| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose.
|
Introduction
This page introduces the possible effects of maternal use of herbal drugs (therapeutic chemicals/agents) on development. In some cases these drugs are "prescribed" to treat pre-existing or pregnancy related maternal medical conditions. In all cases, a discussion with a medical practioner should be had prior to any reproductive decision.
The following herbal drugs have been used for a number of different maternal conditions: Ginkgo Biloba, Kava (Piper methysticum), St. John's wort (Hypericum perforatum), Tian Ma (Gastrodia elata), Valerian (Valeriana officinalis). In some cases very little is known about the potential teratogenic effects of these drugs. Furthermore, care should be made when comparing evaluation of drugs in different species.[1]
The National Center Complementary and Alternative Medicine (USA) provides excellent summary information sheets on many of the commonly used herbal treatments, though many of these information sheets do not include information about herbal drug use during pregnancy. This current page gives some examples of herbs which may impact on development, for further information see NIH - Herbs at a Glance PDF.
| Educational Use Only - Embryology is an educational resource for learning concepts in embryological development, no clinical information is provided and content should not be used for any other purpose.
|
Some Recent Findings
- Dose-dependent beneficial and harmful effects of berberine on mouse oocyte maturation and fertilization and fetal development[2] "Previous studies have shown that berberine, an isoquinoline alkaloid isolated from several traditional Chinese herbal medicines, suppresses growth and induces apoptosis in some tumor cell lines. It has also been shown that berberine possesses anti-atherosclerosis and antioxidant activities in hyperlipidemic model rats. Our previous study in mice found that berberine causes harmful effects on preimplantation and postimplantation embryonic development, both in vitro and in vivo, by triggering reactive oxygen species (ROS)-mediated apoptotic cascades in mouse blastocysts. In the current investigation, we further showed that berberine treatment has distinct dose-dependent effects on oocyte maturation and subsequent development. Preincubation of oocytes with 2.5 μM berberine significantly enhanced maturation and in vitro fertilization (IVF) rates, with subsequent beneficial effects on embryonic development. In contrast, preincubation with 10 μM berberine negatively impacted mouse oocyte maturation, decreased IVF rates and impaired subsequent embryonic development. Similar dose-dependent effects were also demonstrated in vivo. Specifically, intravenous injection of berberine significantly enhanced mouse oocyte maturation, IVF rate and early-stage embryo development after fertilization at a dose of 1 mg/kg body weight but significantly impaired oocyte maturation and IVF rates and caused harmful effects on early embryonic development at a dose of 5 mg/kg. Mechanistically, we found that berberine enhanced intracellular ROS production and apoptosis of oocytes at a concentration of 10 μM but actually significantly decreased total intracellular ROS content and had no apoptotic effect at a concentration of 2.5 μM. Moreover, pretreatment of oocytes with Ac-DEVD-cho, a caspase-3-specific inhibitor, effectively blocked berberine-induced negative impacts on oocyte maturation, fertilization and subsequent development. Collectively, these findings establish the dose-dependent beneficial versus deleterious effects of berberine and suggest that the mechanism underlying the deleterious effects of berberine involves a caspase-3-dependent apoptotic process acting downstream of an increase in intracellular ROS levels."
- Evaluation of in Vitro Embryotoxicity Tests for Chinese Herbal Medicines[3] "Chinese herbal medicines (CHMs) have been widely used during pregnancy, but feto-embryo safety tests are lacking. Here we evaluated in vitro embryotoxicity tests (IVTs) as alternative methods in assessing developmental toxicity of CHMs. Ten CHMs were selected and classified as strongly, weakly and non-embryotoxic. Three well validated IVTs and prediction models (PMs), including embryonic stem cell test (EST), micromass (MM) and whole embryo culture (WEC), were compared. All strongly embryotoxic CHMs were predicted by MM and WEC PM2. While all weakly embryotoxic CHMs were predicted by MM and WEC PM1. All non-embryotoxic CHMs were classified by EST, MM, but over-classified as weakly embryotoxic by WEC PM1. Overall predictivity, precision and accuracy of WEC determined by PM2 were better than EST and MM tests. Compared with validated chemicals, performance of IVTs for CHMs was comparable. So IVTs are adequate to identify and exclude embryotoxic potential of CHMs in this training set."
