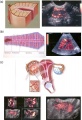
Uterus Development
| Embryology - 9 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
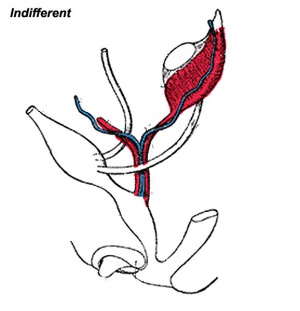
This page introduces the uterus as part of the internal female reproductive tract development. Two paramesonephric ducts form from coelomic epithelium extending beside the mesonephric ducts. In the absence of Mullerian Inhibitory Factor these ducts proliferate and grow extending from the vaginal plate on the wall of the urogenital sinus to lie beside the developing ovary. The paired ducts begin to fuse from the vaginal plate end, forming the primordial body of the uterus and the unfused lateral arms form the uterine tubes. Recent research points to the paramesonephric ducts also being the entire embryonic origin of the vagina. For the pregnant uterus see implantation and maternal decidua.
| Historic Embryology |
| Johannes Peter Müller (1801 - 1858) in 1830 was the first to describe the female genital duct that develops as the uterus and vagina, historically named after him as the "Müllerian duct". The current terminology is the "paramesonephric duct". |
| Menstrual Cycle Links: Introduction | menstrual histology | ovary | corpus luteum | oocyte | uterus | Uterine Gland | estrous cycle | pregnancy test | ||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Uterus Development | Uterus Embryology | Müllerian Duct | Uterine Tube Development | Cervix Development | Broad Ligament Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Paramesonephric Duct
The Müllerian duct (= paramesonephric duct, preferred terminology) paired ducts that form the epithelial lining of female reproductive organs: utererine tube, uterus, upper vaginal canal. The term "paramesonephric" duct means beside the mesonephric (Wolffian) duct, which is its anatomical location in early development. Mullerian refers to Johannes Peter Müller (1801-1858) a German scientist who specialised in comparative anatomy. These ducts initially form and then degenerate in the male.
A recent study using both chicken and mouse embryos has shown that these initially paired tubular structures derive from the coelomic epithelium.[8]
- "Müllerian epithelial tube derived from an epithelial anlage at the mesonephros anterior end, which then segregates from the epithelium and extends caudal of its own accord, via a process involving rapid cell proliferation. This tube is surrounded by mesenchymal cells derived from local delamination of coelomic epithelium."
Mullerian ducts have three elements:
- a canalised epithelial tube
- mesenchymal cells surrounding the tube
- coelomic epithelial cells
Duct Molecular Development
The paired paramesonephic ducts (Müllerian ducts) go through a series of developmental changes recently identified as regulated by a number of molecular factors.
Initiation
Coelomic epithelium Lim1 expressing cells are specified to a duct fate.[10]
- Lim - proteins named for 'LIN11, ISL1, and MEC3,' are defined by the possession of a highly conserved double zinc finger motif called the LIM domain.
- LIM domain-binding factors - interact with the LIM domains of nuclear proteins are capable of binding to a variety of transcription factors.
Invagination
- Wnt4 - induces duct invagination to reach the mesonephric (Wolffian)
Elongation
- WNT9b - from mesonephric duct to guide paramesonephric duct elongation. Cysteine-rich secreted glycoprotein.
- Pax2 - also acts in elongation and duct maintenance. Member of the paired box protein family.
Cells at the leading tip proliferate and form the duct elongating to reach the cloaca (urogenital sinus).
- Links: OMIM - WNT9b | OMIM - Pax2 | OMIM - paired box gene
Uterine Development Movie
| Anterior view of development of the female uterus and vagina between Week 9 and 20.
The paramesonephric ducts (red) fuse in the midline to form the genital canal. The urogenital sinus (yellow), in contact with the paramesonephric duct, thickens to form the sinusal tubercle which extends as a solid vaginal plate, then becomes hollow as the sinovaginal bulb, finally forming the vagina.
