Developmental Signals - Sox
| Embryology - 4 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The SRY (480000) and SOX proteins share a DNA-binding domain known as the HMG box, defined by a 79-amino acid region.
All SOX proteins have a single HMG box and bind linear DNA (transcription factor) in a sequence-specific manner, resulting in the bending of DNA through large angles. Bending causes the DNA helix to open for some distance, which may affect binding and interactions of other transcription factors. SOX1, SOX2 (184429), and SOX3 (313430) show the closest homology to SRY. They share maximum homology within the HMG domain and are expressed mainly in the developing nervous system of the mouse (Collignon et al., 1996). These genes share significant homology outside the HMG box also and are highly conserved throughout their evolution.
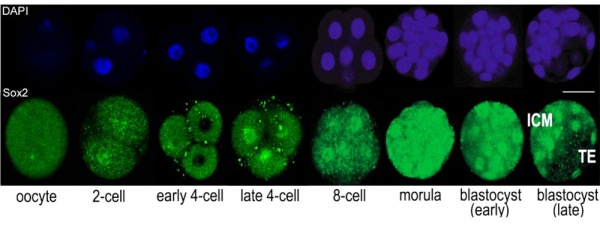
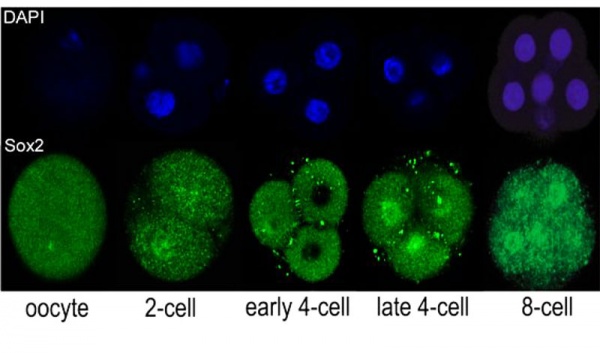
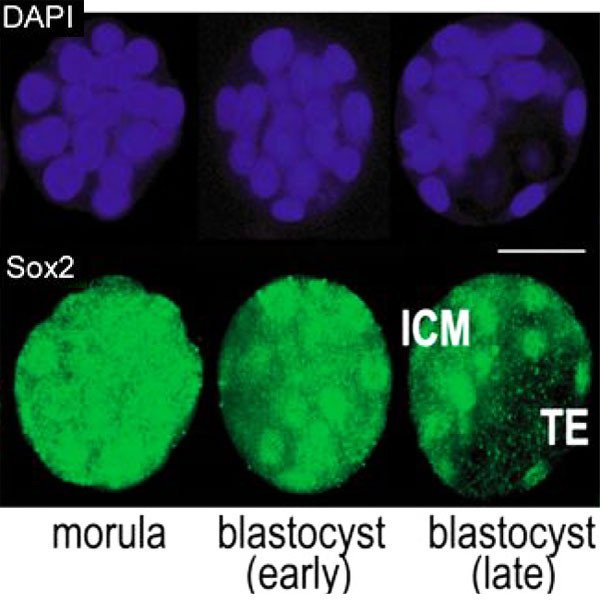
Sox2 is first expressed in very early (morula, blastocyst) development, and has also been identified as one of the 4 "Yamanaka Factors" required to generate an induced pluripotential stem cell (iPS cell). It also forms a trimeric complex with OCT4, yet another "Yamanaka Factor".
Mammals have 20 different SOX proteins that can be subdivided into 8 groups: A, B1, B2, C, D, E, F, G, H.
