Paper - Histochemical horizons in human embryos - Stage 13
| Embryology - 30 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Mckay DG. Adams EC. Hertig AT. and Danziger S. Histochemical horizons in human embryos. I. Five millimeter embryo, Streeter horizon XIII. (1955) Anat. Rec. 122(2): 125-51. PMID 13238850
| Online Editor | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This historic 1955 paper by Mckay and co-authors describes human Carnegie horizon (stage) 13 embryos. Currently only a brief abstract is included on this page.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Histochemical horizons in human embryos. I. Five millimeter embryo, Streeter horizon XIII
Donald Gr. Mckay, Eleanor C. Adams, Arthur T. Hertig and Sara Danziger
Department of Pathology, Harvard Medical School, Boston, Massachusetts and the Pathology Laboratories of the Boston Lying-in Hospital and the Free Hospital for Women, Brookline, Massachusetts
Fourteen Figures (1955)
- Aided by the Institutional Grant of the American Cancer Society to Harvard Medical School and by a grant from the United States Public Health Service,
Introduction
The structural alterations of the human organism during the process of embryologieal development have been thoroughly, if not completely described, although the concomitant physiological and chemical alterations remain less completely explored. As Needham (’50) has pointed out, the regularities discovered by morphological investigations will always have their validity, and will, in a sense, be unaffected by anything that biochemistry may discover. He further states,
“But the important point is that although the regularities established at the level of experimental morphology are irrefragable, they will, in the absence of biochemical experimentation, remain forever meaningless . . . Only in the light of the conception of integrative levels can the saecular gulf between morphology and chemistry be bridged.”
In an attempt to take a short step toward bridging this broad but gradually narrowing gulf, a study of human embryos at the integrative level of histochemistry has been undertaken. It is recognized that the techniques of histochemistry, although somewhat circumscribed in their interpretation, have a potentially broad scope but at present lack quantitative precision. Especially in dealing with such small objects as early embryos, these techniques offer the distinct advantage of precision of localization. It is because of their abilit.y precisely to localize chemical substances and metabolic activities that these methods have been deemed worthy of trial in the study of embryos.
The mode of presenting the observations is conditioned by the techniques used, by the number of specimens available for study, and by the observations of Strceter (’51). He has emphasized the importance of “thinking of the embryo as a living organism which in its time takes on many guises, always progressing from the smaller and simpler to the larger and more complex . . . It is to be remembered that it is by means of their structural organization that embryos are able to carry on as living organisms, for embryos not only develop but they must also live. The requisite functions are carried on during alterations. In order that embryos may maintain themselves at their respective biological levels, it is necessary that their structure be so designed for each developmental period that an adequate physiological performance is insured. So equipped, it follows that they might live indefinitely at any respective plane, so long as no change in themselves, or in their environment, rendered that particular level of organization inadequate. But changes do occur, notably increase in size of the embryo. Therewith the requirements for existence progressively change, and the new needs are cared for by the development of new devices which one after another are discarded or remodeled when the needs are past. Thus one meets with a series of increasingly complex ephemeral organs and structural arrangements characterizing the periods of development that space of the anabasis of the embryo, from the microscopic one-cell egg up to the large highly specialized fetus of later stages . . .”
It seems likely that a similar increasingly complex biochemical evolution accompanies these morphological alterations. These are the considerations which have prompted the following report of a limited histochemieal survey of a 5mm human embryo, which represents the first of a series of such embryos.
Horizon XIII (Streeter)
For the purposes of anatomical orientation and classification, the following summary by Streeter (’5]) is presented of the characteristics of the group into which this embryo fits.
The ovulation age of the group is estimated at 28 i 1 days; t.he embryo as measured in t.he fixative is in most cases about 4 or 5 mm long; the greatest diameter of the chorion is usually between 20 and 30 mm; there is a definite arm—bud ridge and a recongnizable beginning leg bud; the heart chambers are distended; the pore of the otic invagination is now closed; the lens vesicle is not yet indented.
