Paper - The development of the olfactory and the accessory olfactory formations in human embryos and fetuses
| Embryology - 30 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Humphrey T. The development of the olfactory and the accessory olfactory formations in human embryos and fetuses. (1940) J. Comp. Neurol. 431-468.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Olfactory and the Accessory Olfactory Formations in Human Embryos and Fetuses
Tryphena Humphrey
Department of Anatomy, University of Pittsburgh, Pennsylvania
- Aided by grants from the Penrose Fund of the American Philosophical Society, the Carnegie Corporationof New York and the University of Pittsburgh. Publication no. 7, Physiological and morphological studies on human prenatal development.
Introduction
In a recent study of the adult human olfactory bulb the accessory olfactory formation was found to be absent (Humphrey and Crosby, ’38; Crosby and Humphrey, ’39 a, ’39 b), but it was stated that a primordial accessory olfactory bulb would probably be present during embryonic development. The unusual collection of human embryonic material available in the Department of Anatomy of the University of Pittsburgh has made it possible to verify this suggestion and to study the development of both the olfactory and the accessory olfactory formations. The results of this research are presented in the present paper.
Material and Methods
The material used consists of serial sections of the brains of human fetuses varying in size from approximately 14 mm. to 145 mm. in crown-rump length, or from about 6.5 to 18.5 weeks of menstrual age. The thirteen series described, all but the last five of which were horizontally sectioned, are listed chronologically in table 1. The brains of the last five fetuses were frontally sectioned. All of the fetuses under 11 weeks of menstrual age were sectioned in toto. The central nervous system was removed from all those over 11 weeks of age and sectioned separately. The pyridine silver series were stained according to the method of Ranson. The series made with activated protargol follow the method of Bodian (’36 and ’37).
Table 1
| Table 1- Fetus Details | |||
|---|---|---|---|
| No. of Fetus in Collection |
C.R. Length mm |
Approximate Menstrual Age Weeks |
Stain |
| P | 14+ | 6.5 | (Stain - Haematoxylin Eosin) |
| 1 | 16.0 | 7.0 | Erythrosin & toluidin blue |
| 0 | 22.5 | 8.0 | Activated protargol |
| 22 | 26.0 | 8.5 | Erythrosin & toluidin blue |
| 19 | 26.5 | 8.5 | Pyridine silver |
| 33 | 32.0 | 9.5 | Activated protargol |
| 16 | 35.0 | 9.5 | Pyridine silver & Lyon ’s blue |
| 34 | 37.0 | 10.0 | Activated protargol |
| 51 | 49.0 | 11.0 | Activated protargol |
| 39 | 61.5 | 12.0 | Activated protargol & erythrosin |
| 37 | 85.5 | 14.0 | Activated protargol |
| 47 | 112.0 | 15.5 | Erythrosin & toluidin blue |
| 3 | 145.0 | 18.5 | Pyridine silver |
All but the first and third fetuses listed in this table are from the series on which cinematographic records of fetal behavior have been made by Hooker (’36, et seq.). The approximate menstrual age of each was computed by the use of the Streeter tables (Streeter, ’20). The material wasprepared by Dr. Ira D. Hogg, Mr. Reinhardt Rosenberg, and Miss Beryl Dimmick.
The outlines for the drawings and the major structures therein were made with the aid of a slightly modified Edinger projection apparatus. The drawings were then completed with the use of the compound microscope. All computations of cell size were made with the oil immersion objective. In ach case at least ten or more cells were measured and the average taken.
Literature
Aside from such general questions of development as gross appearance, position, relation to the ventricles of the brain, and development of related fissures, the structure of the olfactory bulb in human embryos has received little attention. An exception is Von K6lliker’s (1882 and 1883) description of the bulb in an 8-weeks embryo. The microscopic structure of the olfactory bulb in adult man, however, except for the absence of the accessory olfactory formation (Humphrey and Crosby, ’38 ; Crosby and Humphrey, ’39 a, ’39 b), follows in all essentials the lamination pattern characteristic for such mammals as marsupials, rodents, carnivores, ungulates, insectivores, chiropteres, and other primates as described by numerous observers (Ramon y Cajal, ’11; Winkler and Potter, ’11 and ’14; McCotter, ’12; Herrick, ’24; Gurdjian, ’25; Obenchain, ’25; Hines, ’29; Le Gros Clark, ’31; Humphrey, ’36; Young, ’36; Crosby and Humphrey, ’39 b; Fox, ’40, and many others). The accessory olfactory formation, or accessory olfactory bulb as it is also termed, is absent not only in adult man, but also in the bat (Humphrey, ’36), in most of the macaque material which has been described (although a minute vestigial structure was found in a young macaque; Crosby and Humphrey, ’39 a, ’39 b), in some reptiles (Crosby, ’17; Ariéns Kappers, Huber and Crosby, ’36; Crosby and Humphrey, ’39 b), and in birds (Huber and Crosby, ’29 ; Ariéns Kappers, Huber and Crosby, ’36; Crosby and Humphrey, ’39 b).
The suggestion of the last named authors (Crosby and Humphrey, ’39 b) that an accessory olfactory formation would probably be present in early human embryos only to disappear at some later time is supported by the fact that the vomeronasal or J acobson’s nerve (connected centrally with the accessory olfactory bulb; McCotter, ’12 and ’17) is present not only in early human embryos (Von Kiilliker, 1882 and 1896; Keibel, ’lO; Schaeffer, ’20 and ’28, and many others), but has been demonstrated in a human fetus as old as 6 months (McCotter, ’15) and Jacobson’s organ has been described often in the adult (Peter, ’01; Mangakis, ’02; Kallius, ’05; Keibel, ’10, and others). However, the relation of this nerve to any specific part of the olfactory bulb in human embryos has not been indicated by these authors, although according to von Kolliker (1882 and 1896), Kallius (’05), and Read (’O8) a branch of the olfactory nerve goes to Jacobson ’s organ. Likewise Hertwig (’15) observed that the nerve from this organ is connected with the “Regio olfactoria.”
Description of Material
The present account is concerned with the development of the microscopic configuration of the olfactory and accessory olfactory formations and not with the entering nerve fibers or their areas of peripheral distribution. Since recognition of the accessory olfactory formation itself in the earlier embryos is actually determinable only on the basis of its relationship to the vomeronasal nerve, however, it has been necessary to refer to it and to the general state of development of the organ of Jacobson.
No attempt has been made to study the development of the nervus terminalis. Nevertheless it should be stated that the vomeronasal nerve as used here is limited to the fibers passing from the vomeronasal organ to a special part of the olfactory bulb (the accessory olfactory formation) and does not include the nervus terminalis as this term is used by such observers as McCotter (’12), Huber and Guild (’13), Johnston (’l3 and ’14), Larsell (’18), and Herrick (’31). These two nerves are so intimately associated in the earlier human embryos studied, however, that it is not always possible to differentiate them. In such early embryos the fibers which terminate adjacent to the area of penetration of the olfactory fila, in a region which later shows the developing lamination pattern of the accessory olfactory formation, are considered to be vomeronasal. Those entering caudal to the developing olfactory bulb are probably part of the nervus terminalis. Such a distinction appears to be in harmony with the recent observations of Pearson (’40).
The primitive olfactory bulb before the lamination pattern begins to develop
14+ mm embryo (6.5 weeks)
At this age the vomeronasal organ is represented by a deep groove extending almost directly medialward into the wall of the developing nasal septum. From the much thicker epithelium of the cephalic (medial) wall of this groove the fibers of the vomeronasal nerve extend forward to the forebrain vesicle. At this time no fibers are contributed from the thinner caudal (lateral) wall of the developing vomeronasal organ.
Neither the primordial olfactory formation nor the primitive accessory olfactory bulb present any evidences of beginning lamination such as occurs in the adult. Instead the wide ependymal zone around the ventricle is separated from a narrow, peripherally situated acellular layer by a region of intermediate width which is formed by cells migrating out from the ependyma. The developing accessory olfactory formation has the same three regions, but the narrow peripheral zone is less definite in character and the intermediate region contains fewer migrating cells. Apparently these three regions just indicated correspond to the ependymal, mantle, and marginal layers described elsewhere in the developing neural tube.
