: 22371266 DOI.
- ↑ Galli A, Robay D, Osterwalder M, Bao X, Bénazet JD, Tariq M, Paro R, Mackem S & Zeller R. (2010). Distinct roles of Hand2 in initiating polarity and posterior Shh expression during the onset of mouse limb bud development. PLoS Genet. , 6, e1000901. PMID: 20386744 DOI.
- ↑ Fisher M, Downie H, Welten MC, Delgado I, Bain A, Planzer T, Sherman A, Sang H & Tickle C. (2011). Comparative analysis of 3D expression patterns of transcription factor genes and digit fate maps in the developing chick wing. PLoS ONE , 6, e18661. PMID: 21526123 DOI.
- ↑ Farin HF, Lüdtke TH, Schmidt MK, Placzko S, Schuster-Gossler K, Petry M, Christoffels VM & Kispert A. (2013). Tbx2 terminates shh/fgf signaling in the developing mouse limb bud by direct repression of gremlin1. PLoS Genet. , 9, e1003467. PMID: 23633963 DOI.
- ↑ Arora R, Metzger RJ & Papaioannou VE. (2012). Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. , 8, e1002866. PMID: 22876201 DOI.
- ↑ van den Boogaard M, Wong LY, Tessadori F, Bakker ML, Dreizehnter LK, Wakker V, Bezzina CR, 't Hoen PA, Bakkers J, Barnett P & Christoffels VM. (2012). Genetic variation in T-box binding element functionally affects SCN5A/SCN10A enhancer. J. Clin. Invest. , 122, 2519-30. PMID: 22706305 DOI.
- ↑ Mc Laughlin D, Murphy P & Puri P. (2014). Altered Tbx1 gene expression is associated with abnormal oesophageal development in the adriamycin mouse model of oesophageal atresia/tracheo-oesophageal fistula. Pediatr. Surg. Int. , 30, 143-9. PMID: 24356861 DOI.
- ↑ Kispert A. (2017). T-Box Genes in the Kidney and Urinary Tract. Curr. Top. Dev. Biol. , 122, 245-278. PMID: 28057266 DOI.
Search Bookshelf Tbx
Reviews
Sheeba CJ & Logan MP. (2017). The Roles of T-Box Genes in Vertebrate Limb Development. Curr. Top. Dev. Biol. , 122, 355-381. PMID: 28057270 DOI.
Kispert A. (2017). T-Box Genes in the Kidney and Urinary Tract. Curr. Top. Dev. Biol. , 122, 245-278. PMID: 28057266 DOI.
Buckingham M & Relaix F. (2007). The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu. Rev. Cell Dev. Biol. , 23, 645-73. PMID: 17506689 DOI.
Mansouri A, Goudreau G & Gruss P. (1999). Pax genes and their role in organogenesis. Cancer Res. , 59, 1707s-1709s; discussion 1709s-1710s. PMID: 10197584
Search Pubmed
Search Pubmed Now: Tbx
Search OMIM Tbx
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- OMIM - http://omim.org/entry/602054
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Developmental Signals - Tbx. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Developmental_Signals_-_Tbx
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G