Materials and Methods
This embryo, fetus no. 5 (Free Hospital for Women Path nos.—52-1019), was dissected free of its membranes and immediately fixed in chilled acetone. The chorionie a11d amniotic membranes were similarly fixed. Serial 7 p paraffin sections were cut and every 25th section was mounted for each reaction. The adjacent section was used as a control. A detailed description of the techniques used has been previously reported (McKay, Hertig, Adams and Danziger, ’53). During the process of dissection the ve11tral body wall was torn causing the distortion seen in figures 3 and 4.
Observations
The observations are presented in the following table. The symbol (+) indicates the presence of a chemical constituent or metabolic activity; (++) indicates larger amounts of these materials; and (—) indicates that the material or activity was not detected in this embryo by the method used. It should be noted that the alkaline glycerophosphatase technique produced a great deal of diffusion and is not considered as reliable a method as the alpha naphthyl alkaline phosphatase in which there appears to be little or no diffusion 011 the slide. The recording of the observations of the alkaline glycerophosphatase is only included because of the possibility that this method may i11dicat.e a.n enzyme of a slightly different nature from the alpha naphthyl alkaline phosphatase. In the following text whenever the term alkaline phosphatase is used, it refers to both alpha naphthyl alkaline phosphatase and alkaline glycerophosphatase unless otherwise specified.
-Tables here-
Two control slides for 5—nucleotidase activity were run at pH 7.5 on adjacent sections. One was processed as a water blank and in the other glycerophosphate was substituted for the muscle adenylic acid. Both controls were always negative except for an occasional but minimal glycerophosphatase activity at pH 7.5 on the syncytial surface of the tropho— blast. Due to the well—known diffusion artifacts of a.cid pl1osphatase only those locations in which the reaction appeared consistently positive are recorded. Reactions for inorganic calcium and iron were performed but were negative, and hence l1ave been omitted from the table in this embryo.
Description
Intcgumeent. Glycogen and ribonucleoprotein are present in the cytoplasm of the epithelial cells of the e11tire skin but occur in greater concentration in the limb bud epidermis. Alkaline phosphatase is found at the distal tips of the cells in tlie periderm in the major portion of the skin, alt.hougl1 a much greater activity is observed in the thickened areas over the limb buds and iii the pharyngeal region where this enzyme is present in all the cells throughout the cytoplasm. A slight acid pl1ospl1a.tase activity can be seen in the epidermal layer and the basement. membrane contains glycoprotein.
Connective tissue. There is considerable variation in the reactions of the mesenchyme in different body regions. In general, the most enzymatically active mesenchyme is that in the developing limb buds, the pharyngeal arch regions, the body stalk, and a loose connective tissue in the coelomic wall just dorsal to the liver. The least reactive region is that just ventral to the neural tube.
Alkaline phosphatase is present in the cells in bilateral symmetrical streaks and condensations in the region ventral to the pharynx. A similar active mesenchyme occurs around the tracheo-esophageal passage, around the otic a.nd optic vesicles and posteriorly around the developing lung buds. The connective tissue of the mesentery of the gut shows moderate alkaline phosphatase activity a.nd it is most intense where the mesentery is becoming twisted. The mesenchyme of the ventral body wall and the body stalk especially around the veins exhibits marked alkaline phosphatase activity.
The mesenchymal cells containing glycogen in greatest quantity are found in the connective tissue of the developing limb buds, the adventitia of large blood vessels, the ventral body wall, and tl1e body stalk. Glycogen occurs in the connective tissue cells of other regions but in much lesser amounts.
Ribonucleoprotein is most abundant in the mesenchymal cells of the developing limb buds, the mesentery of the gut, the ventral body wall a11d the body stalk. It is present in the mesenchymal cells of other regions in lesser amounts. Slight acid phosphatase activity can be seen in most connective tissue cells.
The notochord cells contain ribonueleoprotein, abundant glycogen and the membrane surrounding this structure contains glycoprotein.
The somites of the anterior portions of the body differ in their histochemical reactions from those of the tail region. The latter still retain a lumen which is lined by a zone of alpha naphthyl alkaline phosphatase activity. The fibrils of the myotomes of all somites show alkaline phosphatase activity and a.n abundance of glycogen. Conversely the dermatomes and sclerotomes are enzymatically inactive but the cells contain more ribonucleoprotein than those of the myotomes.