- Phytochemical Evaluation, Embryotoxicity, and Teratogenic Effects of Curcuma longa Extract on Zebrafish ( Danio rerio)[4] "Curcuma longa L. is a rhizome plant often used as traditional medicinal preparations in Southeast Asia. The dried powder is commonly known as cure-all herbal medicine with a wider spectrum of pharmaceutical activities. In spite of the widely reported therapeutic applications of C. longa, research on its safety and teratogenic effects on zebrafish embryos and larvae is still limited. ... Overall, the result revealed that plants having therapeutic potential could also pose threats when consumed at higher doses especially on the embryos. Therefore, detailed toxicity analysis should be carried out on medicinal plants to ascertain their safety on the embryos and its development."
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- The Effect of Cynara Scolymus (Artichoke) on Maternal Reproductive Outcomes and Fetal Development in Rats[5] "Cynara scolymus (C.scolymus) is a plant employed worldwide as an herbal medicine. However, there is a paucity of data related to the evaluation of its toxicity in commercial preparations; thus, the aim of this study was to evaluate the possible teratogenic effect of the dry extract of C.scolymus leaves in Wistar rats. Females were treated, from gestation day (GD) 6 until GD19, with 0.0, 1.0, 2.0 or 4.0 g/kg body weight of C.scolymus extract. At GD20, a cesarean section was performed for evaluation of maternal and fetal parameters. C.scolymus did not induce changes in food consumption, preimplantation or postimplantation losses, placental weight or biochemical profile. An increase in water consumption was observed in pregnant females treated with the higher doses of C.scolymus. Experimental groups showed lower body weight gain during pregnancy and lower gravid uterus weight. Maternal body weight minus the gravid uterus weight did not result in significant differences. Reductions in fetal weight and length were observed in experimental groups. The number of live pups per litter was lower in the highest dose group. No fetal skeletal or visceral malformations were detected. The results showed that the consumption of artichoke during pregnancy clearly has a negative impact on fetuses."
- Maternal and developmental toxicity of the hallucinogenic plant-based beverage ayahuasca (AYA) in rats[6] "Rats were treated orally with ayahuasca (AYA) on gestation days (GD) 6-20 at doses corresponding to one-(1X) to eight-fold (8X) the average dose taken by a human adult in a religious ritual, and the pregnancy outcome evaluated on GD21. ...This study suggested that AYA is developmentally toxic and that its daily use by pregnant women may pose risks for the conceptus."
- Prenatal exposure of a girl with autism spectrum disorder to 'horsetail' (Equisetum arvense) herbal remedy and alcohol: a case report[7] "We describe the pediatric environmental history of a three-year-old Caucasian girl with an autism spectrum disorder. We utilized her pediatric environmental history to evaluate constitutional, genetic, and environmental factors pertinent to manifestation of neurodevelopment disorders. Both parents reported prenatal exposure to several risk factors of interest. A year prior to conception the mother began a weight loss diet and ingested 1200mg/day of 'horsetail' (Equisetum arvense) herbal remedies containing thiaminase, an enzyme that with long-term use can lead to vitamin deficiency. The mother reported a significant weight loss during the pregnancy and a deficiency of B-complex vitamins. Thiamine (vitamin B1) deficiency could have been potentiated by the horsetail's thiaminase activity and ethanol exposure during pregnancy. No other risk factors were identified."
- Effects of Ginkgo biloba extract on the embryo-fetal development in Wistar rats[8] "Ginkgo biloba extract (GBE) is an herbal medicine used for treating neurodegenerative diseases, cerebrovascular insufficiency, peripheral arterial occlusive disease, and also vestibular disturbance. ...The GBE treatment in pregnant Wistar rats, during the tubal transit and implantation period, caused no toxic effect on the maternal organism and did not induce embryonic death, growth retardation, and/or fetal malformations."
|
Artichoke
Artichoke (Cynara scolymus, C.scolymus) is a plant used as an herbal medicine.