|
Development Overview
|
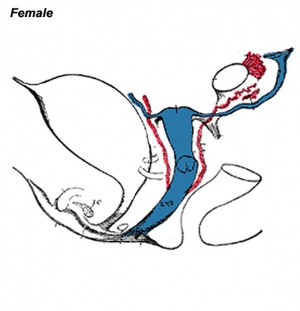
|
| Internal Genital Tract Differentiation |
The data below gives an overview of the timecourse of embryonic human uterine development.[11]
- Carnegie stage 18 - Mullerian duct to the coelomic cavity was formed as the result of an invagination of the coelomic epithelium - stage 18
- Carnegie stages 19 - 23 - duct grows independently from the invagination - stage 19
- Week 20 - uterine horn fimbrial development begins and continues after birth - second trimester
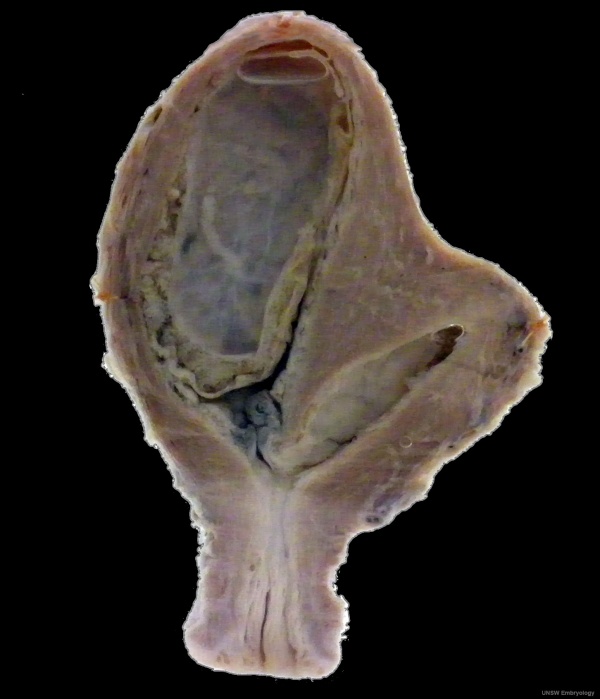
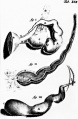
Fetal Uterus
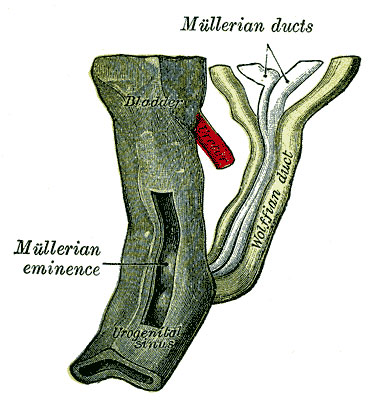
|
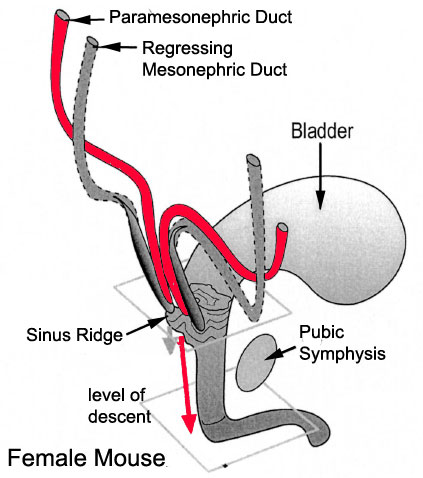
|
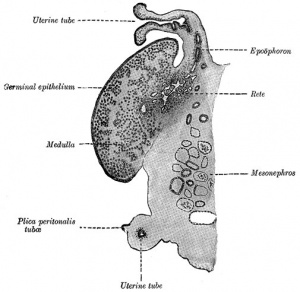
| Urogenital sinus of female human embryo of eight and a half to nine weeks old (From model by Keibel) (Image: Gray's Anatomy) | (Image modified from: Drews U, Sulak O, Schenck PA. Androgens and the development of the vagina.Biol Reprod. 2002 Oct;67(4):1353-9. PMID: 12297555) |
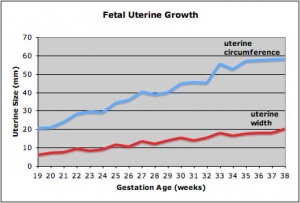
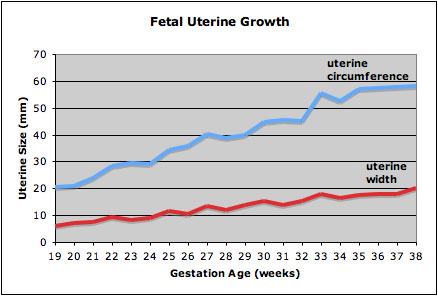
Fetal Uterus Growth
Graph shows the growth during the fetal period of the uterus between week 19 and 38.[12] During this time the uterine circumferunce increases from about 20 mm to just under 60mm and the width increases from less than 10mm to just over 20 mm.
Uterine horn fimbrial development begins after week 20 and continues after birth.
Uterine growth continues postnatally, increasing outer muscle thickness and cyclic changes in the lining with puberty.
Adult external uterine orifice to the fundus is approximately 6.25 cm.