Nervous system. The neural tube is the site of marked alkaline phosphatase activit.y. The enzyme is not found in the nuclei but appears to be confined to the cytoplasm and cytoplasmic processes of the neuroblasts. The dorsal and dorsolateral areas a.re most. reactive while the small ventral streak which extends the entire length of the nervous system is free of alpha naphthyl alkaline phosphatase but contains abundant glycogen and acid phosphatase. The remainder of the neuroblasts contain little or no glycogen or acid phosphatase. All the cells of the central nervous system contain large amounts of cytoplasmic ribonucleoprotein. The endothelium of the small blood vessels surrounding the neural tube exhibits marked alkaline phosphatase activity.
The brain region is essentially the same as the spinal cord anlage in its histochemical nature except that the thin dorsal roof (roof of the IVth ventricle) shows no alkaline phosphatase activity.
Fig. 1 Fetus no. 5, 5 mm. Alpha naphthyl alkaline phosphatase. Lung buds. Enzyme activity is found in the cells of the pleura as well as in the epithelium of the lung buds, the gut, the endothelium of the dorsal portion of the paired aorta, the dorsal region of the myotomes and in the cells of the spinal cord. X 46.
Non-specific esterase is seen in two small cell clusters in the tissue of the floor of the IVth ventricle bilaterally. This enzyme appears as a faint reaction in the cytoplasm of these cells. Non—specific esterase is also found in the cytoplasm of clusters of cells in the connective tissue of the mesentery and in that surrounding the gut epithelium. These seem to represent the nerve cells of the myenteric plexus and the developing coeliac plexus.
The spinal ganglia are characterized by the presence of abundant ribonucleoprotein, alkaline phosphatase, acid phosphatase and non—specific esterase. The spinal nerve fibers exhibit prominent alkaline phosphatase activity.
The otic and optic vesicles present the same histochemical reactions as the neuroblastic tissue of the dorsal and lateral portions of the neural tube. The lens epithelium contains glycogen, alkaline glycerophosphatase but no alpha naphthyl alkaline phosphatase.
Gut tract and (lerivcltivcs. The epitholia of the pharynx, lu11g, and gut present the same histochemical pattern. These cells all contain abundant glycogen and cytoplasmic ribonucleoprotein a11d exhibit alkaline phosphatase activity. The alkaline phosphatase in the gut epithelium is concentrated at the luminal tips of the cells. The basement membrane of these structures contains glycoprotein.
The liver at this stage of development is histochemically as well as morphologically a non—homogeneous organ. It is divided into a dorsal portion, derived from the coelomic mesoblast and a ventral portion derived from the epithelium of the gut tra.ct. The mesenchymal portion, derived from the coelomic mesoblast, is characterized by the presence of a high alkaline phosphatase activity, abundant cytoplasmic glycogen and ribonucleoprotein and no acid phosphatase. On the other hand, the liver cells proper, i.e., those derived from the gut tract epithelium, show no alkaline phosphatase activity and contain no glycogen. However, these cells exhibit the most intense acid phosphatase activity of any in the whole body and contain large amounts of ribonucleoprotein in the form of prominent cytoplasmic basophilia.
The sinus endothelium presents a histochemical pattern unique among cells found in the liver. These cells exhibit alkaline phosphatase activity and contain glycogen, glycoprotein and ribonucleoprotein. The one reaction that sets the endothelium apart from all the other cells of the liver is the presence of 5—nucle0tidase activity. The Kupffer cells or macrophages of the liver are distinguishable from the endothelial cells by the presence of acid phosphatase activity. The coelomic epithelium covering the surface of the liver exhibits the same reactions as the dorsal mesenchymal por tion of the liver for it is, of course, embryologically related.
