User:Z5020117
| Student Information (expand to read) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual Assessments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Please leave this template on top of your student page as I will add your assessment items here. Beginning your online work - Working Online in this course
Click here to email Dr Mark Hill | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 1 Assessment - Researching a Topic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the lab I showed you how to find the PubMed reference database and search it using a topic word. Lab 1 assessment will be for you to use this to find a research reference on "fertilization" and write a brief summary of the main finding of the paper.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 2 Assessment - Uploading an Image | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
OK you are now in a group
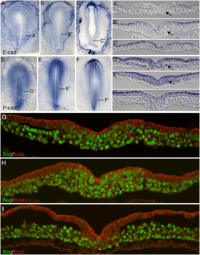
Initially the topic can be as specific or as broad as you want. Chicken embryo E-cad and P-cad gastrulation[1] References
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 4 Assessment - GIT Quiz | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
ANAT2341 Quiz Example | Category:Quiz | ANAT2341 Student 2015 Quiz Questions | Design 4 quiz questions based upon gastrointestinal tract. Add the quiz to your own page under Lab 4 assessment and provide a sub-sub-heading on the topic of the quiz. An example is shown below (open this page in view code or edit mode). Note that it is not just how you ask the question, but also how you explain the correct answer. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 5 Assessment - Course Review | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Complete the course review questionnaire and add the fact you have completed to your student page. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 6 Assessment - Cleft Lip and Palate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 7 Assessment - Muscular Dystrophy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 8 Assessment - Quiz | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A brief quiz was held in the practical class on urogenital development. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 9 Assessment - Peer Assessment | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 10 Assessment - Stem Cells | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
As part of the assessment for this course, you will give a 15 minutes journal club presentation in Lab 10. For this you will in your current student group discuss a recent (published after 2011) original research article (not a review!) on stem cell biology or technology.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab 11 Assessment - Heart Development | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Read the following recent review article on heart repair and from the reference list identify a cited research article and write a brief summary of the paper's main findings. Then describe how the original research result was used in the review article.
<pubmed>26932668</pubmed>Development | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lab Attendance
Z5020117 (talk) 14:34, 5 August 2016 (AEST) Z5020117 (talk) 14:41, 12 August 2016 (AEST) Z5020117 (talk) 14:07, 19 August 2016 (AEST) Z5020117 (talk) 14:11, 26 August 2016 (AEST) Z5020117 (talk) 13:21, 9 September 2016 (AEST) Z5020117 (talk) 15:01, 16 September 2016 (AEST) Z5020117 (talk) 13:27, 23 September 2016 (AEST)
Lab 1 Assessment
'Preimplantation genetic screening for all 24 chromosomes by microarray comparative genomic hybridization significantly increases implantation rates and clinical pregnancy rates in patients undergoing in vitro fertilization with poor prognosis' Summary
<pubmed>27382234</pubmed>
The use of Preimplantation Genetic Screening (PGS) in association with IVF has not been prevalent due to its expensive and highly invasive nature, almost doubling the cost of IVF. Currently, morphology evaluation is predominantly used due to its non-invasive nature despite its variable efficacy. Majumdar et al. designed an experiment to evaluate an improved PGS system that analyses all 24 chromosomes. They believe the incorporation of chromosomal analysis will increase pregnancy and implantation rates in patients with poor prognosis. The twenty subjects of this study were classified into one of three groups, advanced maternal age (AMA), repeated miscarriage (RI) and recurrent implantation failure (RIF).
This study found that the transfer of only a few embryos, particularly euploid embryos, resulted in higher implantation rates in those receiving PGS in comparison to the control non-PGS group. Overall, it was found that in comparison to the traditional morphology evaluation previously used, the incorporation of PGS allows for improved outcomes following IVF even when no euploid embryos were transferred. With recent research establishing the correlation between high prevalence of aneuploidy embryos in patients with AMA and unsuccessful implantations, PGS can be used to successfully identify and eliminate the possibility of aneuploidy embryo transfer, thus allowing for increased implantation and pregnancy rates. In saying this, Majumdar et al. emphasise how successful implantation cannot be guaranteed with PGS as some pregnancy failures occur as a result of factors other than chromosomal abnormalities. Though further research is needed to consolidate the benefits of PGS, this study identified the possibility of achieving successful implantation and pregnancy in a shorter time period with fewer miscarriages when utilising PGS rather than morphology evaluation in association with IVF.
| Mark Hill 18 August 2016 - You have added the citation correctly and written a reasonable summary of the papers findings. Why would you thing PGD would improve implantation rates and clinical pregnancy rates? Note that this is not a high impact Journal, try those first for your article selections. | Assessment 5/5 |
Lab 2 Assessment
Primitive streak development in chick embryo[1]
| Mark Hill 29 August 2016 - All information Reference, Copyright and Student Image template correctly included with the file and referenced on your page here. The citation on the page here could also have appeared in the image legend as shown below. I have also added a reference sub-heading to fix the formatting issue. | Assessment 5/5 |
Lab 3 Assessment
| Mark Hill 31 August 2016 - Lab 3 Assessment Quiz - Mesoderm and Ectoderm development. | Assessment 2.5/5 |
References
Lab 4 Assessment
GIT Abnormalities Quiz
Lab 6 Assessment
Completed course questionnaire
Cleft Palate
Genetic mutation of the transcription factor TBX22, which encodes for the DNA binding-domain, T-Box[1], causes cleft palate. [2]. It has been shown that during palatogenesis TBX22 is found within the tongue and palatal shelves, thus indicating its role in the development of both the tongue and palate. Various mutations of TBX22 can occur, including frameshift mutations resulting in the production of truncated proteins, as well as missense mutations causing a change of a single nucleotide. These mutations result in an inefficient or reduced capability of DNA to bind to the T-Box. In the case of missense mutations, it could also lead to the inability to activate transcription factors. As a result, the lack of formation of functional proteins leads to a dysfunctional palatogenesis process and thus, significantly affecting signalling in normal development to cause formation of cleft palate.