A recent study in rats[5] identified lower maternal body weight gain during pregnancy and lower gravid uterus weight. Fetal weight and length reduction also occurred with the number of live pups/litter lower in the highest dose group. No fetal skeletal or visceral malformations were detected.
Ayahuasca

|
Ayahuasca (AYA, iowaska, yagé) is a psychoactive substance made by South American natives from of Banisteriopsis caapi vine and other ingredients. A recent study in rats[6] at a range of ayahuasca concentrations, corresponding from 1x to 8x the human dosage, demonstrated a number of fetotoxic effects:
- 2X dose survivors had neuronal loss in hippocampal regions CA1.
- 4X and 8X doses died during the treatment period (44 and 52%), and those that survived showed kidney injury.
- 8X dose survivors showed delayed intrauterine growth, increased occurrence of fetal anomalies, neuronal loss in hippocampal regions and in the raphe nuclei.
- Links: Brazil Statistics
|
Black Cohosh

|
Black cohosh (Actaea racemosa, Cimicifuga racemosa, black snakeroot, macrotys, bugbane, bugwort, rattleroot, rattleweed) is a member of the buttercup family and is native to North America. It is not clear if black cohosh is safe for women with a liver disorder, hormone-sensitive conditions, or for pregnant women or nursing mothers.
Black cohosh should not be confused with blue cohosh (Caulophyllum thalictroides).
- Links: USA Office of Dietary Supplements - Black Cohosh | Search PubMed Black Cohosh Teratogen
|
Cat’s Claw
Chasteberry
Chasteberry (Vitex agnus-castus, chaste-tree berry, vitex, monk’s pepper) is the fruit of the chaste tree. This herb may affect certain hormone levels, women who are pregnant or taking birth control pills or who have a hormone-sensitive condition (such as breast cancer) should not use chasteberry.
Search PubMed: Chasteberry Teratogen | Chasteberry | Vitex agnus-castus
Ephedra

|
Ephedra (Ephedra sinica) is an evergreen shrub-like plant, the principal active ingredient ephedrine can powerfully stimulate the nervous system and heart. In 2004, the FDA banned the U.S. sale of dietary supplements containing ephedra.
Specific ingredients include alkaloids, flavonoids, tannins, polysaccharides, organic acids, volatile oils, and many other active compounds.[9]
Search PubMed: Ephedra Teratogen | Ephedra
|
Fenugreek
Fenugreek (Trigonella foenum-graecum) is a legume plant who's seeds are commonly as a food spice. It was used historically for inducing childbirth.
Search PubMed: Fenugreek Teratogen | Fenugreek
Feverfew

|
Feverfew (Tanacetum parthenium, Chrysanthemum parthenium) is a short bush with daisy-like flowers. This herb may cause the uterus to contract, increasing the risk of miscarriage or premature delivery.
|
Ginkgo Biloba

|
Ginkgo leaf extract has been used to treat a variety of ailments and conditions, including asthma, bronchitis, fatigue, and tinnitus (ringing or roaring sounds in the ears).
Extracts are usually taken from the ginkgo leaf and are used to make tablets, capsules, or teas. Occasionally, ginkgo extracts are used in skin products.
Effects of Ginkgo biloba extract on the embryo-fetal development in Wistar rats[8] "Ginkgo biloba extract (GBE) is an herbal medicine used for treating neurodegenerative diseases, cerebrovascular insufficiency, peripheral arterial occlusive disease, and also vestibular disturbance. ...The GBE treatment in pregnant Wistar rats, during the tubal transit and implantation period, caused no toxic effect on the maternal organism and did not induce embryonic death, growth retardation, and/or fetal malformations."
Search PubMed: Ginkgo Teratogen
|
Goldenseal
|
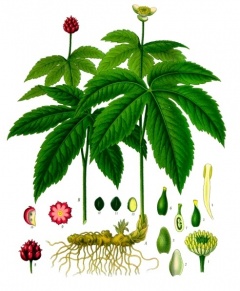
Goldenseal (Hydrastis canadensis, orange-root, yellow-root) is a perennial herb in the buttercup family, it contains the chemical berberine that can cause or worsen jaundice in newborns and may lead to a life-threatening problem called kernicterus.