Fig. 2 Fetus no. 5. 5 mm. Alpha naphthyl alkaline phosphatase. This section is through the point of bifurcation of the trachea and gut. Phosphatase activity is found in the cells of the spinal cord, the spinal nerves, the capillaries of the connective tissue ventral to the cord, the dorsal portions of the somites (myotomes) and in the tracheal and gut epithelium. X 46.
Blood vascular system. The Various anatomical components of the heart reveal several histoehemical differences. The myocardial cells contain deposits of glycogen that are more proniinent than iii any other tissue of this embryo. In addition, the cytoplasm contains ribonucleoprotein. The myocardium appears free of alkaline phosphatase except for the auricular niyocardium which shows slight alkaline glycerophosphatase activity.
Fig. 3 Fetus no. 5, 5 mm. Alpha naphthyl alkaline ph0sph:1ta.so. This enzyme is concentrated in the cells of the spinal cord, the epithelium over the limb bud (on the left), the dorsal liver cells, the endothelium of the liver sinuses in the ventral portion of the liver, and in the gut epithelium. It is also found in the nerves emerging from the spinal ganglion and in the capillaries of the c.o1mect.ive tissue ventral to the cord, X 46
The cardiac endothelium on the other hand appears enzymatically more active than the myocardium. Not only does it reveal alkaline phosphatase activity but 5-nucleotidase is also present. Glycogen, glycoprotein and ribonucleoprotein are also present in the cytoplasm of these cells. The gelatinous myoendocardial cushion contains abundant glycoprotein but no glycogen or enzyme. activity.
The endothelial cells lining the various blood vessels of this embryo present a variety of histochemical patterns. In general, the endothelium of the larger vessels is enzymatically less active than that of the smaller vessels. The endothelium of the aorta and small arteries (capillary vessels) shows a prominent alkaline phosphatase activity, and contains glycogen, glycoprotein and ribonucleoprotein. The aorta is not homogeneous throughout. since the ventral endothelium is free of alkaline phosphatase although this enzyme is found in the endothelium of the dorsal region. In some areas even the dorsal endothelium is negative and presents a striking‘ contrast to small arteries emerging from its lumen which show a marked enzyme activity. The umbilical arteries show no alpha naphthyl alkaline phosphatase activity.
The endothelium lining such veins as the cardinal veins, venous plexi of the limb buds, liver, lateral pharyngeal wall and the yolk sac contains glycogen, glycoprotein and cytoplasmic ribonucleoprotein. In this respect. it is similar to arterial endothelium. Nevertheless, it is strikingly different since it exhibits 5-nucleotidase activity and is free of alpha naphthyl alkaline phosphatase. The umbilical vein is unique since it does show alpha naphthyl alkaline phosphatase. activity.
Germ cells. The germ cells of this embryo are found in the connective tissue of the root of the mesentery, within and beneath the coelomic epithelium, between the epithelial cells lining the gut, within the gut lumen, and in the connective tissue and coelomic epithelium of the developing gonadal folds. These cells are characterized by a cytoplasmic rim of alkaline pliospliatase activity which sharply demarcates them from surrounding tissues. They also contain glycogen and a rim of 1-ibonucleoprotein. These findings have been described in detail in a previous report (McKay et al., ’53).
Fig. 4 Fetus no. 5, 5mm. Alpha iiaplitliyl alkaline pliosplnltasc. The epithelium of the skin over the limb bud on the left is prominently reactive with the alkaline phosphatase technique. Primitive germ cells in the mesentery of the gut are clearly outlined. The syncytial trophoblast of the villi in the left lower region exhibits enzyme activity. X 46.
Mes01z,e]_)h.7*0.9. Alkaline phospliatase. activity is prominent at the luminal tips of the cells of the secretory tubules. These cells contain ribonucleoprotein but no glycogen. The mesonephric duct, 011 the other hand, contains glycogen but is free of alkaline phosphatase activity. \ Coelomic cpithelimn. The coelomic lining over the liver and lung Inesoblast contains glycogen, ribonucleoprotein and alkaline phosphatase. It, therefore, is similar to the epithelium lining the gut tract. ln cont.rast, that covering the parietal abdominal wall and the gut is free of glycogen and phosphatase a.ctivity.