16 mm embryo (7 weeks)
The degree of development of the olfactory areas of the nasal fossa in this embryo is essentially the same as in the preceding one, although the groove forming the organ of Jacobson is less definite in character. As in the first embryo, nerve fibers pass centralward only from the thickened cephalic (medial) wall of the developing Jacobson’s organ (the wall nearer the forebrain). No indication of lamination is to be found in either the primordial olfactory or accessory olfactory formations, both of which show no demonstrable advance in differentiation. These two areas of the primitive olfactory bulb, or pars anterior of the rhinencephalon, are definitely distinguishable from each other only on the basis of the entering nerve fibers.
22.5 mm fetus (8 weeks)
At 8 weeks of menstrual age Jacobson’s organ had deepened and elongated sufficiently to appear as a closed—off pocket for a part of its extent, and both its cephalic (medial) and caudal (lateral) walls have a tall lining epithelium. Fascicles of the vomeronasal nerve pass to the rhinencephalon from both the cephalic and caudal surfaces of the Vomeronasal organ in this fetus, as also is the case in all of the older fetuses in this account which are Seetioned in toto.
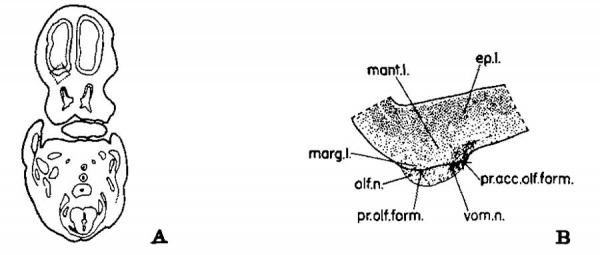
The vomeronasal fibers enter the developing olfactory bulb just medial and slightly dorsal to the fila olfactoria (fig. 1 B).
Abbreviations
acc.olf.form., accessory olfactory forma- mant.l., mantle layer
tion marg.l., marginal layer ant.o1f.nuc., anterior olfactory nucleus mit.c.l., mitral cell layer ep., epeudyma o1f.f., olfactory fila ep.l., ependymal layer olf.n., olfactory nerve fibers ext.gran.l., external granular layer olf.v., olfactory ventricle ext.molec.l., external molecular layer outw.n1it.c., outwandered mitral cells glom., glomerulus pr.acc.olf.form., primordial accessory glom.l., glomerular layer olfactory formation int.gran.l., internal granular layer pr.olf.form., primordial olfactory forint.gran.l.(olf.form.), internal granular mation
layer of olfactory formation vest.acc.olf.form., vestigial accessory int.molec.l., internal molecular layer ‘olfactory formation
l.gran. +mit.c., layer of granule and \'on1.n., vomerpuasal nerve fibers mitral cells
Fig. 1 Drawings to illustrate the location and structure of the primordial olfactory and accessory olfactory formations in a 22.5 mm. embryo. Activated protargol preparation. A, 9. horizontal section through the forebrain vesicles and the developing nasal fossae to show the plane of the sections and the location of drawing B. X 3. B, the area indicated by the dotted lines in figure A, enlarged to show the structure of the primordial olfactory and accessory olfactory formations. X 30.
Even through the center of the primitive olfactory and accessory olfactory formations as yet no indication of beginning lamination exists. Only the three regions representing the ependymal, mantle, and marginal layers found elsewhere in the early development of the neural tube are present in either area, although a definite advance in development occurs in Jacobson’s organ (see p.— 436). The differences between the developing olfactory and accessory olfactory formations at this age are the fewer cells migrating outward from the ependyma, the slightly lesser width of this zone of migrating cells (mantle layer), the greater width of the peripheral, acellular region (marginal zone), and the coarser, more deeply penetrating nerve fibers in the primitive accessory olfactory bulb as compared with the primordial olfactory formation. At levels through the middle of the developing accessory olfactory formation this structure forms a minute elevation on the surface. Von K6lliker’s description (1882, 1883) of the olfactory lobe at this age also indicates no development of the bulbar lamination pattern.
The olfactory bulb before regressive changes occur in the accessory olfactory formation
26 mm fetus (8.5 weeks)
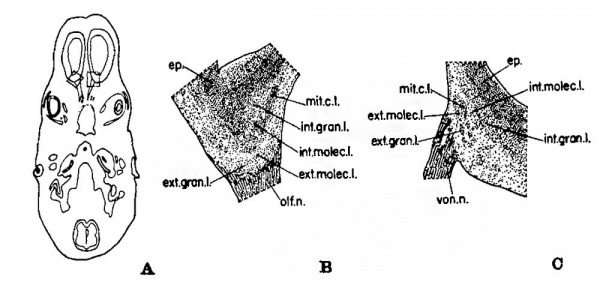
The vomeronasal organ in the 26 mm. human fetus has elongated and deepened sufficiently to appear as a saccular structure having a definite tube—like connection with the nasal fossa. In both the developing olfactory and accessory olfactory formations some indications of beginning layer formation may be seen (fig. 2, B and C). In each region the wide ependyma and the adjacent area of neuroblasts proliferating from it are still present about the ventricle, but toward the surface changes have occurred. In the olfactory formation (fig. 2 B) numerous cells at the surface form a layer in intimate relation with the entering olfactory fila, which, however, do not extend more deeply. This peripheral .cellular layer is separated from the deeper portion of the olfactory formation by a narrow, less cellular lamina.
Central to the latter is a relatively wide lamina of cells which is slightly separated from the neuroblasts migrating out from the ependyma by another less cellular region. Later developmental changes in the olfactory formation indicate that the glomerular and external granular layers differentiate in the cellular region of termination of the entering fibers. Thus this zone forms the anlage for the external granular layer, the deeper acellular region is the primordial external molecular layer, the adjacent cellular lamina represents the future mitral cell layer, and the neuroblasts still migrating out from the ependyma form the primordial internal granular layer. These indications of lamination are present practically all through the region into which the olfactory fila are entering, although no difference in the size of the cells in the developing layers is as yet demonstrable.
Fig. 2 Illustrations to show the position and morphology of the developing olfactory and accessory olfactory formations in the 26 mm. fetus. Erythrosin and toluidin blue preparation. A, a horizontal section through the forebrain to indicate the plane of the sections and the location of the areas enlarged in B and C. X 3. B, an enlargement of the region shown within the dotted lines on the left side of figure A, but at a level 80;; inferior to that of A. This drawing illustrates the beginning of lamination in the olfactory formation. X 30. C, the area enclosed in the dotted lines on the right side of figure A, enlarged to illustrate the beginning of lamination in the accessory olfactory formation. X 30.
The accessory olfactory formation in this fetus retains its dorsomedial position on the caudal surface of the developing olfactory bulb; there it again forms a definite eminence (fig. 2 C). As compared with the olfactory formation (fig. 2 B) in the same fetus, the accessory olfactory bulb (fig. 2 C) has less numerous entering nerve fibers, fewer cells throughout, and certain minor difierences in the developing lamination pattern. The vomeronasal fibers are coarser than the olfactory fila and many of them penetrate more deeply, but some terminate also in the region of the few cells at the surface. However, the primordial external granular layer in this part of the olfactory bulb is more deeply situated, so that a relatively acellular zone, with only a few cells among the entering fibers, is present at the surface. At this time the primitive external granular layer presents evidences of forming a lamina separated from the deeper layers in only a small part of the accessory olfactory formation. In such regionsa still deeper cell band (the primordial mitral cell layer) may be seen and this in turn is separated from the neuroblasts still migrating out (the developing internal granular layer) from the ependyma by a relatively less cellular zone (the region of the internal molecular layer). In view of the developmental changes which occur in these regions later it is possible to state that the Various laminae of the accessory olfactory formation are represented at this age as indicated by the names given in parentheses above.
26.5 mm Fetus (8.5 weeks)
The accessory olfactory formation in this embryo is essentially the same as that in the preceding one of approximately the same size. A similar degree of differentiation is present, although in certain respects somewhat less clearly seen due to the difference in staining of the two series, the pyridine silver impregnation of the series now under consideration being less favorable for cellular study.