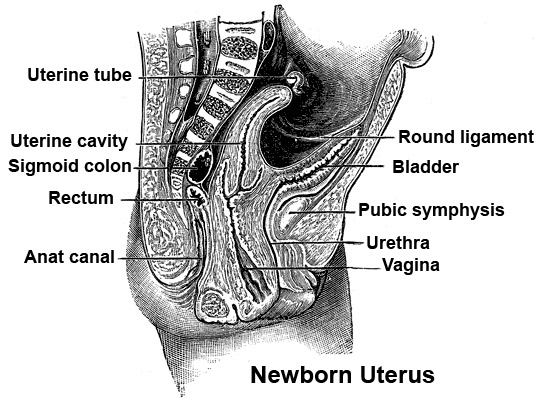
Newborn Uterus
| Growth of the Uterus in the Postfetal Period | ||||
|---|---|---|---|---|
| Age | Length of corpus (mm) | Length of isthmus (mm) | Length of cervix (mm) | Total length (mm) |
| Fetus of 7 months | 22 | |||
| Child of 5 weeks | 27 | |||
| 1 year | 10 | 23 | ||
| 14 months | 10 | 5 | 12 | 27 |
| 2.5 years | 8 | 6 | 12 | 26 |
| 3 years | 9-10 | 5-6 | 10 | 25 |
| 3.5 years | 6 | 5 | 16 | 27 |
| 9 years | 9 | 4.5 | 13 | 27 |
| 11 years | 12 | 6 | 19 | 37 |
| 13 years | 27 | 56 | ||
| 15 years | 59 | |||
| 16 years | 41 | 12 | 25 | 78 |
| 17 years | 27 | 6 | 22 | 55 |
| 17 years | 20 | 4 | 16 | 40 |
| 18 years | 36 | 5 | 31 | 72 |
| 19 years | 27 | 5 | 28 | 60 |
| 19 years | 28 | 6 | 27 | 61 |
| 19 years | 24 | 8 | 21 | 53 |
| 20 years | 30 | 6 | 16 | 52 |
| 20 years | 30 | 7 | 21 | 58 |
| 22 years | 35 | 5 | 29 | 69 |
| 28 years | 40 | 10 | 28 | 78 |
| 29 years (nulliparous wife) | 34 | 10 | 34 | 78 |
| 30 years (virgin) | 38 | 7 | 29 | 74 |
| Data compiled from Hegar (1908) | Uterus Growth Table | Collapsible Table | Uterus Development | ||||
Uterine Tubes
The unfused portion of the paramesonephric ducts will form the uterine tubes. Note that there are several synonyms used for the paired uterine tubes or Fallopian tubes or oviducts or uterine horns.
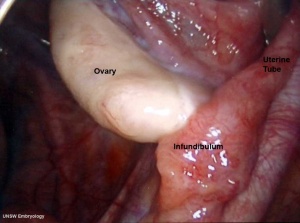
In the adult, the uterine tube has been described in 4 anatomical regions.
| infundibulum | ampulla | isthmus | intramural |
|---|---|---|---|
| funnel-shaped (up to 10 mm in diameter) end of the oviduct. Finger-like extensions of its margins, the fimbriae, are closely applied to the ovary. Ciliated cells are frequent. | mucosal folds, or plicae, and secondary folds which arise from the plicae divide the lumen of the ampulla into a very complex shape. Fertilization usually takes place in the ampulla. | narrowest portion (2-3 mm in diameter) of the tube located in the peritoneal cavity. Mucosal folds are less complex and the muscularis is thick. An inner, longitudinal layer of muscle is present in the isthmus. | penetrates the wall of the uterus. The mucosa is smooth, and the inner diameter of the duct is very small. |
Mucosa
- formed by a ciliated and secretory epithelium resting on a very cellular lamina propria.
- The number of ciliated cells and non-ciliated secretory cells varies along the oviduct.
- Secretory activity varies during the menstrual cycle, and resting secretory cells are also referred to as peg-cells.
- Some of the secreted substances are thought to nourish the oocyte and the very early embryo.
Muscularis
- inner circular muscle layer and an outer longitudinal layer.
- An inner longitudinal layer is present in the isthmus and the intramural part of the oviduct.
- Peristaltic muscle action seems to be more important for the transport of sperm and oocyte than the action of the cilia.
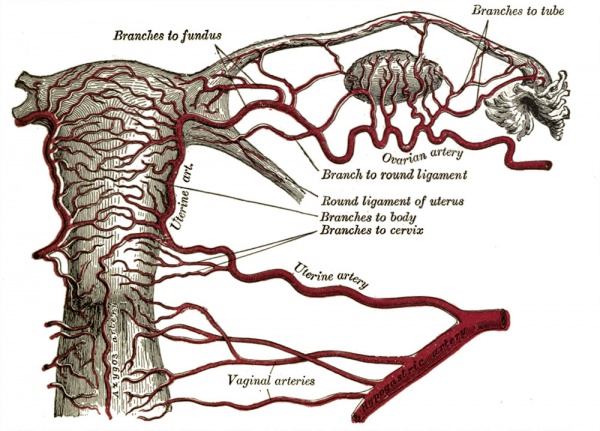
Uterine Blood Supply
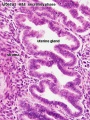
Uterine Glands
Uterine adenogenesis is the term used to describe the formation of uterine glands from the epithelial lining of the uterus that begins prenatal in humans. In other species, the overt development occurs postnatally and has been described through a 3 step the sequence:
- differentiation and budding of the glandular epithelium.