Kernicterus is a form of brain damage caused by excessive jaundice.
Search PubMed: Goldenseal Teratogen
|
Horsetail

|
Field Horsetail or Common Horsetail (Equisetum arvense, rhizomatous stem formation) is a bushy perennial herb native to the northern hemisphere.
The herb contains containing thiaminase, an enzyme that with long-term use can lead to vitamin deficiency.
Has been used in weight loss diets, and a case report has identified that combined with alcohol linked to autism spectrum disorder.[7]
Search PubMed: Horsetail Teratogen
|
Licorice Root
Licorice (Glycyrrhiza glabra, Glycyrrhiza uralensis, gan zao (Chinese licorice)) contains a compound called glycyrrhizin (or glycyrrhizic acid) and consuming large amounts of licorice as food may alter cortisol and related steroid drug levels, as well as increase the risk of preterm labor.
Red Clover

|
Red clover (Trifolium pratense) is a legume and contains phytoestrogens, compounds that are similar to the female hormone estrogen.
|
St. John's Wort

|
St. John's Wort (Hypericum perforatum, hypericum, Klamath weed, goatweed) a plant with yellow flowers used to prepare teas, this herb interacts with many medications in ways that can interfere with their intended effects.
HSTAT St. John's Wort | Appendix II: Side Effects, Adverse Effects, Precautions, and Warnings "The safety of using hypericum during pregnancy or lactation has not been proven so it should be avoided." "St. John's wort induces the CYP 450 3A4 metabolic pathway which is also used by many prescription drugs used to prevent conditions (transplant rejection or pregnancy oral contraceptives), health care providers should alert patients about these potential drug interactions."
|
Yohimbe
|
Yohimbe (Pausinystalia yohimbe ) is derived from the bark of a tall evergreen tree native to western Africa. Women who are pregnant or breastfeeding should not take yohimbe.
- Links: MedlinePlus - Yohimbe
|
References
- ↑ Nau H. (1986). Species differences in pharmacokinetics and drug teratogenesis. Environ. Health Perspect. , 70, 113-29. PMID: 3104022
- ↑ Huang CH, Wang FT & Chan WH. (2020). Dose-dependent beneficial and harmful effects of berberine on mouse oocyte maturation and fertilization and fetal development. Toxicol Res (Camb) , 9, 431-443. PMID: 32905254 DOI.
- ↑ Li L, Yin Tang L, Liang B, Wang R, Sun Q, Bik San Lau C, Chung Leung P, Fritsche E, Liebsch M, Seiler Wulczyn AEM, Spielmann H & Wang CC. (2019). Evaluation of in vitro embryotoxicity tests for Chinese herbal medicines. Reprod. Toxicol. , 89, 45-53. PMID: 31228572 DOI.
- ↑ Alafiatayo AA, Lai KS, Syahida A, Mahmood M & Shaharuddin NA. (2019). Phytochemical Evaluation, Embryotoxicity, and Teratogenic Effects of Curcuma longa Extract on Zebrafish (Danio rerio). Evid Based Complement Alternat Med , 2019, 3807207. PMID: 30949217 DOI.
- ↑ 5.0 5.1 Gotardo AT, Mattos MIDS, Hueza IM & Górniak SL. (2019). The effect of Cynara scolymus (artichoke) on maternal reproductive outcomes and fetal development in rats. Regul. Toxicol. Pharmacol. , 102, 74-78. PMID: 30611817 DOI.
- ↑ 6.0 6.1 da Motta LG, de Morais JA, Tavares ACAM, Vianna LMS, Mortari MR, Amorim RFB, Carvalho RR, Paumgartten FJR, Pic-Taylor A & Caldas ED. (2018). Maternal and developmental toxicity of the hallucinogenic plant-based beverage ayahuasca in rats. Reprod. Toxicol. , , . PMID: 29522798 DOI.