- Sox Links: Sox transcription factors cartoon | Image 1 - Preimplantation Mouse | Image 2 - Preimplantation Mouse | Image 3 - Preimplantation Mouse | Sox | Induced Stem Cells | Yamanaka Factors
| Factor Links: AMH | hCG | BMP | sonic hedgehog | bHLH | HOX | FGF | FOX | Hippo | LIM | Nanog | NGF | Nodal | Notch | PAX | retinoic acid | SIX | Slit2/Robo1 | SOX | TBX | TGF-beta | VEGF | WNT | Category:Molecular |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Sox Expression | Morula+Sox2 | Blastcyst+Sox2 |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Human SOX Family
| Table - Human Sox Family | ||||
| Approved Symbol |
Approved Name | Previous Symbols | Synonyms | Chromosome |
|---|---|---|---|---|
| SOX1 | SRY-box 1 | 13q34 | ||
| SOX2 | SRY-box 2 | 3q26.33 | ||
| SOX3 | SRY-box 3 | PHP | Xq27.1 | |
| SOX4 | SRY-box 4 | 6p22.3 | ||
| SOX5 | SRY-box 5 | "L-SOX5, MGC35153" | 12p12.1 | |
| SOX6 | SRY-box 6 | 11p15.3 | ||
| SOX7 | SRY-box 7 | 8p23.1 | ||
| SOX8 | SRY-box 8 | 16p13.3 | ||
| SOX9 | SRY-box 9 | "CMD1, CMPD1" | SRA1 | 17q24.3 |
| SOX10 | SRY-box 10 | "DOM, WS4, WS2E" | 22q13.1 | |
| SOX11 | SRY-box 11 | 2p25.2 | ||
| SOX12 | SRY-box 12 | SOX22 | 20p13 | |
| SOX13 | SRY-box 13 | "Sox-13, ICA12, MGC117216" | 1q32.1 | |
| SOX14 | SRY-box 14 | SOX28 | 3q22.3 | |
| SOX15 | SRY-box 15 | SOX20 | "SOX27, SOX26" | 17p13.1 |
| SOX17 | SRY-box 17 | 8q11.23 | ||
| SOX18 | SRY-box 18 | 20q13.33 | ||
| SOX21 | SRY-box 21 | SOX25 | 13q32.1 | |
| SOX30 | SRY-box 30 | 5q33.3 | ||
| SRY | sex determining region Y | TDF | Yp11.2 | |
| Links: Developmental Signals - Sox | OMIM | HGNC | Tbx Family | Bmp Family | Fgf Family | Pax Family | R-spondin Family | Sox Family | Tbx Family | ||||
| Human SOX Family | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SOX2
In the mouse blastocyst Sox2 forms a complex with Oct4 to maintain self-renewal of the pluripotent inner cell mass (ICM) and is also required for the formation of trophectoderm.[7]
|
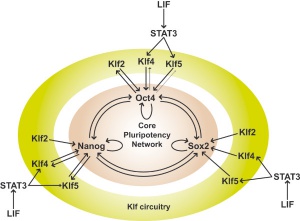
Embryonic stem cell signaling SOX2 |
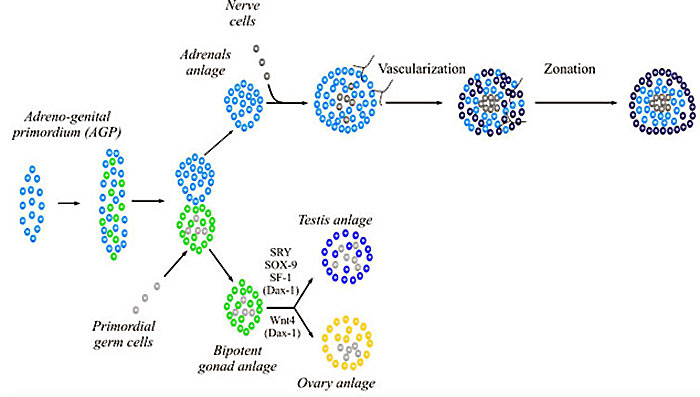
SOX9
SOX9 adrenal and gonad early development[8][9]
| Table - Human Sox Family | ||||
| Approved Symbol |
Approved Name | Previous Symbols | Synonyms | Chromosome |
|---|---|---|---|---|
| SOX9 | SRY-box 9 | "CMD1, CMPD1" | SRA1 | 17q24.3 |
Early Mouse Expression
Limb Expression
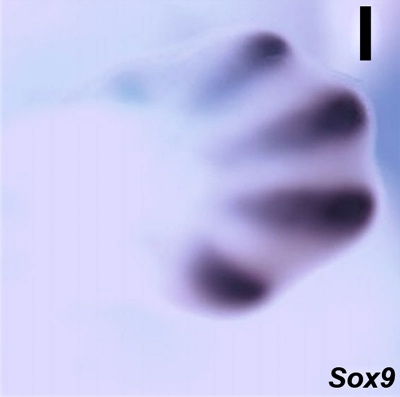
Sox9 expression in E12.5 wild-type mouse embryonic forelimb.[10]
Function
Stem Cells
Sox2
- one of the 4 "Yamanaka factors" (OCT4, SOX2, KLF4, cMyc) required to make a stem cell.