- invagination and tubular coiling of the epithelium.
- branching of the glandular elements and their expansion throughout the endometrial stroma toward the myometrium.
Epithelial-mesenchymal interaction occurs through Wnt signalling during this process:
- Wnt7a - expressed in the luminal epithelium
- Wnt5a - expressed in the mesenchyme
In mice, this development sequence occurs between postnatal day (PND) 5 to 7 and involves Wnt up-regulation of Lymphoid Enhancing Factor 1 (Lef1).[13]
Postnatally both prolactin and estradiol-17 beta (and their receptors) regulate gland development. There are some gland species gestational differences, in both sheep and pigs the glands provide additional histotrophic support by undergoing extensive hyperplasia and hypertrophy.[14]
- Links: Wnt | Epithelial Mesenchymal Interaction
Postnatal Growth
| Growth of the Uterus in the Postfetal Period | ||||
|---|---|---|---|---|
| Age | Length of corpus (mm) | Length of isthmus (mm) | Length of cervix (mm) | Total length (mm) |
| Fetus of 7 months | 22 | |||
| Child of 5 weeks | 27 | |||
| 1 year | 10 | 23 | ||
| 14 months | 10 | 5 | 12 | 27 |
| 2.5 years | 8 | 6 | 12 | 26 |
| 3 years | 9-10 | 5-6 | 10 | 25 |
| 3.5 years | 6 | 5 | 16 | 27 |
| 9 years | 9 | 4.5 | 13 | 27 |
| 11 years | 12 | 6 | 19 | 37 |
| 13 years | 27 | 56 | ||
| 15 years | 59 | |||
| 16 years | 41 | 12 | 25 | 78 |
| 17 years | 27 | 6 | 22 | 55 |
| 17 years | 20 | 4 | 16 | 40 |
| 18 years | 36 | 5 | 31 | 72 |
| 19 years | 27 | 5 | 28 | 60 |
| 19 years | 28 | 6 | 27 | 61 |
| 19 years | 24 | 8 | 21 | 53 |
| 20 years | 30 | 6 | 16 | 52 |
| 20 years | 30 | 7 | 21 | 58 |
| 22 years | 35 | 5 | 29 | 69 |
| 28 years | 40 | 10 | 28 | 78 |
| 29 years (nulliparous wife) | 34 | 10 | 34 | 78 |
| 30 years (virgin) | 38 | 7 | 29 | 74 |
| Data compiled from: Hegar K. Anatomical investigations on the nullipara uterus with special consideration of isthmus (Anatomische Untersuchungen am nullipara Uterus mit besonderer Berücksichtigung der Isthmus). (1908) Beitraae zur Geburtsh. u. Gynak., vol. 13, 1908. reference list | Uterus Growth Table | Collapsible Table | Uterus Development | ||||
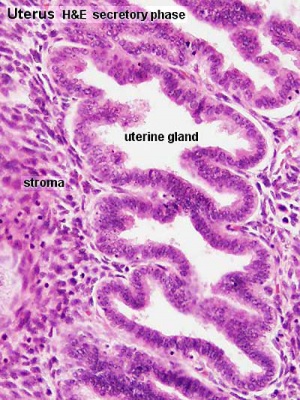
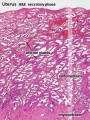
Uterus Histology
See also Menstrual Cycle - Histology
Abnormalities
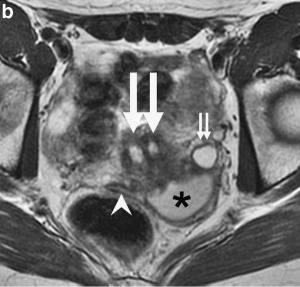
There are at least two clinical society classifications for female genital tract abnormalities:
- European Society of Human Reproduction and Embryology—European Society for Gynaecological Endoscopy (ESHRE-ESGE)[16]
- American Society for Reproductive Medicine (ASRM) [17]
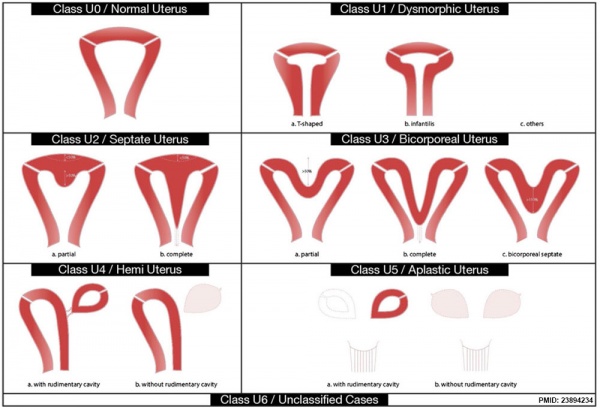
ESHRE-ESGE Classification
European Society for Gynaecological Endoscopy (ESHRE-ESGE)[16]
Uterine anatomical deviations deriving from the same embryological origin:
- U0 - normal uterus
- U1 - dysmorphic uterus
- U2 - septet uterus
- U3 - bicorporeal uterus
- U4 - hemi-uterus
- U5 - aplastic uterus
- U6 - for still unclassified cases
Main classes have been divided into sub-classes expressing anatomical varieties with clinical significance. Cervical and vaginal anomalies are classified independently into sub-classes having clinical significance.
