Cat Development
Introduction
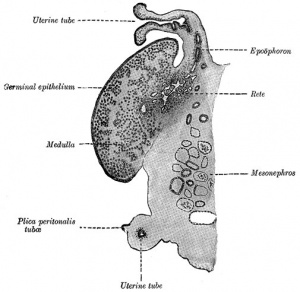
Development of external genitalia in fetal and neonatal domestic cats
J Vet Med Sci. 2009 Feb;71(2):139-45.
Inomata T, Ariga M, Sakita K, Kashiwazaki N, Ito J, Yokoh K, Ichikawa M, Ninomiya H, Inoue S.
Department of Laboratory Animal, School of Veterinary Medicine, Azabu University, Sagamihara, Kanagawa, Japan. inomata@azabu-u.ac.jp Abstract "Development of the external genitalia of fetal and neonatal cat were studied macroscopically, paying attention to the formation of the labia and the sexual differentiation. The female urogenital folds budded from each side of the genital tubercle and, gradually extended to the tip of the genital tubercle by the 6.8 cm stage in crown-rump length. Then, the well-developed urogenital folds ensheathed completely the genital tubercle to form the prepuce of clitoris and the labia, flanking the external opening of vagina as the folds of skin which were equivalent to the labia minora in humans. The genital swellings known to become the labia majora in humans were clearly recognized in the caudolateral region of the genital tubercle during the fetal stage. These swellings became flat and obscure after birth. Thus, in cats the genital swellings did not join to the formation of the labia in the same way as in humans. The sex difference in the external genitalia was first observed at the 3.2-3.3 cm stages. In the male, the anogenital raphe appeared and the caudal portion of the genital swellings moved and fused each other at the caudal region of the genital tubercle. In the female, both features were not easy to observe."
Effect of protein supplementation on development to the hatching and hatched blastocyst stages of cat IVF embryos
Reprod Fertil Dev. 2002;14(5-6):291-6.
Karja NW, Otoi T, Murakami M, Yuge M, Fahrudin M, Suzuki T.
Department of Veterinary Sciences, Yamaguchi University, Japan. Abstract The effects of protein supplementation in culture medium on development to the hatching and hatched blastocyst stages of cat in vitro-fertilized embryos were investigated. In the first experiment, presumptive zygotes derived from in vitro maturation and in vitro fertilization (IVF) were cultured in modified Earle's balanced salt solution (MK-1) supplemented with 0.4% bovine serum albumin (BSA) or 5% fetal bovine serum (FBS) for 9 days. There were no significant differences between the BSA and FBS groups with respect to the proportion of cleavage and development to the morula and blastocyst stages of zygotes. However, the presence of FBS in the medium enhanced development to the hatching blastocyst stage of zygotes compared with the BSA group (31.4% v. 7.8%). Moreover, 2.9% of zygotes cultured with FBS developed to the hatched blastocyst stage. The mean cell number of blastocysts derived from zygotes cultured with FBS was significantly higher (P<0.01) than that from zygotes cultured with BSA (136.6 v.101.5). In the second experiment, embryos at the morula orblastocyst stage, which were produced by culturing in MK-1 supplemented with 0.4% BSA after IVF, were subsequently cultured in MK-1 with 0.4% BSA or 5% FBS. Significantly more morulae developed to the blastocyst (P<0.05) and hatching blastocyst stages (P<0.01) in the FBS group than in the BSA group (71.5% and 53.6% v. 44.9% and 6.0%, respectively). Although none of the morulae cultured with BSA developed to the hatched blastocyst stage, 11.5% of morulae cultured with FBS developed to the hatched blastocyst stage. Moreover, the proportion of development to the hatching blastocyst stage of blastocysts was significantly higher (P<0.01) in the FBS group than in the BSA group (68.7% v. 9.8%). None of the blastocysts cultured with BSA developed to the hatched blastocyst stage, whereas 7.3% of blastocysts cultured with FBS developed to the hatched blastocyst stage. The results of the present study indicate that supplementation with FBS at different stages of early embryo development promotes development to the hatching and hatched blastocyst stages of cat IVF embryos.
PMID: 12467353 http://www.ncbi.nlm.nih.gov/pubmed/12467353
Developmental competence of domestic cat embryos fertilized in vivo versus in vitro
Roth TL, Swanson WF, Wildt DE. Biol Reprod. 1994 Sep;51(3):441-51.
Development of in vitro-fertilized (IVF) cat embryos was compared to that of naturally produced cat embryos in vivo and in vitro. To obtain in vivo-fertilized embryos, queens were mated three times daily on the second and third days of natural estrus and ovariohysterectomized at 64, 76, 100, 124, or 148 h after the first copulation. Embryos were flushed from the reproductive tract, evaluated for developmental stage, and cultured. For IVF, oocytes from gonadotropin-stimulated queens were inseminated with electroejaculated cat sperm in Ham's F-10 and evaluated for fertilization (cleavage to > or = 2 cells) at 30 h. In vitro development of embryos fertilized in vivo (n = 109) and in vitro (n = 46) was evaluated every 24 h for up to 10 days. High-quality embryos recovered at 64, 76, 100, 124, and 148 h after the first copulation were typically 1 to 2 cells (13 of 20), 5 to 8 cells (18 of 28), 9 to 16 cells (14 of 24), morulae (15 of 21), and compact morulae (11 of 18), respectively, suggesting blastomere cleavage once per day in vivo after the first three rapid cell divisions. A similar developmental rate to the morula stage (p > or = 0.05) was achieved in vitro by embryos derived from both in vitro and in vivo fertilization. Additionally, the proportion (p > or = 0.05) of in vivo-generated embryos (2 to 16 cells) that developed to morulae (64 of 83; 77.1%) was similar to that of IVF embryos (28 of 46; 60.9%). However, none of the IVF embryos (0/46), but 70.6% (77 of 109) of the in vivo-produced embryos, achieved blastocyst formation in culture (p < or = 0.05). Furthermore, 66.2% (51 of 77) of these blastocysts exhibited zona hatching. Incidence of morula and blastocyst formation in the in vivo group was influenced by stage of the embryo at collection. Embryos that were at the 9- to 16-cell stage at recovery were more likely (p < or = 0.05) to achieve morula or blastocyst status and emerge from the zona pellucida than younger-stage counterparts. In summary, the in vivo and in vitro growth rate of cat embryos produced after natural mating was comparable to that of embryos fertilized and cultured in vitro. However, developmental ability to the blastocyst stage was superior for embryos produced in vivo after natural mating.
PMID: 7803615
http://www.ncbi.nlm.nih.gov/pubmed/7803615
References
Search Pubmed: cat development
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 30) Embryology Cat Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cat_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G