Respiratory System - Abnormalities
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Abnormalities of the respiratory system include not only lung development but also the upper respiratory tract, the supporting musculoskeletal system and the vascular and neural system. In addition, some respiratory problems arise from prematurity of birth or difficulty with the birth process itself.
The functional part of the respiratory system, the alveoli, continue to develop the postnatal period and through childhood (Postnatal alveoli number graph).
International Classification of Diseases - Respiratory
| Abnormality Links: abnormal development | abnormal genetic | abnormal environmental | Unknown | teratogens | ectopic pregnancy | cardiovascular abnormalities | coelom abnormalities | endocrine abnormalities | gastrointestinal abnormalities | genital abnormalities | head abnormalities | integumentary abnormalities | musculoskeletal abnormalities | limb abnormalities | neural abnormalities | neural crest abnormalities | placenta abnormalities | renal abnormalities | respiratory abnormalities | hearing abnormalities | vision abnormalities | twinning | Developmental Origins of Health and Disease | ICD-11 | ||
|
| System Abnormalities | ||||
|---|---|---|---|---|
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Abnormal Respiratory Development | Newborn Respiratory Distress Syndrome | congenital diaphragmatic hernia | Tracheoesophageal Fistula |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
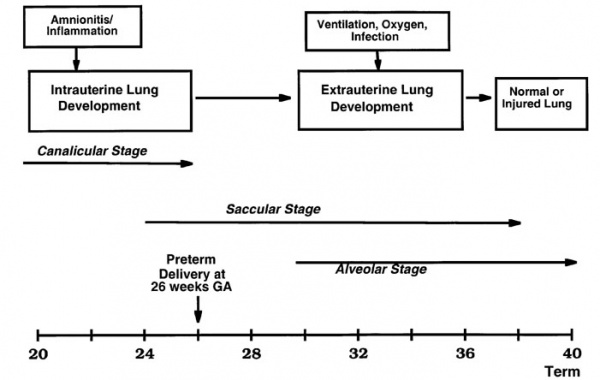
Premature Birth
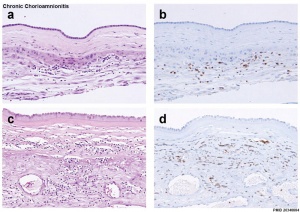
Preterm delivery and overview of related potential fetal and neonatal infections can effect lung development.[9]
After very preterm birth, the chorioamnionitis associated commensal organism is usually Ureaplasma urealyticum.[10]
- Links: Chorioamnionitis | Bacterial Infection
Tracheoesophageal Fistula
(Tracheo-Oesophageal Fistula, Oesophageal Atresia) - Oesophageal Atresia with or without tracheo-oesophageal fistula
Laryngeal-tracheo-oesophageal Cleft
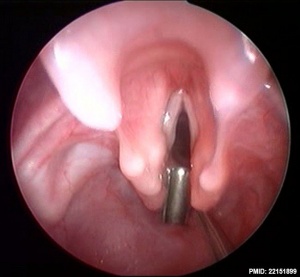
(LC, laryngeal cleft) A rare foregut abnormality allowing digestive tract and the airway to communicate causing chronic cough, aspiration and respiratory distress.
The downward extension of the cleft determines the classification of the abnormality,[12][11]
- Type 0 - submucosal cleft
- Type I - supraglottic, interarytenoid cleft, above the vocal fold level
- Type II - cleft extending below the vocal folds into the cricoid cartilage
- Type III a - cleft extending through the cricoid cartilage but not into the trachea
- Type III b - cleft extending through the cricoid cartilage and into the cervical trachea
- Type IV - cleft extending into the thoracic trachea, potentially down to the carina
Lobar Emphysema (Overinflated Lung)
Congenital lobar emphysema |
|
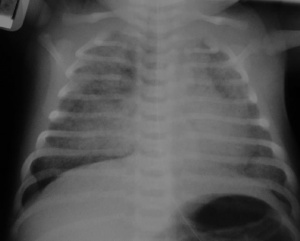
LB00.0 Congenital Diaphragmatic Hernia

| ICD-11 |
|---|
|
LB00 Structural developmental anomalies of diaphragm
|
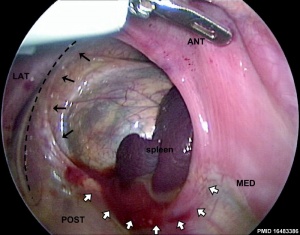
Really a musculoskeletal abnormality, but included here due to the associated respiratory effects. Failure of the pleuroperitoneal foramen (foramen of Bochdalek) to close allows viscera into thorax, most common (80-85%) on the left side of diaphragm. Intestine, stomach or spleen can enter the pleural cavity, compressing the lung.