| ESHRE/ESGE Classification of Uterine Anomalies | |
|---|---|
European Society of Human Reproduction and Embryology (ESHRE) and the European Society for Gynaecological Endoscopy (ESGE)
|
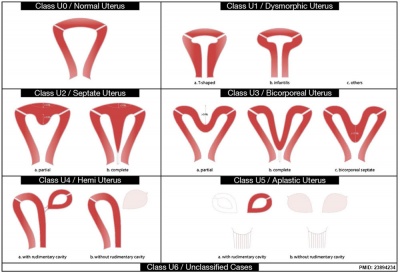
|
| <pubmed>23894234</pubmed>
See also ICD10 Congenital malformations of genital organs (Q50-Q56) | |
Uterine Duplication
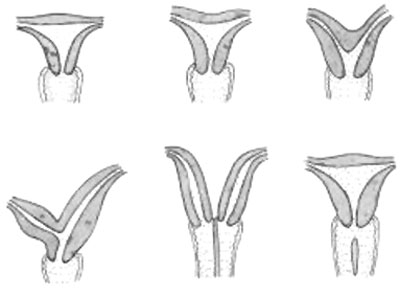
|
A range of uterine and vaginal anatomical anomalies based upon the abnormal development and fusion of the paramesonephric ducts and vaginal plate development.
| |||
|
Unicornate Uterus - failure of the paramesonephric ducts to fuse. A single paramesomnephric duct has fused with the vaginal plate and now opens into the vagina, while the other forms a diverticulum.
|
Bicornuate uterus containing conceptus chorionic sac with placental cord on one side.
Septate Uterus
Uterine residual septum classification:
- American Society for Reproductive Medicine (ASRM) criterion with an internal fundal indentation length equal or greater than 1 cm[18]
- European Society of Human Reproduction and Embryology—European Society for Gynaecological Endoscopy (ESHRE-ESGE) classification of female genital tract congenital anomalies with an internal indentation at the fundal midline greater than 50% myometrial thickness.[16]
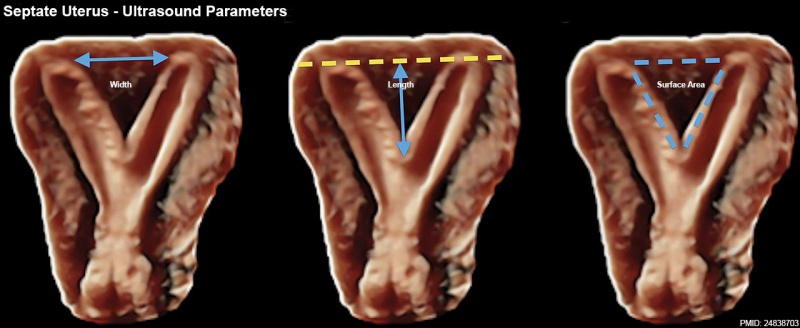
Septate Uterus Ultrasound[19]
Uterine Duplication
(uterus didelphys, double uterus, uterus didelphis) A rare uterine developmental abnormality where the paramesonephric ducts (Mullerian ducts) completely fail to fuse generating two separate uterus parts each connected to the cervix and having an ovary each.
Uterus/Vaginal
Mayer-Rokitansky-Kuster-Hauser syndrome (MRKH, MRK anomaly, Rokitansky-Kuster-Hauser syndrome, RKH syndrome, RKH) consists of congenital aplasia of the uterus and the upper part of vagina due to anomalous development of Müllerian ducts, either isolated or associated with other congenital malformations, including renal, skeletal, hearing and heart defects. Has an incidence of approximately 1 in 4500 newborn girls and has been associated with a microdeletion at 17q12.[20]
There has been recently a single report of a MRKH syndrome woman giving a live-birth after uterus transplantation from a deceased donor.[3]
Cervical: cervical agenesis, cervical duplication
Environmental Abnormalities
DES Diethylstilbestrol or diethylstilbetrol, is a drug that was prescribed to women from 1938-1971 to prevent miscarriage in high-risk pregnancies. The drug acted as a potent estrogen (mimics natural hormone) and therefore could also act as a potential endocrine disruptor. This led to a number of developing fetal reproductive tract and other abnormalities. In the female fetus, it increased risk of abnormal reproductive tract and also carcinogenic (cancer forming). In the male fetus, it increased the occurance of abnormal genitalia. The drug was banned by FDA (USA) in 1979 as a teratogen, it had previously also been used as livestock growth promoter and could have potentially entered the human food chain. (More? endocrine abnormalities | chemicals | drugs)
- Links: Childrens Hospital Boston - Congenital Anomalies of the Uterus | Medical Education Image Link - Cervical agenesis | OMIM - Rokitansky-Kuster-Hauser syndrome |
Cervical Cancer
| ICD-11 2C77 Malignant neoplasms of cervix uteri - Primary or metastatic malignant neoplasm involving the cervix. |
|---|
|
| Links: cervical cancer | human papillomavirus | viral infection | uterus |
In Australia, the "Pap Smear" test was replaced in 2017 (1 December) by a new "National Cervical Screening Program". This new program will use new technologies to detect HPV DNA rather than pathological screening for abnormal cells from a "Pap Smear". See the last report Cervical screening in Australia 2019[21]Australian Institute of Health and Welfare 2019. Physical activity during pregnancy 2011–12. Cat. no. PHE 243. Canberra: AIHW. PDF</ref>, that used Pap tests as the screening tool (data for women screened between 1 January 2016 and 30 June 2017)
For more information see the external links below.