32 mm. fetus (9.5 weeks)
At this age, although not in two older fetuses at least (35 mm. and 37 mm.), the organ of Jacobson no longer opens into the nasal fossa, but is connected with it only by a solid cord of epithelial cells. According to Schaeffer ( ’20) after 10 weeks of embryonal life Jacobson’s organ “varies considerably” and “complete degeneration in early fetal life may ensue.” Both this author (Schaeffer, ’2O and ’28) and Kallius (’05) state, however, that the vomeronasal organ reaches the height of its development in the twentieth week, an age comparable to that at which a fully differentiated accessory olfactory formation is also found in the present study (18.5 weeks old fetus; see p. 456).
The developing olfactory bulb now extends medialward as well as ventralward. The primitive accessory olfactory formation again occupies its dorsomedial portion and again forms a slight superficial eminence (fig. 3 B). At this time there is a degree of differentiation similar to that seen in this region in the preceding fetus (see p. 438), but for a somewhat greater portion of the area. The most superficial layer of cells constitutes the external granular layer, a lamina which is more deeply situated than in the olfactory formation. In this fetus it is more definitely separated from the deeper layers, however, and consists of cells averaging 6.4;: in their greatest diameter as compared with 8.9 u for the average diameter of the cells of the wider and more definite primitive mitral cell layer. The primordial external molecular, internal molecular, and internal granular layers are essentially unchanged as compared with these laminae in the 8.5 weeks old fetus. It is of interest that in the lateral part of the differentiating accessory olfactory formation the developing mitral cell layer is wider and more distinct than elsewhere although the primitive external granular layer noted medially cannot be identified throughout the same area.
Although having increased greatly in both size and ventrafrontal extent as compared with the olfactory formation in the 26 mm. fetus, the olfactory formation of the 32 mm. fetus has no marked advance in the differentiation of its lamination pattern (fig. 3 C). In fact there is even less indication of a primordial internal molecular layer. Thus the region of the primitive mitral cell layer is in continuity, for the most part, with the neuroblasts migrating out from the ependyma to form the internal granular layer. The cells in the region allocated to the mitral cell layer, however, are somewhat more compactly arranged and now average 8.6 u in size as compared with 7.2 u for tl1ose more deeply located. At this age the fila olfactoria are more intimately associated with the cell layer at the surface, the external granular layer. Actually the two appear as a single band of intermingled cells and fibers although the proportion of cells is greater on the deeper sur— face. As at all earlier ages considered the developing olfactory formation is much more cellular than is the primitive accessory olfactory formation in the same fetus.
35 mm fetus (9.5 weeks)
The developing lamination pattern of the accessory olfactory formation is not only more clear than is that in the preceding fetus but also more extensive. A slight eminence on the surface continues to mark the outline of the accessory olfactory formation. In its best differentiated part the primordia of those laminae previously observed in this region are now demonstrated more clearly. A comparable difference in size (2.5 u) between the cells of the primordial mitral cell layer and those of the developing external granular layer, observed in the 32 mm. fetus, also is found here; the former average 8.7 u, the latter 6.2 u in diameter. The apparent decrease in the size of the cells may be due to the greater shrinkage in the present preparation, which is stained with pyridine silver.
Fig. 3 Drawings to illustrate the olfactory and accessory olfactory formations in the 32 mm. fetus. Activated protargol preparation. A, a diagram of a. horizontal section through the entire fetus to indicate the plane of the sections and the areas enlarged in B and C. X 3. B, an enlargement of the accessory olfactory formation from the area enclosed in dotted lines in figure A. X 30. C, a drawing of the olfactory formation from an area comparable to that of figure B, but from a section 380 u inferior to the plane of that figure. X 30.
Except for an increase in size and the marked extension medially as well as ventrally, the olfactory formation in the 35 mm. fetus has not advanced in development. In fact, the slightly larger size of the cells forming the primordial mitral cell layer, as compared with the developing granule cells, cannot be noted here (possibly due to the type of preparation), although a similar difference in size can be observed in the developing accessory olfactory formation.
37 mm Fetus (10 weeks)
Although the opening is narrow, the organ of Jacobson retains its tubular connection with the nasal fossa in this fetus as well as in that of 35 mm. crownrump length. In other respects it resembles the organ of Jacobson in the 32 mm fetus, in which, however, the connection with the nasal fossa has been lost (see p. 439).
The lamination pattern noted in the olfactory and accessory olfactory formations in the younger fetuses is now much plainer. The surface eminence formed by the accessory olfactory formation is still demonstrable (fig. 4B). The more lateral portion of the vomeronasal nerve sends fibers even farther lateralward near the surface after entering the accessory olfactory formation (fig. 4B). In the region nearest the olfactory formation (fig. 4 C) the mitral cell layer extends for a considerable distance lateralward to come into relation with the anterior olfactory nucleus (see p. 444). Although a comparison of figure 4 C with figure 9 B (both of which are drawn at the same magnification) appears to indicate that the accessory olfactory formation in the 37 mm. fetus is larger than in the 145 mm. fetus this appearance is probably due in part at least to differences in the plane of section.
The laminae of the accessory olfactory formation are now much better differentiated (fig. 4, B and C). The external granular layer is less compact in nature but wider and more definitely demonstrable. Again its cells are distinctly smaller than are those of the mitral cell layer. The average diameter of the cells in the mitral cell layer is 8.9 u as compared with 7.0;: for those in the external granular layer and 6.7 u for those in the primitive internal granular layer. Numerous fibers of the Vomeronasal nerve extend directly inward to the external granular layer; a few pass medially and laterally among the scattered cells just within the surface. Some mitral cell processes extend out into the external granular layer also. The developing external molecular layer is now wider and definitely separates the external granular and mitral cell layers. The mitral cell layer itself is the most clearly differentiated of all the laminae. It appears as a relatively wide band of neurons of larger size than the remainder of the cells in the accessory olfactory formation. That these cells do not exhibit the shape typical for mitral cells in the adult is probably due to the relatively small amount of cytoplasm present as yet. In the region of entrance of the vorneronasal fila the mitral cell layer is broken up and less clear, but a more distinct lamina extends lateralward from this area. Here the mitral cell layer is thicker and more definite, a condition in harmony with the greater number of fibers entering this region. In the more lateral part of the accessory olfactory formation, however, the developing external granular layer is less clear than in the more medial portion (fig. 40). Central to the mitral cell layer the internal molecular layer consists of a narrow zone in which there are relatively few cells. The primitive internal granular layer at this age is still represented by almost uniformly scattered small neuroblasts migrating out from the ependyma.
Fig. 4. Drawings to illustrate the olfactory and accessory olfactory formations in the 37 mm. fetus. Activated protargol preparation. A, a diagram of a horizontal section through the fetus to indicate the planeof the sections and the location of the detailed drawings which follow. X 3. B, an enlargement of the more dorsamedial portion of the accessory olfactory formation, the area shown within the dotted lines in figure A. X 30. C, the accessory olfactory formation on the same side of the brain, as seen in a section 100p. inferior to A and B. X 30. D, a section through the olfactory formation, 480;: inferior to figure C. X 30.
As the mitral cell layer of the accessory olfactory formation extends lateralward, its clarity in outline decreases and there appears deep to it a second cell band (fig. 4 C) which becomes more distinct farther lateralward. The cells in this lamina average 8.1 u in diameter, a size intermediate between that of the mitral cells (8.9 H) and the granule cells (6.7 u). From its position and other relations, this deeply situated cell layer represents the anterior olfactory nucleus.
The olfactory fila enter the olfactory formation (fig. 4D) frontally on its ventral, medial, and lateral surfaces. A greater degree of cellular proliferation than is observed in the accessory olfactory formation continues to exist in the olfactory formation of this fetus. The highest differentiation is present in the areas of entrance of the nerve fibers. There is little change in the lamination pattern as yet as compared with that in the 32 mm. fetus. No clumping of cells (average size, 7.6 u) can be observed in the internal granular layer, into which many neuroblasts are still migrating from the ependyma. The most definite change is in the mitral cells, some of which are now beginning to assume a more triangular outline and average 11.0 p in diameter as compared with 8.6 p in the 32 mm. fetus. They do not form a sharply defined layer either deeply or superficially, however, and evidence of an internal molecular layer is present only rarely. Developing mitral cells are found either singly or in small groups within the external molecular layer. Rarely such a cell is present even in the inner border of the cell layer at the surface of the olfactory formation, the external granular layer. As yet no glomeruli have developed.