|
Left posterolateral diaphragmatic hernia[13]
|
Australian Statistics
A recent Western Australian study[14] of congenital diaphragmatic hernia (CDH) outcomes showed:
- 35% of live-born infants died before referral or transport.
- population of infants reaching center represented only 40% of the total cases
- 92% percent of postoperative infants survived beyond 1 year of age
- 80% of infants who reached the surgical referral center
- only 52% of live-born infants, 32% of all cases, and 16% of all prenatally diagnosed cases survived.
- the overall mortality rate for this condition remains high
- 33% of all cases of CDH and 49% of prenatally diagnosed fetuses underwent elective termination of pregnancy
- the number of fetal terminations confounds the accurate assessment of the true outcomes of this condition
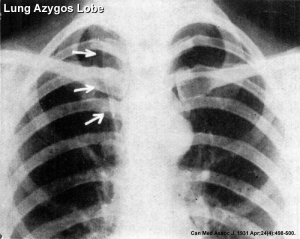
Azygos Lobe
Common anatomical variation occurring in about 0.5% of the population. The right lung upper lobe expands either side of the posterior cardinal. There is also some course variability of the phrenic nerve in the presence of an azygos lobe.
Congenital Laryngeal Webs
Laryngeal abnormality due to embryonic (week 10) incomplete recanalization of the laryngotracheal tube during the fetal period. Rare abnormality occuring mainly at the level of the vocal folds (glottis).
Meconium Aspiration Syndrome
(MAS) Meconium is the gastrointestinal contents that accumulate in the intestines during the fetal period. Fetal stress in the third trimester, prior to/at/ or during parturition (birth) can lead to premature meconium discharge into the amniotic fluid and sunsequent ingestion by the fetus and damage to respiratory function. Damage to placental vessels meconium myonecrosis may also occur.
- meconium is formed from gut and associated organ secretions as well as cells and debris from the swallowed amniotic fluid.
- Meconium accumulates during the fetal period in the large intestine (bowel). It can be described as being a generally dark colour (green black) , sticky and odourless.
- Normally this meconium is defaecated (passed) postnatally over the first 48 hours and then transitional stools from day 4.
- Abnormally this meconium is defaecated in utero, due to oxygen deprivation and other stresses. Premature discharge into the amniotic sac can lead to mixing with amniotic fluid and be reswallowed by the fetus. This is meconium aspiration syndrome and can damage both the developing lungs and placental vessels.
Australian Statistics
The following Australia and New Zealand (1995 - 2002) data is from a recent (2009) study, the epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome.[15]
- Data were gathered on all of the infants in Australia and New Zealand who were intubated and mechanically ventilated with a primary diagnosis of MAS (MASINT) between 1995 and 2002, inclusive.
- MASINT occurred in 1061 of 2,490,862 live births (0.43 of 1000), with a decrease in incidence from 1995 to 2002.
- A higher risk of MASINT was noted at advanced gestation, with 34% of cases born beyond 40 weeks, compared with 16% of infants without MAS.
- Fetal distress requiring obstetric intervention was noted in 51% of cases, and 42% were delivered by cesarean section.
- There was a striking association between low 5-minute Apgar score and MASINT.
- Risk of MASINT was higher where maternal ethnicity was Pacific Islander or indigenous Australian and was also increased after planned home birth.
- Uptake of exogenous surfactant, high-frequency ventilation, and inhaled nitric oxide increased considerably during the study period, with >50% of infants receiving > or =1 of these therapies by 2002.
- Risk of air leak was 9.6% overall, with an apparent reduction to 5.3% in 2001-2002.
- The duration of intubation remained constant throughout the study period (median: 3 days), whereas duration of oxygen therapy and length of hospital stay increased.