| DOH Information Video |
|---|
|
<html5media width="480" height="360">https://www.youtube.com/embed/a22VIXp3cxc</html5media> Australian Department of Health (Published on Nov 1, 2017) |
- "The two yearly Pap test for women aged 18 to 69 will change to a five yearly human papillomavirus (HPV) test for women aged 25 to 74. Women will be due for the first Cervical Screening Test two years after their last Pap test."
- "The Cervical Screening Test detects infection with human papillomavirus (HPV). Partial genotyping is used to determine the type of HPV into one of two groups: oncogenic HPV 16/18 or oncogenic HPV types other than 16/18 as a pooled result." (NCSP Factsheet)
- Links: National Cervical Screening Program | Factsheet | Compass Trial |
ABC radio program Monday 27 March 2017 - Death of the pap smear? | ABC Audio - Death of the pap smear?
History of the Pap Smear
The information below relates to the original "Pap Smear" (Papanicolaou smear, pap test, cervical smear) The text below is from the ABC - Great Moments In Science.
- "Luckily, we have the famous Pap Smear - an excellent way to find cancer of the cervix before it digs in locally and/or spreads throughout the body. The Pap Smear is named after a certain Dr. Papanicolaou - who did a Pap Smear on his wife virtually every day for 20 years.
- George Nikolas Papanicolaou was born in 1883 in Kymi, a small town overlooking the Aegean Sea on the Island of Euboea in Greece. His father, Nikolas Papanicolaou was both the Major of Kymi and a medical doctor. His older brother, Naso, had studied law, so his father convinced George to continue in the family medical tradition. So George studied medicine, and did well, graduating with a degree in honours in 1904............"
- Links: Menstrual Cycle - Histology | Dilatation and curettage (D&C) | ABC - Great Moments In Science
Broad Ligament
| The broad ligament is found associated with the internal human female genital tract. It forms a mesentery consisting of a double fold of the peritoneum that connects the uterus to the peritoneal floor and walls.
Anatomically it has three parts:
Abnormalities include peritoneal endometriosis. |
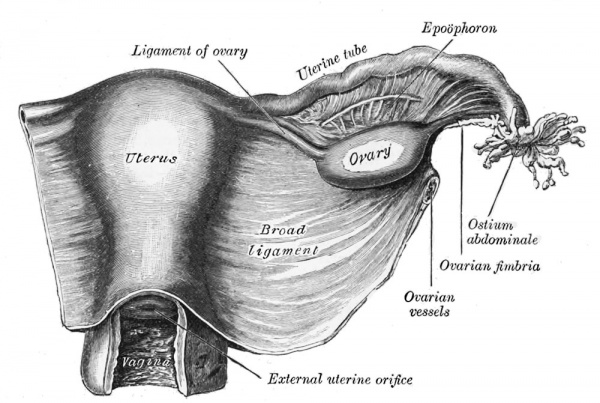
|
Molecular
Wnt genes - Wnt4, Wnt5a, and Wnt7a implicated in the formation and morphogenesis of the Müllerian duct.
Wnt7a - mediates the patterning of the oviduct and differentiation of the uterus.
beta-catenin - manufactured in the mesenchyme is a downstream effector of Wnt7a.
Bmp2 - decidualization regulator of gene expression and function (shown in mouse uterus).
Lim1, Lhx9, Emx, Pax-2, Hox-A9, Hox-A10, Hox-A11, Hox-A13, WT1, SF-1, GATA-4. TGF-beta
References
- ↑ Pitot MA, Bookwalter CA & Dudiak KM. (2020). Müllerian duct anomalies coincident with endometriosis: a review. Abdom Radiol (NY) , , . PMID: 32179978 DOI.
- ↑ Nanjappa MK, Mesa AM, Medrano TI, Jefferson WN, DeMayo FJ, Williams CJ, Lydon JP, Levin ER & Cooke PS. (2019). The histone methyltransferase EZH2 is required for normal uterine development and function in mice. Biol. Reprod. , , . PMID: 31201420 DOI.