The olfactory bulb after regressive changes appear in the accessory olfactory formation
49 mm fetus (11 weeks)
According to Von Kiilliker (1882) at the age of 3 months the olfactory bulb is directed posteriorly as well as downward but later extends medialward and forward. Such a position for the developing olfactory bulbs is observed in this and in the next two fetuses studied. In the fetuses of 15.5 weeks of menstrual age or over the bulb is directed frontally.
The most striking advance in the development of the olfactory formation (fig. 5 C) occurs in the mitral cell layer. Although there has been no significant increase in the size of the mitral cells, 11.3 in in diameter as compared with 11.1 p in the 37 mm. fetus) and but little more separation from the internal granular layer of the bulb, the mitral cells are now arranged in the sectors seen iii the olfactory bulbs of all older specimens studies (see pp. 447, 449, 452 and 454) and in the adult (Humphrey and Crosby, ’38; Crosby and Humphrey, ’39 b). Two such sectors, concave toward the surface of the bulb, are illustrated in figure 5 C. Outwandered mitral cells are present in the external molecular layer and, more rarely, at the border of the external granular layer. Glomeruli have not as yet begun to differentiate.
fig. 5 Drawings illustrating the olfactory and accessory olfactory formations in the 49 mm. fetus. Activated protargol preparation. A, an outline drawing to indicate the plane of the sections and the location of figures B and C. X 3. B, a detailed drawing of the right accessory olfactory formation from the area enclosed in dotted line on the left side of figure A. X 30. C, a drawing of the left olfactory and accessory olfactory formations taken from the area enclosed in dotted lines on the right side of figure A. X 30.
The accessory olfactory formation (fig. 5, B and C) is about equally well developed on both sides of the brain and on each side regressive changes have begun to appear. The mitral cell layer is more compact and clearly delimited although the component neurons have not changed significantly in size (8.4 u as compared with 8.9 u in the preceding fetus). Neither accessory olfactory formation has an external granular layer such as is present earlier and glomeruli are not differentiated as might be expected had further development occurred. Deep to the mitral cell layer is the anterior olfactory nucleus in which the slightly smaller cells are more loosely arranged than are those in the mitral cell layer. 011 both sides of the brain, although more clearly on the left (fig. 5 C), the medial part of the mitral cell layer is in intimate relation with the anterior olfactory nucleus, often appearing either to be a part of it or to turn deeply to join it.
61.5 mm fetus (12 weeks)
In the 61.5 mm. fetus the olfactory ventricles are still patent throughout although proportionately smaller in size. All the layers of the olfactory formation (fig. 6 B) cannot be recognized definitely as yet, but certain advances in lamellar differentiation have taken place. Among the most noteworthy of these is the greater number of outwandered mitral cells and the change in the shape of many mitral cells to the more typical triangular outline of the adult neuron. l\Ieasurement of the mitral cells of the olfactory formation now gives an average of 11.5 u, not enough of an increase in size to be significant. Another advance in development is the greater clarity in the arrangement of the mitral cells in the various sectors which were first noted in the 49 mm. fetus (see also pp. 452 and 454). The dorsal and ventral sectors are the most prominent, but lateral and medial ones are also present. A glomerular layer has not yet appeared. The external molecular layer contains many mitral cells and fewer cells of the granule type. The internal molecular layer, although narrow, may be seen in such areas as the middle of the sectors of the mitral cell layer, but it is not distinguishable elsewhere. N o clumping of granule cells, such as is present in the adult, is demonstrable in the internal granular layer and neuroblasts are still migrating out into it from the ependyma.
fig. 6 Illustrations of the olfactory and accessory olfactory formations of the 61.5 mm. fetus. Activated protargol and erythrosin preparation. A, an outline drawing of a section through the anterior commissure to show the plane of section of the series and the general location of the detailed figure which follows. X 3. B, the left olfactory and accessory olfactory formations enlarged from the area within the dotted lines in figure A. X 30.
The accessory olfactory formation (fig. 6B) of this fetus exhibits, on the Whole, degenerative rather than developmental changes. An approximately equivalent degree of differentiation is present on both sides although the difference in the angle at which the two olfactory bulbs are sectioned (see fig. 6A) makes comparison difiicult. Apparently the accessory olfactory formation on the left side of figure 6A is cut in a plane approaching the sagittal; the one on the right side, however, is sectioned more nearly frontally.
The accessory olfactory formation on the left side (fig. 6 B) is situated at the angle at which the developing olfactory bulb turns medialward and, at this age, caudalward (although later frontally; see p. 445). Most of the vomeronasal fila enter it at this point of turning although some enter both cephalic and caudal to the area in question. The most clearly developed part of the accessory formation is situated just dorsal to the ventricle in figure 6 B. Both ends of the curved band of cells in this region come into relation with the anterior olfactory nucleus on its deeper aspect. At the level illustrated the cells in this layer are larger (8.7 p) at the narrow end of the lamina and grade off into smaller ones (6.1 u) at the thicker end of the layer, the part in more intimate contact with the anterior olfactory nucleus in this figure. Between the two parts is a zone intermediate as to cell size. Superficial to this cell layer are uniformly scattered cells, which, although equal in size (8.9 p) to the larger ones in the cell layer deep to them, have a different staining reaction. Cells of this same type, which are present throughout the area of entrance of the vomeronasal fibers in figure 6B, and in the more frontal area into which fibers penetrate at other levels, obviously belong to the accessory olfactory formation. At least part of the curved cell lamina represents the mitral cell layer of the accessory olfactory bulb, but the portions which pass over into the anterior olfactory nucleus without any demonstrable change appear either to have failed to develop sufiiciently to form a mitral cell layer and to have acquired instead the characteristics of the anterior olfactory nucleus, or to be undergoing regressive changes such that they are taking on the characteristics of this center. Superficial to the mitral cell layer the scattered cells probably represent cells of the external granular layer which have increased in size with the changes in the mitral layer and become uniformly scattered through the area.
The right accessory olfactory formation resembles the left in all essentials. The plane of the sections of this olfactory bulb permits observation of the fact that the vomeronasal fila enter on the dorsomedial surface and that many of them extend across the dorsal aspect of the area after entrance. Cells of the same type as those in the area into which fibers are entering in figure 6 B are scattered along these fibers. At one level, in the medial part of the field and again less clearly lateralward, these cells are clumped into a mass resembling the vestigial accessory olfactory formation illustrated in figures 7 C, 7 D, and 8 A for the 85.5 mm. and 112 mm. fetuses.
85.5 mm. fetus (14 weeks)
At 14 weeks of menstrual age the olfactory formation is but little changed as compared with that of the 12 weeks old fetus. The only significant differences are the clumping of the granule cells in a Very limited portion of the peripheral part of the internal granular layer and the onset of closure of the olfactory ventricle by the growth of cells into it from the ependyma. The sector arrangement of the mitral cells first observed in the 11 weeks old fetus is present here also, although less clearly defined in this case than in either the 12 or 15.5 weeks old fetuses.
A rudimentary accessory olfactory formation is demonstrable on both sides of the brain. On the left side three different stages in the regression of this structure are present. Dorsomedially it is possible to trace the vomeronasal nerve to a very limited area (fig. 7 A) similar to the accessory formation in the 49 mm. fetus; this area is comparable to the medial part of the mitral cell layer illustrated in figure 5 0. Here, in addition to the mitral cell layer which is present in the accessory olfactory formation in the 49 mm. fetus, a layer of smaller cells representing the external granular layer is situated more superficially and extends farther lateralward. A second vestige (fig. 7 B) of the accessory olfactory formation is represented by a single small glomerulus resembling the left accessory olfactory formation of the 112 mm. fetus (fig. 450 8 B). The position of this glornerulus, into relation with which Vomeronasal fibers can also be traced, is equivalent to the middle of the mitral cell layer in figure 5 C. It has a frontecaudal extent of approximately 50 u, a mediolateral diameter of about 70 u, and consists of a single glomerulus with its accompanying intra— a11d periglomerular cells. None of the cells, however, have acquired the character of mitral cells.
fig. 7 Drawings of the vestigial remains of the accessory olfactory formation of an 85.5 mm. human fetus. Activated protargol preparation. X 30. A, the best developed part of the left accessory olfactory formation. This area is comparable in its location to the most medial portion of the accessory formation illustrated in figure 5 C. B, vestiges of the accessory olfactory formation in the form of a single glomerulus located in an area comparable to the middle of the accessory olfactory formation of figure 5 C. C, the undifferentiated remains of the accessory olfactory formation found at the region corresponding to the most lateral part of the accessory olfactory formation of figure 50. D, the vestiges of the right accessory olfactory formation. The area drawn is comparable in position to the middle of the accessory olfactory formation of figure 5B.