Yolk sac. The endoderm contains glycogeii, glycoprotein, ribonucleoprotein, marked a.cid phosphatase, alkaline phosphatase and non-specific esterase activity. The yolk sac endothelium differs i11 that it shows 5-nucleotidase activity but is free of a.cid phosphatase and alpha naphthy] alkaline phosphatase.
('Izori(m,ic tissue. The syncytial trophoblast contains 0*ly— coprotein, ribonucleoprotein, alkaline phosphatase, acid phosphatase and 5-nucleotidase activity. The alkaline phosphatase and 5-nucleotidase appear confined to the region of the brush border of the syncytium, while the acid phosphatase is present. throughout the entire cytoplasm of this tissue. The cytotrophoblast also contains ribonucleoprotein and slight a.cid phosphatase activity. It. differs from the syncytium since it contains abundant glycogen but is free of alkaline phosphatase and ‘.')-nucleotidase. Macrophages in the villi contain acid phosphatase and 11on-specific esterase. The endothelium of the vessels of the villi exhibits :3—nucleotidase activity. Fibrin deposits attached to a few of the villi exhibit an intense pink color with the periodic acid Schiff stain after amylase digestion.
Discussion
Allx‘(.II/me ])}l0.s‘))h(lf(($e (Ind fluid mtcrf(1ccs
At the interfaces or membrane barriers between extracellular fluids of major difi"erence there is a high alkaline pliesphatase activity. Standing between the maternal blood and the extracellular fluid of the villi (placenta) is the syncytial trophoblast with a very high alkaline phosphatase activity. Because the nature of alkaline pl1ospl1a.tase is that of a hydrolytic enzynie whose function is to relea.sc inorganic phosphate from organic phosphate radicals, it seems likely that much inorganic phosphate is released by this membrane. McKay et a.l. (’54b) have demonstrated that the villous (placental) connective tissue fluid has a much higher inorganic phosphate content than the maternal plasma. It therefore seems likely that the inorganic phosphate released by the trophoblast is largely directed toward the embryonic circulation and the chorionic fluid.
The next location where high alkaline phosphatase activity stands between two different extracellular fluids is in the coelomic mesoblast of the dorsal region of the liver near the hepatocardiac veins. Streeter (’51) has pointed out that in this age group the hepatocardiac veins appear to be functioning chiefly in connection with the specialized coelomic walls separating them from the coelomic fluid.
“The permeable character of the tissue overlying the hepatocardiac veins facilitates the passage of fluid from the coelomic tract. to these large veins, just as they are about to enter the heart. At this time the chorionic circulation is but poorly established, and therefore the main source of wa.ter and food substances for the embryonic tissues must still be the fluid circulating in the coelomic channel.”
This fluid is essentially chorionic fluid. The histochemical observation therefore suggests that phosphate ions are being concentrated at this membranous interface between coelomic (chorionic) fluid and the plasma of the venous blood of the embryo itself. “A similar specialization of the coelomic surface is also found in the body-stalk region and on the inner surface of the body wall over the saclike distention of the umbilical veins. ” Alkaline phosphatase activity is very prominent in these regions also.
The anteriolar or capillary membrane separating arterial plasma from the intercellular fluid of the embryo itself in certain regions is also the site of prominent alkaline phosphatase activity.
In summary, it appears that phosphate ions are being concentrated in or near the membranes standing between maternal plasma and chorionic fluids; ohorionic fluid and embryonic venous plasma; and between embryonic arterial plasma and embryonic intercellular fluid. This principle could be extended to the cell membrane dividing embryonic intercellular fluid and intracellular fluid i11 tl1e case of certain cells such as the germ cells, the neuroblasts, the gut epithelium, and the epithelium covering the limb buds.
Alkaline phosphatase anti-regions of rapid growth
The tissue localization of the highest alkaline phosphatase activities can be related to tl1e relative growtli rate of tissues in this embryo. One of the most rapidly growing tissues at this stage of development is the central nervous system. Streeter (’51) has noted that the neural tube up to and including this stage of development has grown so rapidly that it largely determines the shape of the embryo. Alkaline phosphatase activity is very prominent in the cells of the neural tube. The other tissues that are growing most rapidly in this age group are the gut tract, the limb buds, the dorsal region of the liver and the chorionic trophoblast. These are the regions of greatest alkaline phosphatase activity.