- ↑ 7.0 7.1 Ortega García JA, Angulo MG, Sobrino-Najul EJ, Soldin OP, Mira AP, Martínez-Salcedo E & Claudio L. (2011). Prenatal exposure of a girl with autism spectrum disorder to 'horsetail' (Equisetum arvense) herbal remedy and alcohol: a case report. J Med Case Rep , 5, 129. PMID: 21453474 DOI.
- ↑ 8.0 8.1 Fernandes ES, Pinto RM, de Paula Reis JE, de Oliveira Guerra M & Peters VM. (2010). Effects of Ginkgo biloba extract on the embryo-fetal development in Wistar rats. Birth Defects Res. B Dev. Reprod. Toxicol. , 89, 133-8. PMID: 20437472 DOI.
- ↑ Miao SM, Zhang Q, Bi XB, Cui JL & Wang ML. (2020). A review of the phytochemistry and pharmacological activities of Ephedra herb. Chin J Nat Med , 18, 321-344. PMID: 32451091 DOI.
Books
- Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Benzie IFF, Wachtel-Galor S, editors. Boca Raton (FL): CRC Press; 2011. Bookshelf
Reviews
- Saunders EJ, Saunders JA. Drug therapy in pregnancy: the lessons of diethylstilbestrol, thalidomide, and bendectin. Health Care Women Int. 1990;11(4):423-32.
Articles
- McBride WG. Prescription drugs in the first trimester and congenital malformations. Aust N Z J Obstet Gynaecol. 1992 Nov;32(4):386.
Search Pubmed
June 2010 "herbal drugs in pregnancy" All (395) Review (50) Free Full Text (30)
Search Pubmed: herbal drugs in pregnancy | herbal drug teratogen
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Terms
| Drug Terms
|
- adverse reaction - (adverse event) An unwanted effect caused by the administration of drugs. Onset may be sudden or develop over time (See Side Effects).
- approved drugs - In the U.S., the Food and Drug Administration (FDA) must approve a substance as a drug before it can be marketed. The approval process involves several steps including pre-clinical laboratory and animal studies, clinical trials for safety and efficacy, filing of a New Drug Application by the manufacturer of the drug, FDA review of the application, and FDA approval/rejection of application (See Food and Drug Administration).
- AUC - acronym for Area Under the plasma concentration versus time Curve is an important parameter when determining drug effects, both therapeutic and teratogenic.
- clinical trial - A research study to answer specific questions about vaccines or new therapies or new ways of using known treatments. Clinical trials (also called medical research and research studies) are used to determine whether new drugs or treatments are both safe and effective. Carefully conducted clinical trials are the fastest and safest way to find treatments that work in people. Trials are in four phases: Phase I tests a new drug or treatment in a small group; Phase II expands the study to a larger group of people; Phase III expands the study to an even larger group of people; and Phase IV takes place after the drug or treatment has been licensed and marketed. (See Phase I, II, III, and IV Trials).
- cohort - In epidemiology, a group of individuals with some characteristics in common.
- contraindication - A specific circumstance when the use of certain treatments could be harmful.
- double-blind study - A clinical trial design in which neither the participating individuals nor the study staff knows which participants are receiving the experimental drug and which are receiving a placebo (or another therapy). Double-blind trials are thought to produce objective results, since the expectations of the doctor and the participant about the experimental drug do not affect the outcome; also called double-masked study. See Blinded Study, Single-Blind Study, and Placebo.
- drug clearance - measured as the volume of blood or plasma from which a compound is irreversibly removed per unit time.
- drug distribution - movement of a drug from one location in the body to another, generally by passive diffusion down the concentration gradient.
- drug-drug interaction - A modification of the effect of a drug when administered with another drug. The effect may be an increase or a decrease in the action of either substance, or it may be an adverse effect that is not normally associated with either drug.
- drug metabolism - (drug biotransformation)
- efficacy - Referring to a drug or treatment, the maximum ability of a drug or treatment to produce a result regardless of dosage. A drug passes efficacy trials if it is effective at the dose tested and against the illness for which it is prescribed. In the procedure mandated by the FDA, Phase II clinical trials gauge efficacy, and Phase III trials confirm it (See Food and Drug Administration (FDA), Phase II and III Trials).