The third vestige (fig. 7 C) of the accessory olfactory formation is located still farther lateralward in a region corresponding to the most lateral portion of the mitral cell layer of the accessory formation in figure 5 C. It is slightly larger than the glomerulus just described and resembles the rudiment of the accessory olfactory formation on the right side of the brain in this fetus (fig. 7 D) and in the 112 mm. fetus (fig. 8 A). On the right side, however, the vomeronasal nerve is definitely in relation with the mass of cells. Toward the more frontal end of the right bulb scattered cells with no particular arrangement also come into relation with the entering vomeronasal fila. Although a few sections of the right bulb are missing from the series through this area, such sections are scattered sufiiciently so that no further representation of the accessory olfactory formation is believed to be present. In that case it would appear that the accessory olfactory formation on the left. side of the brain in this fetus is more clearly represented than is that on the right side—a situation noted also in the two older fetuses described (112 mm. and 145 mm.; see pp. 452 and 458).
112 mm Fetus (15.5 weeks)
At this age the olfactory ventricle (fig. 8) is still patent in parts of the bulb although comparatively narrow and in many places partially obliterated. For example, in some sections there are two or three small openings instead of a single large one and the remainder of the ventricle is represented either by ependyma or completely obliterated. In other sections only ependymal remains of the ventricle are present. Caudalward, in the region of the olfactory crus, a definite, slit—like ventricle is in continuity with the anterior horn of the lateral ventricle. The olfactory bulb itself is considerably elongated and flattened with a clearly developed crus. It is now directed cephalad (see p. 445).
Although the olfactory formation has not yet attained the degree of differentiation found in that of the 145 mm. fetus, certain distinct advances in development are present (fig. 8). The olfactory fila enter the bulb on all surfaces at its most frontal tip, but in following the series caudally they disappear progressively from the dorsal, lateral, ventral, and medial surfaces respectively, remaining farthest caudalward on the ventromedial aspect as in the macaque (Crosby and Humphrey, ’39 b). Glomeruli are beginning to appear in a few regions deep to the zone of entering fibers on the ventral surface, but no definite glomerular layer is as yet present. Mitral cell dendrites can be traced into many of the developing glomeruli identified. Because of the small number of glomeruli which have developed and the scarcity of granule cells in the region, the external granular layer is still represented primarily by the cells situated deep to the entering fila. The external molecular layer varies in width with the depth of the mitral cell layer and contains many cells of both mitral and granule cell types. The mitral cell layer is more prominent than in either the 145 mm. fetus or in the adult and exhibits Very clearly the arrangement into sectors which began to appear in the 49 mm. fetus. These sectors present a concavity superficially and are best defined just caudal to the accessory olfactory formation. In the middle of each sector the mitral cells are more compactly arranged and the layer is thicker. At the ends, where two sectors join each other, the mitral cells are fewer in number and more scattered. The dorsal sector appears farthest frontalward, extends less far caudally than any of the other sectors, and its neurons are more loosely arranged. The mitral cells now average 12.9 n in diameter, are more definitely triangular in outline, and contain a considerable accumulation of Nissl substance. The layer is usually two or more cells thick with the neurons frequently arranged in small clumps. The internal molecular layer, like the external, varies in width and clearness. It contains many granule cells and a few mitral cells. In the internal granular layer the cells at the periphery are arranged in little clumps between which are small fiber bundles. Centrally such an arraxigement is not present, the granule cells there appearing uniformly distributed.
Vestiges of the accessory olfactory formation may be recognized on both sides of the brain in spite of the fact that marked regressive changes have taken place. It is only on the left side, however, that a discrete area with any organized structure is still retained (fig. 8B). Here the minute vestigial accessory olfactory formation appears as a tiny eminence about 170 p in diameter and of approximately the same frontocaudal extent. It is situated near the middle of the area occupied by the dorsal sector of the olfactory formation farther frontalward, a position comparable to that of the vestigial accessory olfactory formation in the young macaque (Crosby and Humphrey, ’39 b). Morphologically this accessory olfactory formation consists of one to three glomeruli, deep to which small mitral cells (10.1 n) and a few granule cells are intermingled in the form of a limiting layer. A few scattered granule cells in continuity with the internal granular layer of the olfactory formation are found beneath the layer of mitral and granule cells. In structure, then, as well as in location, this rudimentary accessory olfactory formation is strikingly similar to the vestigial one found in the young macaque, the only significant difference being the greater number of mitral cells present. Recognition of the relationship of the vomeronasal fila to this area is not easy since the fibers have become incorporated within the surface of the bulb (see also pp. 449 and 454). The compact arrangement and deeper staining reaction of these fibers, however, make it possible to trace them from the medial surface of the bulb to a position near the vestigial accessory olfactory formation, but the discrete nature of the bundle is lost before it reaches the accessory olfactory formation itself.
fig.8 Illustrations of frontal sections of the olfactory bulbs of a. 112 mm. fetus. Erythrosin and toluidin blue preparation. X 30. A, the right olfactory bulb to show the course of the vomeronasal nerve within its surface and the cells along it which are the only demonstrable vestiges of the accessory olfactory formation. The illustration of the course of the vomeronasal nerve with the accompanying neurons was made by combining the features observed in six consecutive 15p. sections. B, the left olfactory bulb showing a vestigial accessory olfactory formation similar to that observed in the young macaque. Both the plane of the section and its frontocaudal location differ slightly from those of figure A.
On the right side of the brain the accessory olfactory formation (fig. 8 A) has undergone still greater regressive changes, so that the presence of any representation of it at all might be questionable were it not for the relationship with the vomeronasal fila and the presence of similar vestiges in other fetuses (see pp. 450, 451, and fig. 7, C and D). The vo1neronasal nerve, however, is more readily followed than on the left side. It enters the olfactory bulb dorsomedially as a compact bundle (fig. 8A) which extends lateralward just within the dorsal surface to a point somewhat beyond the middle of the bulb. Along this nerve bundle, in addition to scattered cells, there are also two to three little clumps of more deeply staining neurons in relation with which the nerve apparently terminates. These scattered neurons and the small cell groups along the vomeronasal nerve within the olfactory bulb are the only vestiges of the right accessory olfactory formation, which is, then, even less well represented than is the left.
145 mm fetus (18.5 weeks)
The lamination pattern of the olfactory formation in the 18.5 weeks old fetus under consideration is in every respect as well differentiated as is that in the adult bulb (Humphrey and Crosby, ’38) and in some respects even plainer (see p. 455), although the olfactory bulb itself is smaller in size (fig. 9, A and B). As in the adult, the olfactory ventricle is no longer patent in the bulb, but in the caudal part of the olfactory crus in this fetus it is present and retains its connection with the lateral ventricle.
The olfactory fila (fig. 9, A and B) surround the frontal tip of the bulb and enter it on all sides although the dorsal portion of the fibers enter only quite far cephalad. The fila situated lateralward do not extend so far caudalward as do the ventromedial bundles which are still present caudally after all others have entered the bulb. Thus the arrangement of the olfactory fila into the four sectors, noted both in the adult bulb (Humphrey and Crosby, ’38) and in the preceding fetus, is also present here. There is a marked infolding of the mitral cell layer in the ventral, lateral, and medial sectors of the bulb and the mitral cell layer in the dorsal sector is more clearly defined as is the case also in the olfactory formation of the pig (Crosby and Humphrey, ’39 b). At this age there are proportionately fewer outwandered mitral cells present in the external molecular layer than occur in the adult, so that the mitral cell layer is more definite. More outwandered mitral cells are present in this fetus, however, than in the preceding one. The average size of the mitral cells at this time is 19.2 p.
fig.9 Drawings of frontal sections through the olfactory and accessory olfactory formations of a 145 mm. fetus. Pyridine silver preparation. X 30. A, the left olfactory bulb cephalic to the region of the accessory olfactory formation. B, the left olfactory bulb at a level through the middle of the accessory olfactory formation and the frontal tip of the anterior olfactory nucleus. 0, the right accessory olfactory formation in the region of its better difierentiated cephalic portion. D, the right accessory olfactory formation at the level of its less well developed caudal portion.