Since growth requires the production of nucleoprotein in the process of cell multiplication, it seems likely that the inorganic phosphate released in these tissues is being utilized mainly in nucleoprotein synthesis. This can be related to the fact that the areas listed above are also the regions of this embryo which exhibit the most prominent cytoplasmic basephilia (ribonucleoprotein). Also, the cells are smaller and more closely packed in these areas of rapid growth than in other areas. This is 11ot to imply that phosphate ions are being used exclusively in nucleoprotein synthesis, since some of these rapidly growing tissues contain abundant glycogen, and some phosphate is undoubtedly being combined with various carbohydrate moieties.
Metabolic activity of endothelium.
One of the most interesting observations in this embryo is the marked Variation in the metabolic activity of endothelium lining different vascular channels. Morphologically the endothelium presents itself as a homogeneous organ, whereas histochemically it appears to be an extremely non-homogeneous organ. Differences can even be found in the endothelium of the same vessel, as in the aorta, where alkaline phosphatase activity is present in the endothelium of the dorsal portion but cannot be detected in that of the ventral region.
Generally, endothelium of the arteries and arterial capillaries of the embryonic body reveals alkaline phosphatase activity, but no 5—nucleotidase, While the endothelium of veins and venous plexi presents the opposite arrangement. There are interesting exceptions to this rule, namely, (1) the liver sinus endothelium exhibits both alkaline phosphatase and 5—nucleotidase activity; (2) the umbilical arteries show no alkaline phosphatase or 5—nucleotidase While the umbilical vein reveals activity of both these enzymes, and (8) capillaries of the placental villi are either devoid of alkaline phosphatase and 5—nucleotidase or exhibit only 5—nucleotidase activity. These metabolic differences in the endothelium of vessels of the same general class seem to point to distinctly different functional activities of these Vessels, whether they be arterial or venous in nature.
5-Nucleotidase
Considerable interest centers on the localization of this enzyme because of its relationship to nucleoprotein metabolism and because of its specificity. According to Zeller (’51) the specific substrates for 5—nucleotidase are adenosine5—phosphoric and inosine-5-phosphoric acid, and its action is one of dephosphorylation. It therefore seems likely that pl1osphate plus nucleoside are being released in regions of 5nucleotidase activity. However, the possibility of nucleotide synthesis at these points cannot be eliminated. The histochemical observations indicate that at this stage of development this enzyme is confined to the linings of vascular channels, for the most part of a Venous nature. The functional significance of this observation remains to be elucidated.
Liver and yolk sac
It is of some interest to compare the histoehemical pattern of the yolk sac and the liver in this embryo. Needham (’50) states, “The conception of the placenta and also the avian and reptilian yolk—sacs, as the ‘foie transitoire’ remains indeed classical.” This concept may well be applied to the human yolk sac. Streeter has called attention to the intimate and direct. vascular connection between the yolk sac and the liver in embryos of this size.
“It will be seen that the liver plexus intervenes as a filtration. or perhaps an absorption, network between the yolk sac network and the general blood stream (sinus venosus). It foreshadows the portal circulation of later development, when the mesenteric blood from the digestive tract is passed through the liver parenchyma before reaching the heart. At any rate, in age group XIII all the blood from the yolk sa.c passes through the liver plexus, and it is this blood alone that the liver receives. This is a temporary arrangement that coexists With the maximum development of the yolk sac, and one might speak of it as a pre-portal system, pending the day when we are better informed on its functional activities.”
Obviously the yolk sac and liver at this stage of development may be considered members of a functional team, acting togetlier on the blood returned to the heart through this system. Histochemically they present certain outstanding similarities. The epithelial cells of both structures exhibit the most intense acid phosphatase activity of any cells of the embryonic body. The only other tissue that approaches this activity is the syncytial trophoblast. In addition, the vascular endothelium of both structures exhibits 5—nucleotidase activity. Alkaline phosphatase is present in both structures, hovvever, in the liver it is confined to the sinus endothelium, while in the yolk sac it is found at the luminal tips of the epithelial cells of the endoderm. Yolk sac epithelium and the liver cells both contain abundant ribonucleoprotein.