- Death related to MAS occurred in 24 infants (2.5% of the MASINT cohort; 0.96 per 100,000 live births).
Newborn Respiratory Distress Syndrome
The historic name of "Hyaline Membrane Disease" (HMD) described the "glassy" appearance of the premature neonatal lungs due to insufficient surfactant.
Surfactant deficiency in immature lungs leads to:
- alveolar instability and collapse
- capillary leak edema
- hyaline membrane formation
- Links: medline plus | eMedicine
Hyaline Membrane Disease History
See the recent review of Hyaline Membrane Disease (HMD) history[16] and surfactant.[17]
- 1835 - first description in premature babies born with immature “fetal lungs.”
- 1947 - pressure required to inflate deceased newborn lungs lower when saline was introduced into the lungs.
- 1959 - concept that HMD due to lack of surfactant (Prof. Mary Ellen Avery).
- 1980 - first study on endotracheal administration of surfactant in premature infants.
Surfactant Metabolism
(pulmonary surfactant metabolism dysfunctions, surfactant dysfunction disorders) For review of genetic disorders of surfactant dysfunction.[18]
Mutations in the genes encoding:
- surfactant protein B (SP-B)
- surfactant protein C ( SP-C)
- phospholipid transporter ABCA3
Bronchopulmonary Dysplasia
A chronic lung disease which can occur following premature birth and related lung injury. The definition of bronchopulmonary dysplasia (BPD) has in recent years changed from a severe lung injury and associated repair, to more of a disruption of lung growth in older infants.[19]
Most infants who develop BPD are born more than 10 weeks before their due dates, weigh less than 1,000 grams (about 2 pounds) at birth, and have breathing problems. Infections that occur before or shortly after birth also can contribute to BPD.
- Links: NIH - NHLBI
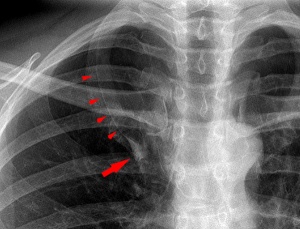
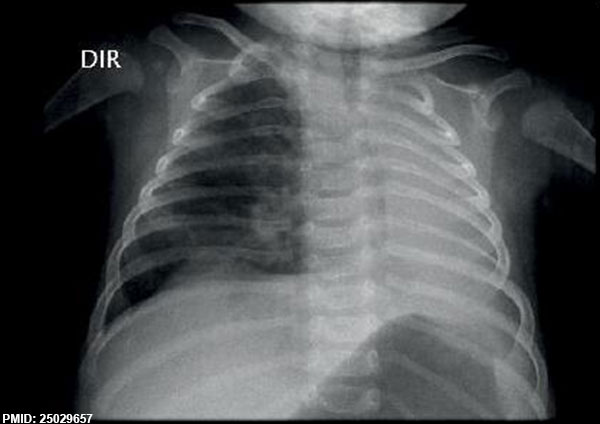
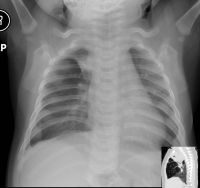
Lung Agenesis
Agenesis of Left lung (X Ray)[20]
Prevalence, including the bilateral and unilateral forms, is 0.5-1.0 per 10,000 live births.
Cystic Fibrosis
Cystic Fibrosis (CF) is a serious genetic disease due to abnormal chloride channel synthesis (cystic fibrosis transmembrane conductance regulator, CFTR), the impact occurs postnatally. Mucus accumulates mainly in the passages of the lungs and in the pancreas.
- Links: PubMed Health | OMIM | USA National Heart Lung and Blood Institute | Cystic Fibrosis Australia
OMIM
List of respiratory related abnormalities Respiratory and Diaphragmatic Hernia.
References
- ↑ 1.0 1.1 Fisher JC & Bodenstein L. (2006). Computer simulation analysis of normal and abnormal development of the mammalian diaphragm. Theor Biol Med Model , 3, 9. PMID: 16483386 DOI.