A glomerular layer is now represented on all aspects of the olfactory formation, but it is better developed on the ventral and lateral surfaces (fig. 9, A and B). In regions in which it is not represented the granule cells deep to the entering fibers are now markedly increased in number. Both peri- and intraglomerular cells can be distinguished. The granule cells form a more definite external granular layer than is found in the adult olfactory bulb. Occasionally an outwandered mitral cell may be seen in the border of this layer but less often than in the adult. The external molecular layer contains both outwandered mitral cells, singly or in groups, and granule cells; the former are more conspicuous although less numerous than the latter. The internal molecular layer is narrow, poorly de~ fined and often so filled with granule cells as to be difficult to distinguish at all. The internal granular layer is still represented centrally by closely massed granule cells which are uniformly arranged. Peripherally, however, the lamellar arrangement of little clumps of granule cells interspersed with fiber bundles is present for a wider distance than in the 112 mm. fetus. VVith this preparation it is not possible to determine what portion of the cells in the internal granular layer consists of neuroglia.
Laterally, ventrally, and particularly medially (fig. 9A), isolated and rather sharply circumscribed masses of neurons are found in the outer part of the internal granular layer, but not necessarily at the same frontocaudal level. These cells are intermediate in size between the mitral cells and the granule cells and are of a type similar to those found in the anterior olfactory nucleus farther caudalward. It seems likely that these neurons may constitute cell groups in the path of the olfactory tracts and hence be comparable, at least, to the isolated parts of the bulbar anterior olfactory nucleus described in the adult human olfactory bulb (Humphrey and Crosby,'38; Crosby and Humphrey, ’41).
The accessory olfactory formation is present and occupies the same position in relation to both olfactory bulbs, but it does not have the same clearness of delineation and lamination on the right side (fig. 9, C and D) as is manifested on the left (fig. 9B). In following this transversely sectioned series of the brain frontocaudally the accessory olfactory formation first appears at levels in which the most cephalic part of the anterior olfactory nucleus is found. Such levels correspond to those in which the accessory olfactory formation is located in the macaque (Crosby and Humphrey, ’39 b) and in many other mammals. Although situated dorsomedially in early human fetuses, the accessory olfactory formation in the 145 mm. fetus occupies a dorsolateral position, adjacent to the region which has no olfactory formation at these levels. This location is just lateral to that suggested as its possible site by Crosby and Humphrey (’39 b, Y of fig. 6 E) for adult man.
In the fetus under consideration the accessory olfactory formation of the left olfactory bulb (fig. 9 B) is clearly delineated through its middle portion, although less clear frontally and still less caudally. ‘All the laminae typical of this structure in other mammals are present. The vomeronasal fila enter from the medial side of the area. The glomeruli are small as compared with those of the olfactory formation, but form more than a single layer, at least through the best developed part of the accessory olfactory formation. The external granular layer has a few mitral cells adjacent to it and periand intraglomerular cells are present. The external molecular layer, although definite and often equal in width to this layer in the olfactory formation, contains more granule cells among the fibers. The mitral cell layer is wide, as is that of the dorsal sector of the olfactory formation. The mitral cells are not as large as are those in the olfactory formation, having attained an average diameter of only 15.6 u (see p. 455). The internal molecular layer is indefinite because of the many granule cells it contains. The internal granular layer consists only of diffusely scattered granule cells which are continuous with those in the olfactory formation. At the frontal end of the accessory olfactory formation the mitral cell layer is in continuity with this layer of the olfactory formation both medially and laterally. Through its caudal part, however, the accessory formation is bordered medially by an area free of olfactory formation and laterally by a small region in which the continuity of the mitral cell layer with that in the olfactory formation is interrupted by a zone containing scattered neurons of both granule and mitral cell types.
The accessory olfactory formation on the right side (fig. 9, C and D) of the brain, although having about equal frontecaudal and mediolateral dimensions as that on the left side, is much less clearly delimited and fails to show a well differentiated lamination pattern. It is more clearly defined toward its frontal end (fig. 9, 0), however, and in such areas the zone of entering fila may be bordered either by an area resembling a large glomerulus or only by a wide band of granule cells which forms the external granular layer. In a part of the accessory formation (fig. 9 C) an indefinite mitral cell layer is distinguishable, but in the remainder of the region (fig. 9D) a wide layer of granule cells with a few small mitral cells is separated from the internal granular layer of the olfactory formation only by a zone containing a few scattered cells. In many areas, and especially farther caudalward, the neurons are all of about equal size although a few larger ones resembling mitral cells may be present.
Summary
- The development of the olfactory and the accessory olfactory formations was studied in thirteen human embryos ranging from approximately 14 mm. to 145 mm. crown-rump length, or from about 6.5 to 18.5 weeks of menstrual age.
- Differentiation within the primordial olfactory and accessory olfactory formations is first observed at approximately 8.5 weeks of menstrual age. In the series of embryos studied, differentiation in the two regions appeared at the same time.
- The accessory olfactory formation is represented in embryos from 6.5 to 18.5 weeks of menstrual age, although beginning at 11 weeks regressive changes occur in this region on one or both sides of the brain.
- In an 18.5 weeks old fetus a fully differentiated accessory olfactory formation is present on the left side of the brain although not on the right and only vestiges of the structure may remain in still younger fetuses. Such vestiges vary from a tiny structure consisting of two or three typical glomeruli with granule and small mitral cells through a single small glomerulus with no mitral cells to clumps of neurons along the course of the vomeronasal nerve within the olfactory bulb.
- The position of the accessory olfactory formation Varies from dorsomedial with relation to the primitive rhinenceph— alon to dorsolateral with reference to the olfactory bulb at 18.5 weeks of menstrual age. At intermediate ages vestiges of the accessory olfactory formation often occupy a middorsal position.
- Differentiation of the lamination pattern is apparently slightly more rapid as Well as more clear cut in the accessory olfactory as compared with the olfactory formation. In the latter region, however, there is a greater proliferation of neuroblasts from the ependyma, particularly in the earlier stages.
- From the time differentiation is first observed in the series of embryos considered, representation of all of the laminae in the adult olfactory and accessory olfactory formations, with the exception of the glomerular layer, is present. This layer, which is the last to develop, does not appear until after lamellar differentiation is present in the superficial part of the internal granular layer.
- An arrangement of the mitral cell layer of the olfactory formation into sectors first appears at 11 weeks of menstrual age and is demonstrable thereafter in all of the fetuses studied.
- A difference in the size of the developing mitral and granule cells is first observed in both the olfactory and the accessory olfactory formations at 9.5 weeks of menstrual age. In the olfactory formation there is a progressive increase in the size of the mitral cells from that time onward. In the accessory olfactory formation regressive changes are accompanied by failure of the mitral cells to increase further in size.
- Outwandered mitral cells are definitely present as early as 11 weeks of menstrual age and occur throughout later stages of development although they are proportionately less numerous than in the adult. The mitral cell layer of the olfactory formation, however, is more clearly delineated at 18.5 weeks of menstrual age than in the adult.
- Closure of the olfactory ventricle by growth of cells into it from the ependyma begins first at 14 Weeks of menstrual age. Except in the caudal part of the crus, closure is complete at 18.5 weeks.
- The anterior olfactory nucleus appears earliest in rela— tion with the accessory olfactory formation. In at least certain cases in which regression occurs in the accessory olfactory formation, some of its mitral cells appear to become a part of the anterior olfactory nucleus.
Discussion And Conclusions
In the series of fetuses considered, differentiation of the primordial olfactory and accessory olfactory formations begins at the same time, about 8.5 weeks of menstrual age (26 and 26.5 mm. embryos). It is of interest that the beginning of differentiation occurs at approximately the same age that fetal activity is first shown in the cinematographic records of Hooker (’36 and ’39a; fetus With a crown-rump length of 25 mm. or between 8 and 8.5 weeks of menstrual age). This initiation of differentiation in the rhinencephalon simultaneously With the appearance of fetal activity is in accordance with the observations of Coghill (’28) on Amblystoma that “In the early—flexure stage the centers of difierentiation have become active in the tectum, the epithalamic, thalamic, infundibular, and rhinencephalic regions.”