One of the major differences between the two structures from the histochemical standpoint is the presence of glycogen in the epithelial cells of the yolk sac and its absence in the epithelial cells of the liver. In subsequent stages of development, tl1e yolk sac disappears and eventually the liver cells take over the glycogen storage function that in this 5mm embryo is managed by the yolk sac of this primitive portal system. A similar transfer of metabolic activity from yolk sac to liver will be seen in the non-specific esteraselactivity. In this embryo, non—specific esterase is found in the yolk sac but not in the liver. At later stages it is also found in the liver, and subsequently, with atrophy of the yolk sac, the liver will have taken over this function.
Thus, at this stage of development, the liver appears to lack certain chemical constituents or metabolic activities that are supplied by the yolk sac and that in time make their appearance in the liver itself.
Summary
- This report contains a description of a 5mm human embryo which was studied by use of procedures for the histochemical demonstration of the following metabolic substances or activities: glycogen, glycoprotein, cytoplasmic ribonucleoprotein, alpha naphthyl alkaline phosphatase, alkaline glycerophosphatase, 5-nucleotidase, non-specific esterase, calcium and iron.
- An attempt has been made to relate some of the observations to functional mechanisms in operation in embryos of this stage of development.
- Alkaline phosphatase activity is concentrated in the membrane interfaces separating intercellular fluids of major difference, i.e., those separating maternal plasma from chorionic fluid, chorionic fluid from embryonic venous plasma, and embryonic arterial plasma from embryonic intercellular fluid. It appears that inorganic phosphate ions are concentrated at these interfaces.
- Alkaline phosphatase activity is concentrated in the most rapidly growing tissues of this embryo, including neural tube, limb buds, gut tract, dorsal portion of the liver and the trophoblast. Since these are regions of most active nucleoprotein synthesis, it is likely that the inorganic phosphate released at these points is largely utilized in this synthetic process.
- The endothelium lining the various vascular channels of this embryo is histochemically non—homogeneous, indicating a varied function of endothelium in different parts of the body.
- 5—nucleotidase activity is confined to the linings of vascular channels in this embryo.
- The liver and yolk sac function as a “pre-portal” unit, and the chemical constituents or metabolic activities in which the liver is deficient are supplied by the yolk sac. In later stages of development, as the yolk sac involutes, the liver assumes these metabolic functions.
Literature Cited
BRACHET, J. 1947 Nucleic acids in the cell and the embryo. In Symposia of the Society for Experimental Biology. No. I. Nucleic Acid. (Jambridge Univ. Press, Cambridge.
McKAY, D. G., A. T. HEMIG, E. C. ADAMS AND S. DANZIGER 1953 Histochemical observations on the germ cells of human embryos. Anat. Rec, 117: 201-220.
MCKAY, D. G., C. C. ROBY, A. T. HERTIG AND M. V. RICHARDSON 1954a Studies of the function of early human trophoblast. I. Observations on the chemical composition of the fluid of hydatidiform moles. Am. J. Obst. and Gyn., in press.
1954b Studies on the function of early human trophoblast. II. Preliminary observations on certain chemical constituents of chorionic and early amniotic fluid. Am. J. Obst. and Gyn.. in press.
NEEDHAM, J. 1950 Biochemistry and Morphogenesis. Cambridge Univ, Press, Cambridge.
Streeter GL. Developmental horizons in human embryos. Description of age groups XIX, XX, XXI, XXII, and XXIII, being the fifth issue of a survey of the Carnegie Collection (prepared for publication by Heuser CH. and Corner GW.). (1951) Carnegie Instn. Wash. Publ. 592, Contrib. Embryol., 34: 165-196.
STREETER, G. L. 1951 Developmental Horizons in Human Embryos. Age Groups XI to XXIII. Carnegie Institution of VVashingt.on, VVashington, I). C.