- ↑ Karolak JA, Vincent M, Deutsch G, Gambin T, Cogné B, Pichon O, Vetrini F, Mefford HC, Dines JN, Golden-Grant K, Dipple K, Freed AS, Leppig KA, Dishop M, Mowat D, Bennetts B, Gifford AJ, Weber MA, Lee AF, Boerkoel CF, Bartell TM, Ward-Melver C, Besnard T, Petit F, Bache I, Tümer Z, Denis-Musquer M, Joubert M, Martinovic J, Bénéteau C, Molin A, Carles D, André G, Bieth E, Chassaing N, Devisme L, Chalabreysse L, Pasquier L, Secq V, Don M, Orsaria M, Missirian C, Mortreux J, Sanlaville D, Pons L, Küry S, Bézieau S, Liet JM, Joram N, Bihouée T, Scott DA, Brown CW, Scaglia F, Tsai AC, Grange DK, Phillips JA, Pfotenhauer JP, Jhangiani SN, Gonzaga-Jauregui CG, Chung WK, Schauer GM, Lipson MH, Mercer CL, van Haeringen A, Liu Q, Popek E, Coban Akdemir ZH, Lupski JR, Szafranski P, Isidor B, Le Caignec C & Stankiewicz P. (2019). Complex Compound Inheritance of Lethal Lung Developmental Disorders due to Disruption of the TBX-FGF Pathway. Am. J. Hum. Genet. , , . PMID: 30639323 DOI.
- ↑ Wigen RB, Duan W, Moraes TJ & Chiu PPL. (2019). Predictors of Long-Term Pulmonary Morbidity in Children with Congenital Diaphragmatic Hernia. Eur J Pediatr Surg , 29, 120-124. PMID: 30583297 DOI.
- ↑ Kemppainen M, Lahesmaa-Korpinen AM, Kauppi P, Virtanen M, Virtanen SM, Karikoski R, Gissler M & Kirjavainen T. (2018). Maternal asthma is associated with increased risk of perinatal mortality. PLoS ONE , 13, e0197593. PMID: 29775476 DOI.
- ↑ Lo J, Zivanovic S, Lunt A, Alcazar-Paris M, Andradi G, Thomas M, Marlow N, Calvert S, Peacock J & Greenough A. (2018). Longitudinal assessment of lung function in extremely prematurely born children. Pediatr. Pulmonol. , 53, 324-331. PMID: 29316378 DOI.
- ↑ Hagadorn JI, Brownell EA, Herbst KW, Trzaski JM, Neff S & Campbell BT. (2015). Trends in treatment and in-hospital mortality for neonates with congenital diaphragmatic hernia. J Perinatol , 35, 748-54. PMID: 25950919 DOI.
- ↑ McFetridge L, McMorrow A, Morrison PJ & Shields MD. (2009). Surfactant Metabolism Dysfunction and Childhood Interstitial Lung Disease (chILD). Ulster Med J , 78, 7-9. PMID: 19252722
- ↑ Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG & Kim JS. (2010). The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod. Pathol. , 23, 1000-11. PMID: 20348884 DOI.
- ↑ Jobe AH & Ikegami M. (2001). Antenatal infection/inflammation and postnatal lung maturation and injury. Respir. Res. , 2, 27-32. PMID: 11686862
- ↑ Goldenberg RL, Hauth JC & Andrews WW. (2000). Intrauterine infection and preterm delivery. N. Engl. J. Med. , 342, 1500-7. PMID: 10816189 DOI.
- ↑ 11.0 11.1 Leboulanger N & Garabédian EN. (2011). Laryngo-tracheo-oesophageal clefts. Orphanet J Rare Dis , 6, 81. PMID: 22151899 DOI.
- ↑ Benjamin B & Inglis A. (1989). Minor congenital laryngeal clefts: diagnosis and classification. Ann. Otol. Rhinol. Laryngol. , 98, 417-20. PMID: 2729823 DOI.
- ↑ Tovar JA. (2012). Congenital diaphragmatic hernia. Orphanet J Rare Dis , 7, 1. PMID: 22214468 DOI.
- ↑ Colvin J, Bower C, Dickinson JE & Sokol J. (2005). Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics , 116, e356-63. PMID: 16140678 DOI.
- ↑ Dargaville PA & Copnell B. (2006). The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics , 117, 1712-21. PMID: 16651329 DOI.
- ↑ Aly H, Mohamed MA & Wung JT. (2017). Surfactant and continuous positive airway pressure for the prevention of chronic lung disease: History, reality, and new challenges. Semin Fetal Neonatal Med , 22, 348-353. PMID: 28818610 DOI.