The accessory olfactory formation, or its vestigial remains, may be demonstrated in human fetuses up to the age of 18.5 weeks. At this time it may have attained a degree of differentiation comparable to that of the accessory bulb of many other adult mammals, that is, typical glomerular, external granular, external molecular, mitral cell, and internal molecular layers may be observed. Before this age is reached, however, and in fact usually before complete differentiation of all its laminae is attained, regressive changes have often appeared. As a result of these changes even the recognition of vestiges of the accessory olfactory formation may be difficult in some fetuses although in others a definite, but tiny rudimentary structure similar to that found in the young macaque is present. From the material available it is not possible to state whether or not all vestiges of the accessory olfactory formation may have disappeared completely in some cases before the age of 18.5 weeks.
Although the accessory olfactory formation may attain a morphologic pattern typical for this structure in one olfactory bulb, it may not reach this degree of differentiation in the other olfactory bulb of the same fetus (18.5 weeks). There appears to be a tendency for the better developed accessory olfactory formation to be retained on the left side of the brain in older fetuses (85.5 mm., 112 mm., and 145 mm.). Although the number of cases in which any distinct diflerence can be noted is too few to be significant, it is more than suggestive of a difference in the rate of development on the two sides of the brain. It is of interest that Kallius (’05) noted a similar difference between the left and the right organ of Jacobson in a 22 weeks old fetus. The left remained fully developed, but the right had lost its connection with the nasal cavity and consisted only of a blind pocket.
Throughout its early development the accessory olfactory formation exhibits a lesser degree of cellular proliferation than is observed in the olfactory formation. This would indicate a slight initial lag in development in the accessory olfactory formation, although later complete differentiation appears to be present before it is found in the olfactory formation (see p. 463). In the earliest stages studied, and wherever a vestige showing definite olfactory structure remains, the accessory olfactory formation appears as an eminence on the surface of the developing olfactory bulb. In the 18.5 weeks old fetus it occupies a dorsolateral position with relation to the olfactory bulb, although in the early embryos it has a medial location and at 15.5 weeks the vestigial accessory olfactory formation occupies a middorsal position. This apparent change in position is possibly the result of rotation of the bulb as it turns first caudalward, then frontally, during development.
It is of interest that the history of the accessory olfactory formation as observed here parallels that of J acobson’s organ in human embryos. The earliest indications of regression in the accessory olfactory formation are found at 11 weeks of menstrual age, while according to Schaeffer (’20) Jacobson’s organ “varies considerably” after 10 weeks. Since, according to Schaeffer (’20 and ’28) and Kallius (’05), J acobson’s organ attains its highest development in the twentieth week in man (if it does not degenerate earlier) the accessory olfactory formation might be expected to be fully differentiated at a similar age. Such is the case in the left accessory olfactory formation of the oldest fetus observed (18.5 weeks), although definite dedifferentiation has occurred on the right side in this fetus as well as in both accessory formations in younger fetuses (14 and 15.5 weeks old fetuses, for example).
The laminae of the olfactory and accessory olfactory formations develop in a similar manner. first marginal, mantle, and ependymal layers, such as are found elsewhere in the neural tube, appear. From the mantle layer cells migrate outward to form two laminae, separated from each other and from the neuroblasts still migrating out from the ependyma by two less cellular zones. The inner of the two cellular bands forms the mitral cell layer and the neuroblasts which continue to migrate out from the ependyma become the internal granular layer in both the olfactory and the accessory olfactory formations. The outer cellular band, which forms the external granular layer in each case, is situated at the surface just beneath the entering fila olfactoria in the olfactory formation. In the accessory olfactory formation it is situated more deeply, being separated from the surface by a less cellular area. Many of the vomeronasal fila penetrate to this layer, however, as is the case with the olfactory fila. A few cells are also found at the surface in relation with other entering vomeronasal fibers. The glomeruli of the olfactory formation develop in relation with the external granular layer. Whether or not this is true in the accessory olfactory formation it is not possible to state definitely, although such appears to be the case. The glomerular layer itself is the last lamina to develop in both the olfactory and the accessory olfactory formations.
A definite explanation for the late development of the glomerular layer is not possible from a study of the material available. It may be that, although at least some mitral cell dendrites extend out into the external granular layer as early as 10 weeks of menstrual age, the complex dendritic branching which constitutes the glomerulus in the adult (cat, Ramon y Cajal, ’11) does not develop until much later. Perhaps also the granule cells in this region do not participate in the synaptic relations or develop extensive branching of their dendrites until later. At least there is an increase in the number of granule cells deep to the entering fila shortly before the development of the glomeruli. Possibly another factor in the late appearance of the glomeruli may be that complete differentiation of the other bulbar laminae is essential before the synaptic relations between mitral cells and olfactory fibers become concentrated in one region. The substantiation of any explanation will depend, of course, on a studyof Golgi preparations.
The primordia of all the laminae in the accessory olfactory formation are more clear cut than are those in the olfactory formation in early development. Apparently all the laminae are represented earliest in the accessory olfactory formation, since in this structure in the 15.5 weeks old fetus typical glomeruli are present although glomeruli are only beginning to develop in the olfactory formation. However, there is not suflicient evidence on this point.
A difierence in the average size of the mitral and the granule cells in the accessory olfactory formation appears as early as 9.5 weeks of menstrual age and increases in clarity in all cases in which development has continued in the accessory olfactory formation. A difference in the size of the developing mitral and granule cells in the olfactory formation occurs at the same time, but the mitral cells attain a greater size than do those in the accessory olfactory formation.
In the olfactory formation indications of a sector arrangement of the mitral cell layer first appears in the 11 weeks old fetus. The sectors are most clearly defined at 15.5 weeks, but are more definite in the 18.5 Weeks old fetus than in the adult olfactory formation. In the older fetuses the dorsal sector is the most distinct and is characterized by a wider mitral cell layer. It is of interest that not only these sectors of the mitral cell layer but also the entire lamination pattern are better developed in the olfactory formation at 18.5 Weeks of menstrual age than in the adult olfactory bulb. Perhaps this is a repetition of phylogeny since the olfactory formation is relatively better developed in subprimates than in primates. It also suggests that regression, which usually has progressed so far in the accessory olfactory formation, is beginning in the olfactory formation as well by the time adult life is reached.
The external molecular layer is narrow and indefinite in the olfactory formation during early development, but at 11 weeks of menstrual age increases in width. At the same time, with the more definite character assumed by the mitral cell layer, outwandered mitral cells are more readily observed, although they are demonstrable in the 10 weeks old fetus (fig. 4 D, unlabeled). Although present either singly or in clumps at all older stages observed, the outwandered mitral cells are not as numerous as in the adult.
The internal molecular layer in the olfactory formation is never clearly defined. The internal granular layer first begins to develop clumps of granule cells interspersed With fibers in the 14 weeks old fetus. These occur only at the periphery of the layer at first but in older fetuses extend farther centrally.
In the oldest fetus studied (18.5 weeks), however, the central part of the internal granular layer consists of uniformly distributed cells as in the adult.
The olfactory ventricle remains Widely patent up to 12 weeks of menstrual age. At 14 weeks cells begin to grow into it from the ependyma and at 15.5 weeks of menstrual age it is partially obliterated in many places and in a few regions completely so. At 18.5 weeks the olfactory ventricle is entirely obliterated except for the connection of the crural part with the lateral ventricle.
The earliest representation of the anterior olfactory nucleus is in relation with the accessory olfactory formation. The position of this part of the nucleus, which is also its most cephalic end, is comparable to that at which its frontal tip is situated in the macaque and in the newborn pig (Crosby and Humphrey, ’39 b). Several isolated masses of cells of similar character, situated farther frontalward, appear to be comparable to the isolated cell masses forming the bulbar part of the anterior olfactory nucleus in the adult (Humphrey and Crosby, ’38; Crosby and Humphrey, ’41). In some cases (49 mm. and 61.5 mm. fetuses) the occurrence of regressive changes in the accessory olfactory formation is accompanied by a more intimate relation of its mitral cell layer with the anterior olfactory nucleus in such a manner as to suggest that with further dedifferentiation this lamina of the accessory olfactory formation may possibly take on the function of the higher center. Details of the development of the anterior olfactory nucleus, however, are not within the scope of the present paper.