- Food and Drug Administration - (FDA) The U.S. Department of Health and Human Services agency responsible for ensuring the safety and effectiveness of all drugs, biologics, vaccines, and medical devices, including those used in the diagnosis, treatment, and prevention of HIV infection, AIDS, and AIDS-related opportunistic infections. The FDA also works with the blood banking industry to safeguard the nation's blood supply. Internet address: http://www.fda.gov/.
- informed consent - The process of learning the key facts about a clinical trial before deciding whether or not to participate. It is also a continuing process throughout the study to provide information for participants. To help someone decide whether or not to participate, the doctors and nurses involved in the trial explain the details of the study.
- oral bioavailability - refers to the percentage of the oral dose of that drug that ends up in the systemic circulation, few drugs have complete oral bioavailability.
- orphan drugs - An FDA category that refers to medications used to treat diseases and conditions that occur rarely. There is little financial incentive for the pharmaceutical industry to develop medications for these diseases or conditions. Orphan drug status, however, gives a manufacturer specific financial incentives to develop and provide such medications.
- pharmacokinetics - The processes (in a living organism) of absorption, distribution, metabolism, and excretion of a drug or vaccine.
- Phase I trials - Initial studies to determine the metabolism and pharmacologic actions of drugs in humans, the side effects associated with increasing doses, and to gain early evidence of effectiveness; may include healthy participants and/or patients.
- Phase II trials - Controlled clinical studies conducted to evaluate the effectiveness of the drug for a particular indication or indications in patients with the disease or condition under study and to determine the common short-term side effects and risks.
- Phase III trials - Expanded controlled and uncontrolled trials after preliminary evidence suggesting effectiveness of the drug has been obtained, and are intended to gather additional information to evaluate the overall benefit-risk relationship of the drug and provide and adequate basis for physician labeling.
- Phase IV trials - Post-marketing studies to delineate additional information including the drug's risks, benefits, and optimal use.
- placebo - A placebo is an inactive pill, liquid, or powder that has no treatment value. In clinical trials, experimental treatments are often compared with placebos to assess the treatment's effectiveness. (See Placebo Controlled Study).
- placebo controlled study - A method of investigation of drugs in which an inactive substance (the placebo) is given to one group of participants, while the drug being tested is given to another group. The results obtained in the two groups are then compared to see if the investigational treatment is more effective in treating the condition.
- placebo effect - A physical or emotional change, occurring after a substance is taken or administered, that is not the result of any special property of the substance. The change may be beneficial, reflecting the expectations of the participant and, often, the expectations of the person giving the substance.
- plasma proteins - can bind drugs, acidic drugs bind to albumin, basic drugs bind to lipoproteins or to alpha-1 acid glycoprotein.
- Preclinical - Refers to the testing of experimental drugs in the test tube or in animals - the testing that occurs before trials in humans may be carried out.
- side effects - Any undesired actions or effects of a drug or treatment. Negative or adverse effects may include headache, nausea, hair loss, skin irritation, or other physical problems. Experimental drugs must be evaluated for both immediate and long-term side effects (See Adverse Reaction).
- statistical significance - The probability that an event or difference occurred by chance alone. In clinical trials, the level of statistical significance depends on the number of participants studied and the observations made, as well as the magnitude of differences observed.
- toxicity - An adverse effect produced by a drug that is detrimental to the participant's health. The level of toxicity associated with a drug will vary depending on the condition which the drug is used to treat.
- treatment IND - Investigational New Drug (IND) application, which is part of the process to get approval from the FDA for marketing a new prescription drug in the U.S. It makes promising new drugs available to desperately ill participants as early in the drug development process as possible. Treatment INDs are made available to participants before general marketing begins, typically during Phase III studies. To be considered for a treatment IND a participant cannot be eligible to be in the definitive clinical trial.
|
|
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Abnormal Development - Herbal Drugs. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Herbal_Drugs
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G





