It was also found that TBX22 serves as a transcriptional repressor and modification of this repressor activity occurs through SUMO-1, a small ubiquitin-like modifier that binds upstream from the T-Box domain[3]. Therefore, mutations of SUMO-1 can also impair the function of TBX22 and can present as the craniofacial defect of X-linked cleft palate as found in many cases. Research shows that loss of function of SUMO-1 occurs as a result of exposure to an array of environmental and other factors during early pregnancy including, smoking, lack of nutritional supplements and maternal age and it is exposure to these factors that can have an effect on normal signalling in development encouraging the formation of cleft palate.
Lab 7 Assessment
1. What is/are the dystrophin mutation(s)?
Majority of the dystrophin mutations, approximately 60%[4], are due to deletions or insertions of nucleotides resulting in a downstream frameshift of the dystrophin gene. The remainder of dystrophin mutations are either point mutations, where there is a substitution of a single nucleotide or minor frameshift errors. These mutations can result in the complete absence or in milder forms, the alteration or reduction of the dystrophin protein.
2. What is the function of dystrophin?
The dystrophin protein found in both skeletal and cardiac muscle plays a structural role by linking the internal cytoskeleton of the muscle with the extracellular matrix. It is also responsible for protecting muscles during contraction and relaxation from injury by strengthening muscle fibres. Dystrophin also plays an additional role in cell signaling through interaction with other proteins involved in sending and receiving chemical signals. Research has shown that dystrophin may be present in minor amounts within the neurons of the brain. Thus, they may be involved in the formation of synapses[5] .
3. What other tissues/organs are affected by this disorder?
DMD can affect the respiratory muscles thus negatively impacting lung function. As the dystrophin protein is also found within cardiac muscle, the heart is also affected in DMD. Due to this dysrhythmia or arrhythmia, irregular heart rhythm and cardiomyopathy, abnormal pumping action can result. The presence of dystrophin in brain tissue can also result in learning and behavioural difficulties[6] .
4. What therapies exist for DMD?
Though there is no known cure for DMD, extensive research is being currently performed to treat DMD. The following are a few examples of research being conducted in the field of DMD.
Some of the therapies available include gene replacement therapy whereby plasmids or viruses are utilised to deliver dystrophin sequences but this is currently a work in progress. Myoblast transplantation where myoblasts are artificially delivered into the affected site can also be offered. This is because it has been shown that myoblasts can fuse to form new muscle fibres but upon exhaustion of the proliferative ability of the myoblasts, the skeletal muscle is converted into connective tissue. Unfortunately, studies have shown unsatisfactory results for such treatment. In saying this, stem-cell therapy has shown to be a good alternative to myoblast transplantation due to the extended proliferative life-span of stem cells.
Administration of aminoglycoside antibiotics is a potential therapy that targets DMD caused by premature stop codons. Results of such treatment have not been promising but have indicated that it may be more useful in only a select few DMD mutations. On the other hand, chimaeraplasts have been utilised as a vehicle to deliver the correct nucleotide to the site of dystrophin mutation. Unfortunately, the viability of such treatment is short-lived and requires further research.
Antisense oligonucleotides have been utilised to help redirect dystrophin splicing to exclude the inclusion of the premature stop codon, the most common cause of DMD. This will allow partial restoration of the reading frame and thus allow formation of the dystrophin, albeit shorter protein. Research has also shown that proteasome inhibitors can be used to improve the integrity of muscle. Lastly, upregulation therapy focuses on replacement of defective genes by increasing expression of alternative genes e.g. utrophin. These are promising areas of research in DMD therapy.
Currently, the only form of therapy available is management of DMD and this can be done through prescription of steroid medication to help maintain muscle integrity. Surgery can also be performed to release tightness of joints as well as treat scoliosis, the lateral curvature of the spine which can come as a result of DMD. Supportive equipment can also be provided including night splints, walking frames, wheelchairs, and other mobility aids. It is also important to regulate the patient’s diet and exercise routine. Muscle relaxants and anti-inflammatory medication can also be provided to help with any pain or discomfort .
5. What animal models are available for muscular dystrophy?
Currently there are two animal models that have been utilised to further understand Duchenne Muscular Dystrophy, the mdx mouse model and the golden retriever muscular dystrophy (GRMD) dog. These models are significant as both these species lack the dystrophin protein.
- ↑ NIH U.S. National Library of Medicine,. (2016). TBX22. Genetics Home Reference. Retrieved 12 September 2016, from https://ghr.nlm.nih.gov/gene/TBX22
- ↑ <pubmed>14729838</pubmed>
- ↑ <pubmed>17846996</pubmed>
- ↑ <pubmed>15470384</pubmed>
- ↑ U.S. National Library of Medicine,. (2016). DMD gene. Genetics Home Reference. Retrieved 19 September 2016, from https://ghr.nlm.nih.gov/gene/DMD
- ↑ Muscular Dystrophy Australia,. (2015). Muscular Dystrophy - "The Home of MDA". Mda.org.au. Retrieved 19 September 2016, from http://www.mda.org.au/disorders/dystrophies/dmd-bmd.asp