Literature Cited
Aiufixs Kspenns, C. U., G. CARL HUBER AND E. C. Caosnv 1936 The comparative anatomy of the nervous system of vertebrates, including man. The Macmillan Company, New York.
BODIAN, D. 1936 A new method for staining nerve fibers and nerve endings in mounted paraflin sections. Anat. Rec., vol. 65, pp. 89-97.
1937 The staining of paraffin sections of nervous tissues with activated protargol. The role of fixatives. Anat. Rec., vol. 69, pp. 153-162.
COGHILL, G. E. 1928 Correlated anatomical and physiological studies of the growth of the nervous system of Amphibia. VIII. The development of the pattern of differentiation in the cetebrum of Amblystoma punctutum. J. Comp. Neur., vol. 45, pp. 227-247.
CROSBY, E. C. 1917 The forebrain of Alligator mississippiensis. J. Comp. Neur., vol. 27, pp. 325-402.
CROSBY, E. (1., AND T. HUMPHREY 1939 a A comparison of the olfactory and the accessory olfactory bulbs in certain representative vertebrates. Papers Mich. Acad. Sci., Arts and Let., vol. 24, pp. 95-104. (1938.)
1939b Studies of the vertebrate telencephalon. I. The nuclear configuration of the olfactory and accessory olfactory formations and the nucleus olfactorius anterior of certain reptiles, birds and mammals. J. Comp. Neur., vol. 71, pp. 121—213.
1941 Studies of the vertebrate telencephalon. II. The nuclear pattern of the anterior olfactory nucleus, tuberculum olfactorium and the amygdaloid complex in adult man. J. Comp. Neur. (In press.)
Fox, 0. A. 1940 Certain basal telencephalic centers in the cat. J. Comp. Neur., vol. 72, pp. 1-62.
GURDJIAN, E. S. 1925 Olfactory connections of the albino rat, special reference to the stria medullaris and anterior commissure. J. Comp. Neur., vol. 38, pp. 127-163.
HERRICK, C. .T. 1924 The nucleus olfactorius anterior of the opossum. J. Comp. Neur., vol. 37, pp. 317-359.
- 1931 A11 introduction to neurology. 5th edition. W. B. Saunders Company, Philadelphia and London.
HERTWIG, O. 1915 Lehrbuch der Entwicklungsgeschichte des Menschen und der Wirbeltiere. G. fischer, Jena.
HINES, M. 1929 The brain of Ornithorhynchus anatinus. Phil. Tr. Roy. Soc., London, ser. B, vol. 217, pp. 155-287.
HOOKER, D. 1936 Early fetal activity in maunnals. Yale J. Biol. and Med., vol. 8, pp. 579-602.
- 1939 a A preliminary atlas of early human fetal activity. Published privately by the author, University of Pittsburgh, Pa.
- 1939b Fetal behavior. Res. Publ. Ass. nerv. ment. Dis., vol. 19, pp. 237-243.
HUBER, G. CARL, AND E. C. CROSBY 1929 The nuclei and fiber paths of the avian diencephalon, with consideration of telencephalic and certain mesencephalic centers and connections. J. Comp. Neur., vol. 48, pp. 1-225.
HUBER, G. CARL, AND S. R. GUILD 1913 Observations on the peripheral distribution of the nervus temiinalis in Mammalia. Anat. Rec., vol. 7, pp. 253272.
HUMPHREY, T. 1936 The telencephalon of the hat. I. The non cortical nuclear masses and certain pertinent fiber connections. J. Comp. Neur., vol. 65, pp. 603-711.
HUMPHREY, T. AND E. C. Cnossv 1938 The human olfactory bulb. Univ. (of Mich.) Hosp. Bull., vol. 4, pp. 61-62.
JOHNSTON, J. B. 1913 The nervus tenninalis in reptiles and mammals. J. Comp. Neur., vol. 23, pp. 97-120.
JOHNSTON, J. B. 1914 The nervus terminalis in man and mammals. Anat. Rec., vol. 8, pp. 185-198.
KALLIUS, E. 1905 Geruchsorgau (Organon olfactus). In Bardeleben’s Handbuch der Anatomie des Menschen, Bd. 5, Abb., 1. G. fischer, Jena.
Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
KEIBEL, F. 1910 The development of the sense organs. Section 16 in Keibel and Mall, Manual of human embryology, vol. 2. J. B. Lippincott 00., Philadelphia and London.
VON KoLLiKER, A. 1882 Der Lobus olfactorius und die Nervi olfactorii bei jungen menschlichen Embryonem. Sitzungsb. d. phys.-med. Gesellsch. zu Wiirzburg, 1881, S. 68-72.
- 1883 Zur Entwicklung des Auges und Geruchsorganes menschlicher Embryonen. Verhandl. d. phys.-med. Gesellsch. in Wiirzb., N.F., Bd. 17, S. 229-257.
- 1896 Handbuch der Gewebelehre des Menschen, Bd. 3. W. Engelmann, Leipzig. LARSELL, O. 1918 Studies on the nervus terminalis: mammals. J. Comp. Neur., vol. 30, pp. 1-68.
LE GROS CLARK, W. E. 1931 The brain of Microcebus murinus. Proc. Zool. Soc., London, vol. 30, pp. 463-486.
MCCOTTER, R. E. 1912 The connection of the vomeromasal nerves with the accessory olfactory bulb in the opossum and other mammals. Anat. Rec., vol. 6, pp. 299-318.
- 1915 A note on the course and distribution of the nervus terminalis in man. Anat. Rec., vol. 9, pp. 243-246.
- 1917 The vomero—nasa1 apparatus in Chrysemys punctata and Rana catesbiana. Anat. Rec., vol. 13, pp. 51-67.
MANGAKIS, M. 1902 Ein Fall von Jacobson’schen Organ beim Erwachsenen. Anat. Anz., Bd. 21, S. 106-109.
OBENCHAIN, J. B. 1925 The brains of the South American marsupials, Caenolestes and Orolestes. Publ. 224 of field Museum of Natural History, Z00]. Series, vol. 14, no. 3, pp. 175-232.
PEARSON, A. A. 1940 The development of the nervus terminalis in man. Anat. Rec., vol. 76, supplement, p. 45.
PETER, K. 1901 Die Entwickelung des Geruchsorgans und Jacobson ’schen Organs in der Reihe der Wirbeltiere. In Bd. 2, Teil 2 of HertWig’s Handbuch der vergleichende und experimentellen Entwickelungslehre der Wirbeltiere. G. fischer, Jena, 1906.
RAMON Y CAJAL, S. 1911 Histologie du systéme nerveux de l’homme et des vertébrés, vol. 2. A. Maloine, Paris.
READ, E. A. 1908 A contribution to the knowledge of the olfactory apparatus in dog, cat and man. Am. J. Anat., vol. 8, pp. 17-48.
SOHAEFFER, .T. P. 1920 The nose, paranasal sinuses, nasolacrimal passageways, and olfactory organ in man. P. Blakist0n’s Son and Co., Philadelphia.
1928 Section III in Special Cytology edited by Edmund V. Cowdry. Paul B. Hoeber, Inc., New York, vol. 1, p. 60.
Streeter GL. Weight, sitting height, head size, foot length, and menstrual age of the human embryo. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. , : 143-170.
STREETER, G. L. 1920 Weight, sitting height, head size, foot length, and menstrual age of the human embryo. Contrib. to Embryol. ‘(Carn. Inst. Wash. Publ. no. 274), vol. 11, pp. 143-170.
WINKLER, C., AND A. POTTER 1911 An anatomical guide to experimental researches on the rabbit brain. W. Versluys, Amsterdam.
- 1914 An anatomical guide to experimental researches on the cat’s brain. W. Versluys, Amsterdam.
YOUNG, M. W. 1936 The nuclear pattern and fiber connections of the non cortical centers of the telencephalon of the rabbit (Lepus cuniculus). J. Comp. Neur., vol. 65, pp. 295-377.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 30) Embryology Paper - The development of the olfactory and the accessory olfactory formations in human embryos and fetuses. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_development_of_the_olfactory_and_the_accessory_olfactory_formations_in_human_embryos_and_fetuses
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G