ZELLER, E. A. 1951. Enzymes as essential components of toxins. In: The En zymr-s—Chemistry and Mechanism of Action, Academic Press Inc., New York.
Plates
Plate 1
Fetus no. 5, 5 mm. Yolk Sac. X 1.50
Glycogen appears as black granules and is found at the luminal tips of the cells of the yolk sac endoderm.
Alkaline glyeerophosphatase is present in the yolk sac endoderm, the endothelium of the vessels and in the granular material in the lumen of the yolk sac.
5-nucleotidase is confined to the endothelium of the blood vessels of this organ.
Plate 2
Fetus no. 5, 57mm. X 1.50
Spinal cord. Alpha implitliyl alkaline pliosphatase. tratod in the cgvtoplasiri oi" the 11(‘112'Ol)l{1StS of the dorsal and lateral portions It is also seen in the nerve fibers eniorging from the central nervous system and in the dorsal region of the soinites.
The enzyme is (-on<'on—
of the ceirtml nervous system and is absent from the vorrtml region.
Lateral pharyngeal Wnll. 5—11u<=lootidase. This enzyme is found in the endotheliurn of the vascular plexus of the coniioctivc tissue of this region.
Section shows liver in lower half and lung buds in the upper half. 5—1n1cloo— tidase. and in small veins in the connective tissue of the developing lung.
This enzyme is found in tho Ondotlieliiim lining the liver sinuses
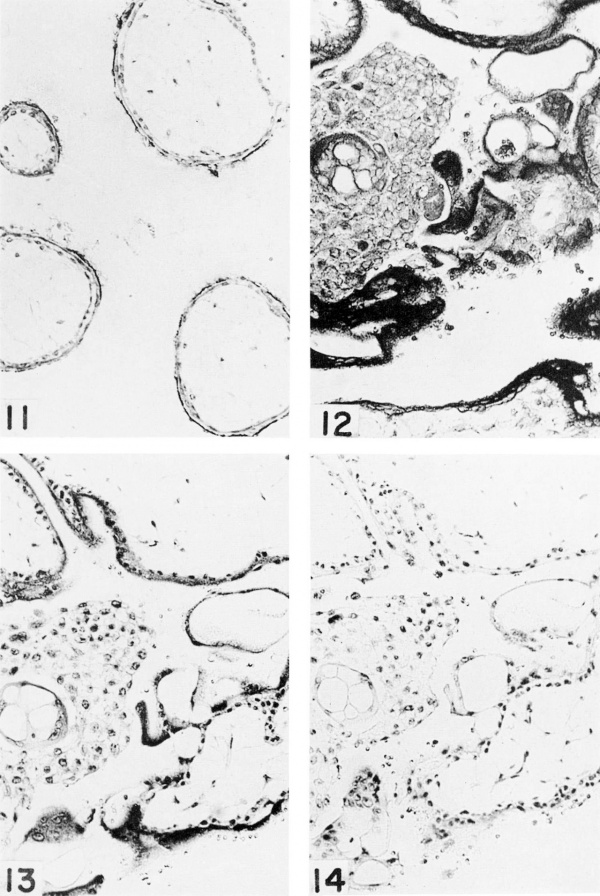
Plate 3
Fetus no. 5, 57 mm. Trophoblast. X 150
5-nucleotidase is present only in the brush border of the syncytium.
Acid phosphatase is present in the syneytial layer and in a few scattered cells of the cytotrophoblastic column. The nuclei do not react.
Eibonucleoprotein is concentrated in the syncytial cytoplasm but is found in moderate amount in the cytoplasm of the cytotrophoblast cells. Compare with figure 14:.
Ribonuclease digestion of a section adjacent to figure 13. Compare with figure 13. The ribonucleoprotein of the cytoplasm of the syncytium and cytotrophoblast has been removed.
Cite this page: Hill, M.A. (2024, April 30) Embryology Paper - Histochemical horizons in human embryos - Stage 13. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Histochemical_horizons_in_human_embryos_-_Stage_13
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G