- ↑ 3.0 3.1 Parikh PM, Charak BS, Banavali SD, Koppikar SB, Giri N, Nadkarni P, Saikia TK, Gopal R & Advani SH. (1988). A prospective, randomized double-blind trial comparing metoclopramide alone with metoclopramide plus dexamethasone in preventing emesis induced by high-dose cisplatin. Cancer , 62, 2263-6. PMID: 3052785
- ↑ Roly ZY, Backhouse B, Cutting A, Tan TY, Sinclair AH, Ayers KL, Major AT & Smith CA. (2018). The cell biology and molecular genetics of Müllerian duct development. Wiley Interdiscip Rev Dev Biol , , . PMID: 29350886 DOI.
- ↑ Prior M, Richardson A, Asif S, Polanski L, Parris-Larkin M, Chandler J, Fogg L, Jassal P, Thornton JG & Raine-Fenning NJ. (2018). Outcome of assisted reproduction in women with congenital uterine anomalies: a prospective observational study. Ultrasound Obstet Gynecol , 51, 110-117. PMID: 29055072 DOI.
- ↑ Prunskaite-Hyyryläinen R, Skovorodkin I, Xu Q, Miinalainen I, Shan J & Vainio SJ. (2016). Wnt4 coordinates directional cell migration and extension of the Müllerian duct essential for ontogenesis of the female reproductive tract. Hum. Mol. Genet. , 25, 1059-73. PMID: 26721931 DOI.
- ↑ Huang CC, Orvis GD, Kwan KM & Behringer RR. (2014). Lhx1 is required in Müllerian duct epithelium for uterine development. Dev. Biol. , 389, 124-36. PMID: 24560999 DOI.
- ↑ 8.0 8.1 Guioli S, Sekido R & Lovell-Badge R. (2007). The origin of the Mullerian duct in chick and mouse. Dev. Biol. , 302, 389-98. PMID: 17070514 DOI.
- ↑ Deutscher E & Hung-Chang Yao H. (2007). Essential roles of mesenchyme-derived beta-catenin in mouse Müllerian duct morphogenesis. Dev. Biol. , 307, 227-36. PMID: 17532316 DOI.
- ↑ Kobayashi A, Shawlot W, Kania A & Behringer RR. (2004). Requirement of Lim1 for female reproductive tract development. Development , 131, 539-49. PMID: 14695376 DOI.
- ↑ Hashimoto R. (2003). Development of the human Müllerian duct in the sexually undifferentiated stage. Anat Rec A Discov Mol Cell Evol Biol , 272, 514-9. PMID: 12740945 DOI.
- ↑ Soriano D, Lipitz S, Seidman DS, Maymon R, Mashiach S & Achiron R. (1999). Development of the fetal uterus between 19 and 38 weeks of gestation: in-utero ultrasonographic measurements. Hum. Reprod. , 14, 215-8. PMID: 10374123
- ↑ Shelton DN, Fornalik H, Neff T, Park SY, Bender D, DeGeest K, Liu X, Xie W, Meyerholz DK, Engelhardt JF & Goodheart MJ. (2012). The role of LEF1 in endometrial gland formation and carcinogenesis. PLoS ONE , 7, e40312. PMID: 22792274 DOI.
- ↑ Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW & Spencer TE. (2001). Developmental biology of uterine glands. Biol. Reprod. , 65, 1311-23. PMID: 11673245
- ↑ Wang ZJ, Daldrup-Link H, Coakley FV & Yeh BM. (2010). Ectopic ureter associated with uterine didelphys and obstructed hemivagina: preoperative diagnosis by MRI. Pediatr Radiol , 40, 358-60. PMID: 19924410 DOI.
- ↑ 16.0 16.1 16.2 Grimbizis GF, Gordts S, Di Spiezio Sardo A, Brucker S, De Angelis C, Gergolet M, Li TC, Tanos V, Brölmann H, Gianaroli L & Campo R. (2013). The ESHRE-ESGE consensus on the classification of female genital tract congenital anomalies. Gynecol Surg , 10, 199-212. PMID: 23894234 DOI.
- ↑ . (1988). The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, müllerian anomalies and intrauterine adhesions. Fertil. Steril. , 49, 944-55. PMID: 3371491
- ↑ Bermejo C, Martínez Ten P, Cantarero R, Diaz D, Pérez Pedregosa J, Barrón E, Labrador E & Ruiz López L. (2010). Three-dimensional ultrasound in the diagnosis of Müllerian duct anomalies and concordance with magnetic resonance imaging. Ultrasound Obstet Gynecol , 35, 593-601. PMID: 20052665 DOI.
- ↑ Ludwin A, Ludwin I, Pityński K, Banas T & Jach R. (2014). Role of morphologic characteristics of the uterine septum in the prediction and prevention of abnormal healing outcomes after hysteroscopic metroplasty. Hum. Reprod. , 29, 1420-31. PMID: 24838703 DOI.