- Links: Stem Cells | Shinya Yamanaka
Genital Development
Sox9
- regulates sex development.
Cartilage Development
Sox9
- regulates cartilage development.
- in chondrogenesis model Sox9 is coexpressed with the gene encoding Col2a1 (type II collagen), the major cartilage matrix protein.
- up-regulated by fibroblast growth factors (FGFs) in primary chondrocytes.
- Links:Cartilage Development
Respiratory Development
Sox2
- regulates patterning of the anterior foregut into ventral (trachea) and dorsal (esophagus) fates
- endoderm expression during formation of foregut derivatives
- declines in regions undergoing lung bud morphogenesis
- declines in ventral region generating the trachea
- Links: Respiratory System Development | StemBook - Specification and patterning of the respiratory system
Rib Development
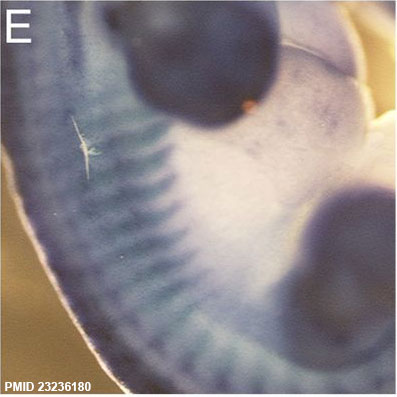
Sox9 expression in the Mouse (E12.5) rib primordial.[11]
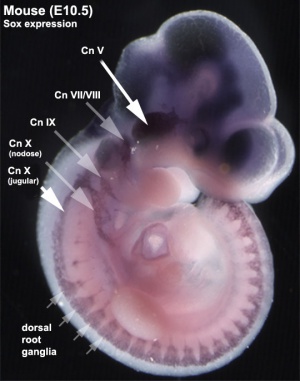
Neural Development
Sox2 binding sites have been identified in mouse cortical (germinal zone) progenitor cells (this study also identified Pax6 and Lhx2 sites)[12]
- Links: Neural System Development | Pax | OMIM Sox2
Hearing Development
Sox2 Lineage tracing of Sox2-expressing progenitor cells in the mouse inner ear reveals a broad contribution to non-sensory tissues and insights into the origin of the organ of Corti[13] "The transcription factor Sox2 is both necessary and sufficient for the generation of sensory regions of the inner ear. ...We find that Sox2-expressing cells in the early otocyst give rise to large numbers of non-sensory structures throughout the inner ear, and that Sox2 only becomes a truly prosensory marker at embryonic day (E)11.5. Our fate map reveals the organ of Corti derives from a central domain on the medial side of the otocyst and shows that a significant amount of the organ of Corti derives from a Sox2-negative population in this region."
- Links: Inner Ear Development | Mouse Development | OMIM Sox2
Signaling Pathway
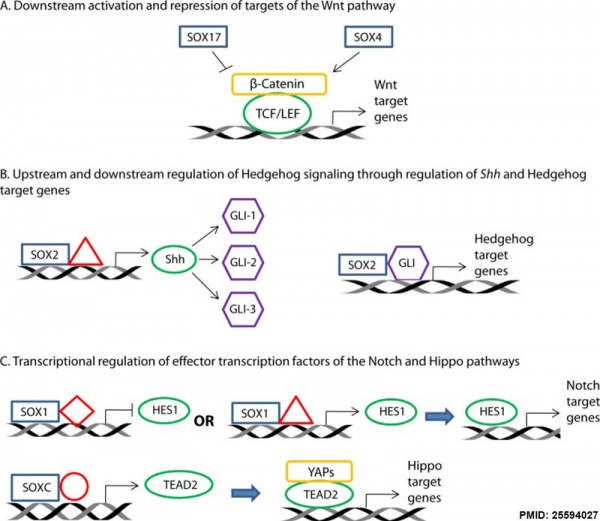
SOX Developmental Signaling Pathways[14]
OMIM
About OMIM "Online Mendelian Inheritance in Man OMIM is a comprehensive, authoritative, and timely compendium of human genes and genetic phenotypes. The full-text, referenced overviews in OMIM contain information on all known mendelian disorders and over 12,000 genes. OMIM focuses on the relationship between phenotype and genotype. It is updated daily, and the entries contain copious links to other genetics resources." OMIM
References
- ↑ Paudyal A, Damrau C, Patterson VL, Ermakov A, Formstone C, Lalanne Z, Wells S, Lu X, Norris DP, Dean CH, Henderson DJ & Murdoch JN. (2010). The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear. BMC Dev. Biol. , 10, 87. PMID: 20704721 DOI.