- ↑ Obladen M. (2005). History of surfactant up to 1980. Biol. Neonate , 87, 308-16. PMID: 15985753 DOI.
- ↑ Wert SE, Whitsett JA & Nogee LM. (2009). Genetic disorders of surfactant dysfunction. Pediatr. Dev. Pathol. , 12, 253-74. PMID: 19220077 DOI.
- ↑ Deakins KM. (2009). Bronchopulmonary dysplasia. Respir Care , 54, 1252-62. PMID: 19712501
- ↑ Jentzsch NS. (2014). Unilateral pulmonary agenesis. J Bras Pneumol , 40, 322-4. PMID: 25029657
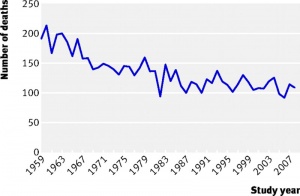
- ↑ Barr HL, Britton J, Smyth AR & Fogarty AW. (2011). Association between socioeconomic status, sex, and age at death from cystic fibrosis in England and Wales (1959 to 2008): cross sectional study. BMJ , 343, d4662. PMID: 21862532
Reviews
Gur M, Hakim F & Bentur L. (2017). Better understanding of childhood asthma, towards primary prevention - are we there yet? Consideration of pertinent literature. F1000Res , 6, 2152. PMID: 29333254 DOI.
Aly H, Mohamed MA & Wung JT. (2017). Surfactant and continuous positive airway pressure for the prevention of chronic lung disease: History, reality, and new challenges. Semin Fetal Neonatal Med , 22, 348-353. PMID: 28818610 DOI.
Hekking PP & Bel EH. (2014). Developing and emerging clinical asthma phenotypes. J Allergy Clin Immunol Pract , 2, 671-80; quiz 681. PMID: 25439356 DOI.
Clement A, Nathan N, Epaud R, Fauroux B & Corvol H. (2010). Interstitial lung diseases in children. Orphanet J Rare Dis , 5, 22. PMID: 20727133 DOI.
Thébaud B & Abman SH. (2007). Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am. J. Respir. Crit. Care Med. , 175, 978-85. PMID: 17272782 DOI.
Hartl D & Griese M. (2005). Interstitial lung disease in children -- genetic background and associated phenotypes. Respir. Res. , 6, 32. PMID: 15819986 DOI.
Articles
Sztanó B, Torkos A & Rovó L. (2010). The combined endoscopic management of congenital laryngeal web. Int. J. Pediatr. Otorhinolaryngol. , 74, 212-5. PMID: 20004027 DOI.
Shannon EH. (1931). THE AZYGOS LOBE OF THE LUNG. Can Med Assoc J , 24, 498-500. PMID: 20318245
Mata J, Cáceres J, Alegret X, Coscojuela P & De Marcos JA. (1991). Imaging of the azygos lobe: normal anatomy and variations. AJR Am J Roentgenol , 156, 931-7. PMID: 2017954 DOI.
Whitfield JM, Charsha DS & Chiruvolu A. (2009). Prevention of meconium aspiration syndrome: an update and the Baylor experience. Proc (Bayl Univ Med Cent) , 22, 128-31. PMID: 19381312
Speckman JM, Gamsu G & Webb WR. (1981). Alterations in CT mediastinal anatomy produced by an azygos lobe. AJR Am J Roentgenol , 137, 47-50. PMID: 6787889 DOI.
Baumgartner FJ. (2009). Thoracoscopic surgery for hyperhidrosis in the presence of congenital azygous lobe and its suspensory web. Tex Heart Inst J , 36, 44-7. PMID: 19436785
Search Pubmed
Search Pubmed: Respiratory System Developmental Abnormalities | Tracheoesophageal Fistula | Bronchopulmonary Dysplasia | Congenital Laryngeal Webs | Hyaline Membrane Disease | Meconium Aspiration Syndrome
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- AAFP - Respiratory Distress in the Newborn
- NZ - Parenchymal Lung Disease
- Cystic Fibrosis Australia
- Medline Plus - Diaphragmatic hernia
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Respiratory System - Abnormalities. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Respiratory_System_-_Abnormalities
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G