- ↑ Bernardini L, Gimelli S, Gervasini C, Carella M, Baban A, Frontino G, Barbano G, Divizia MT, Fedele L, Novelli A, Béna F, Lalatta F, Miozzo M & Dallapiccola B. (2009). Recurrent microdeletion at 17q12 as a cause of Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome: two case reports. Orphanet J Rare Dis , 4, 25. PMID: 19889212 DOI.
- ↑ Australian Institute of Health and Welfare 2019. Cervical screening in Australia 2019. Cancer series no. 123. Cat. no. CAN 124. Canberra: AIHW. PDF
Reviews
Passos IMPE & Britto RL. (2020). Diagnosis and treatment of müllerian malformations. Taiwan J Obstet Gynecol , 59, 183-188. PMID: 32127135 DOI.
Farage M & Maibach H. (2006). Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. , 273, 195-202. PMID: 16208476 DOI.
Cummings AM & Kavlock RJ. (2004). Function of sexual glands and mechanism of sex differentiation. J Toxicol Sci , 29, 167-78. PMID: 15467266
Articles
Deutscher E & Hung-Chang Yao H. (2007). Essential roles of mesenchyme-derived beta-catenin in mouse Müllerian duct morphogenesis. Dev. Biol. , 307, 227-36. PMID: 17532316 DOI.
Guioli S, Sekido R & Lovell-Badge R. (2007). The origin of the Mullerian duct in chick and mouse. Dev. Biol. , 302, 389-98. PMID: 17070514 DOI.
Hashimoto R. (2003). Development of the human Müllerian duct in the sexually undifferentiated stage. Anat Rec A Discov Mol Cell Evol Biol , 272, 514-9. PMID: 12740945 DOI.
Search PubMed
Search May 2007 "embryonic uterine development" 3,025 reference articles of which 491 were reviews.
Search Pubmed: Uterus Development | embryonic uterine development | Paramesonephric Duct | Mullerian Duct | Endocrine Disruptors | uterine+adenogenesis
Additional Images
Terms
Note some of these terms relate to the adult or the maternal uterus during pregnancy.
- cervical cerclage - A clinical birth procedure involving circumferential banding or suture of the cervix early (between 12 -14 weeks) or when required to prevent or treat passive dilation prior to completion of pregnancy (37 weeks), described as cervical insufficiency.
- cervical insufficiency - (CI) A clinical term describing a painless and progressive dilatation and effacement of the cervix that may lead to second trimester abortions or preterm delivery. It has also been described as inability of the uterine cervix to retain a pregnancy in the absence of uterine contractions. The condition may in some instances treated clinically by cervical cerclage. The biological basis is currently undetermined with some evidence showing a genetic relationship.
- cervical length - There is some data that shows the risk of spontaneous preterm labour and delivery increases in women who have a short cervix PMID 8569824.
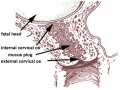
- cervical mucus plug - (CMP) During early pregnancy, maternal glands located at the cervical junction between vagina and uterus secrete mucus that forms a plug or barrier between these two structures.
- cervical pregnancy - A rare type of ectopic pregnancy with implantation at the cervical canal, occurring with an incidence ranging between 1:1,000 and 1:18,000 pregnancies. Clinically, when an associated haemorrhage occurs a hysterectomy is usually performed.
- cervical ripening - Clinical birth term describing the hormonal softening of the cervix to allow expansion in preparation for birth.
- cervix - (Latin, cervix = neck) The female anatomical region of the uterus forming a canal that opens and connects to the vagina.
- fallopian tube obstruction - (tubal occlusion) A blockage of the uterine tube that can affect fertility due to a pathologic occlusion, spasm or plugging and also be either unilateral (single tube) or bilateral (both tubes). Described anatomically as in the proximal, the mid or the distal part of the tube.
- fundus - (Latin, fundus = "bottom") Top part of the uterus body lying between the two uterine tubes and a common implantation site.
- hysterosalpingography - A clinical diagnostic technique used to visualise the uterine cavity by X-ray.
- hysteroscopy - A clinical diagnostic technique used to visualise the uterine cavity by a camera or video.
- peg-cell - (resting secretory cell) A histological term for the non-ciliated secretory epithelial cells located within the uterus.
- Pouch of Douglas - (rectouterine pouch or rectovaginal) Anatomical description of the female peritoneal cavity lying between the back wall of the uterus and rectum.
- rectouterine pouch - (Pouch of Douglas or rectovaginal) Anatomical description of the female peritoneal cavity lying between the back wall of the uterus and rectum.
- sonohysterography - A clinical diagnostic technique used to visualise the uterine cavity by ultrasound. Firstly, fluid is injected through the cervix into the uterus, then ultrasound is carried out to image the uterine cavity.
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Blue Histology Female Reproductive Tract
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 9) Embryology Uterus Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Uterus_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G