- ↑ Wakamatsu Y & Suzuki K. (2019). Sequence alteration in the enhancer contributes to the heterochronic Sox9 expression in marsupial cranial neural crest. Dev. Biol. , 456, 31-39. PMID: 31430446 DOI.
- ↑ LeBel DP, Wolff DJ, Batalis NI, Ellingham T, Matics N, Patwardhan SC, Znoyko IY & Schandl CA. (2017). First Report of Prenatal Ascertainment of a Fetus With Homozygous Loss of the SOX10 Gene and Phenotypic Correlation by Autopsy Examination. Pediatr. Dev. Pathol. , , 1093526617744714. PMID: 29216801 DOI.
- ↑ Corsinotti A, Wong FC, Tatar T, Szczerbinska I, Halbritter F, Colby D, Gogolok S, Pantier R, Liggat K, Mirfazeli ES, Hall-Ponsele E, Mullin NP, Wilson V & Chambers I. (2017). Distinct SoxB1 networks are required for naïve and primed pluripotency. Elife , 6, . PMID: 29256862 DOI.
- ↑ Umeda K, Oda H, Yan Q, Matthias N, Zhao J, Davis BR & Nakayama N. (2015). Long-term expandable SOX9+ chondrogenic ectomesenchymal cells from human pluripotent stem cells. Stem Cell Reports , 4, 712-26. PMID: 25818812 DOI.
- ↑ Cutting A, Chue J & Smith CA. (2013). Just how conserved is vertebrate sex determination?. Dev. Dyn. , 242, 380-7. PMID: 23390004 DOI.
- ↑ 7.0 7.1 Keramari M, Razavi J, Ingman KA, Patsch C, Edenhofer F, Ward CM & Kimber SJ. (2010). Sox2 is essential for formation of trophectoderm in the preimplantation embryo. PLoS ONE , 5, e13952. PMID: 21103067 DOI.
- ↑ Val P, Lefrançois-Martinez AM, Veyssière G & Martinez A. (2003). SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl. Recept. , 1, 8. PMID: 14594453 DOI.
- ↑ Penrad-Mobayed M, Perrin C, L'Hôte D, Contremoulins V, Lepesant JA, Boizet-Bonhoure B, Poulat F, Baudin X & Veitia RA. (2018). A role for SOX9 in post-transcriptional processes: insights from the amphibian oocyte. Sci Rep , 8, 7191. PMID: 29740094 DOI.
- ↑ Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V & Tabin CJ. (2006). Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. , 2, e216. PMID: 17194222 DOI.
- ↑ Plummer NW, Spicher K, Malphurs J, Akiyama H, Abramowitz J, Nürnberg B & Birnbaumer L. (2012). Development of the mammalian axial skeleton requires signaling through the Gα(i) subfamily of heterotrimeric G proteins. Proc. Natl. Acad. Sci. U.S.A. , 109, 21366-71. PMID: 23236180 DOI.
- ↑ <pubmed>26721689</pubmed>
- ↑ Gu R, Brown RM, Hsu CW, Cai T, Crowder AL, Piazza VG, Vadakkan TJ, Dickinson ME & Groves AK. (2016). Lineage tracing of Sox2-expressing progenitor cells in the mouse inner ear reveals a broad contribution to non-sensory tissues and insights into the origin of the organ of Corti. Dev. Biol. , 414, 72-84. PMID: 27090805 DOI.
- ↑ Thu KL, Becker-Santos DD, Radulovich N, Pikor LA, Lam WL & Tsao MS. (2014). SOX15 and other SOX family members are important mediators of tumorigenesis in multiple cancer types. Oncoscience , 1, 326-35. PMID: 25594027 DOI.
Reviews
Lee YH & Saint-Jeannet JP. (2011). Sox9 function in craniofacial development and disease. Genesis , 49, 200-8. PMID: 21309066 DOI.
Kiefer JC. (2007). Back to basics: Sox genes. Dev. Dyn. , 236, 2356-66. PMID: 17584862 DOI.
Search PubMed: Sox
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 4) Embryology Developmental Signals - Sox. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Developmental_Signals_-_Sox
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G