Paper - A human embryo of the pre-somite period from the uterine tube
| Embryology - 8 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Kindred JE. A human embryo of the pre-somite period from the uterine tube. (1933) Amer. J Anat. 53: 221-241.
| Online Editor | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Note this paper was published in 1933 and our understanding of early embryo development has improved since this historic human study. This embryo has been classified as Carnegie Stage 7.
Modern Notes Carnegie Stage 7
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Human Embryo Of The Presomite Period From The Uterine Tube
James E. Kindred
Laboratory Of Histology And Embryology, University Of Virginia
Seven text figures And One Plate (Rwmrry figures)
Introduction
The embryo from a ruptured tubal pregnancy which is described in this paper was obtained through the courtesy of Dr. William H. Goodwin and his assistant, Dr. Antonio Gentile, of the University of Virginia Hospital. The clinical data were supplied by Doctor Gentile and additional personal information was obtained by Dr. Carson Lee fifer, assistant resident surgeon. I wish to acknowledge my indebtedness to these gentlemen for their aid in making this specimen available for study. The embryo has been named the Goodwin embryo and will be so designated in comparing it with other embryos.
History of the Case
The patient was a white woman, 25 years old, a teacher by occupation, and married for 5 months. Her menstrual history began at 13 years and was regular until the present illness. The last normal period started on April 13 and ended on April 18, 1931. The complaint which brought the patient to the hospital was a sudden acute pain of 24 hours’ duration in the lower abdomen. The attending physican diagnosed the condition as appendicitis and advised immediate hospitalization. The physical diagnosis at the hospital was appendicitis and salpingitis. Vaginal bleeding was recorded on admission to the hospital. She was operated on by Doctor Goodwin on May 19th. A large amount of blood, some of it clotted, was found in the peritoneal cavity. The left uterine tube seemed engorged, but not ruptured; blood was oozing from the os. There was evidence of intratubal rupture and the blood clots were removed for examination. One of the clots contained the embryo described below. It was evidently a case of ruptured intratubal pregnancy with passage of the chorion enclosed in a fragment of the tubal wall into the peritoneal cavity via the os abdominale.
Coitus had taken place on_ April 26th and May 5th. Thus the times from insemination to recovery of the ovum by operation (May 19th) were 22 and 13 days, respectively. The menstrual age of the specimen is 36 days.
Methods of Study
The clot containing the ovum was brought from the operating room to the histological laboratory immediately upon removal from the patient. It was received in normal saline solution. No sign of the chorionic sac except the ends of several villi could be seen. It was deemed advisable to fix the mass intact rather than to run the risk of injuring it by trying to remove the blood clot. The whole- mass was fixed in 10 per cent formalin. Since the blood clot was longer than broad, it was thought that the long axis of the embryo, if such were present, would probably correspond with this axis. Therefore, after embedding in paraffin the mass was sectioned parallel With its long axis at a thickness of 6 u. Villi and chorion extend through 462 sections. All but three of the sections passing through the region of the embryo are in good condition. The sections were stained with Delafield’s hematoxylin and eosin. Although grossly well preserved, the cells of the blastoderm, amnion, and umbilical vesicle have the appearance of having been macerated before fixation. No nuclear details can be made out in the cells of these regions, although the tissue has not disintegrated. Only several mitoses can be seen in the cells. The chorionic epithelium, the cytotrophoblast, and the plasmoditrophoblast, and the mesodermal tissue of the chorion are Well preserved and cytological details can be seen. Two models were made of the specimen; one at 100 diameters, including the blastodisc, amnion, umbilical vesicle, embryophore, and the chorion with its villi in the immediate region of the embryophore; the other of the blastodisc alone at a magnification of 350 diameters. The models were made of blotting paper infiltrated with hard paraffin.
Description
General
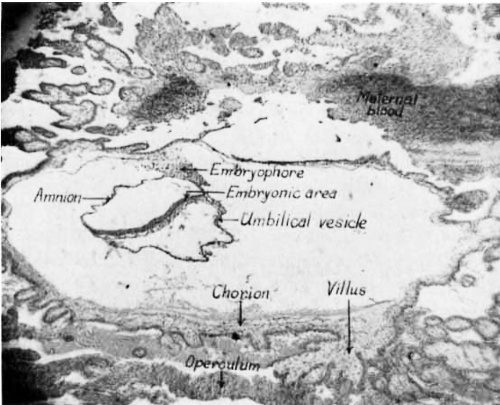
The sections of the blood clot reveal an elongate chorionic sac embedded in a piece of the wall of the uterine tube (fig. 1). The blood clot surrounds the chorion, except on the surface which originally faced toward the lumen of the tube. On this surface an operculum deciduae is present. Part of the tubal mucosa is still attached to the mass and overlies one end of the chorion. Vill.i project beneath this portion of the mucosa and extend parallel with it. Within the chorion lies the embryo with its immediate adnexa, the amnion and umbilical vesicle, suspended from the chorion by a short stubby embryophore. The umbilical vesicle is larger than the amnio-embryonic vesicle and is swung to the left of the embryonic shield. The sections are cut in a sagittal plane. The embryonic disc is roughly elliptical, but the left side is folded at such an angle that the sections of this region pass tangentially through it. The only structures present on the blastodisc are the primitive streak, primitive folds, primitive node, and the beginning of a head process.
Fig. 1 Photomicrograph of a sagittal section through the whole ovum showing the embryo with its adnexa. The surface of the chorion which faces toward the lumen of the uterine tube is at the bottom. X 30.
The Chorion
The chorion is an elongate disc-shaped structure (fig. 1). Its external dimensions including the villi are: 5.8 mm. long, 2.72 mm. broad, and 2.25 mm. deep. Its lumen is flattened so that its length is 2.25 mm., its width 2.44 mm., and its diameter in the region of the embryo is 0.75 mm. These measur-ements of the dimensions of the lumen were made from the inner surface of the mesoderm. The long axis of the chorion lies parallel with the long axis of the tube.
The chorionic surface is covered with villi of different lengths. The longest villi extend from the sides of the chorion up and down beneath the mucosa of the tube. The larger villi project about 2.0 mm. beyond the surface of the chorion. The cores of several of the larger villi contain spaces apparently arising from an edematous condition resulting from liquefaction and dissolution of the stroma. The plasmoditrophoblast and the cytotrophoblast are Well developed and cover the villi and the surface of the chorio11. Cords of plasmoditrophoblast connect the ends of neighboring villi particularly on the surface toward the original lumen of the tube Where they have fused to form a cellular bounding Wall (fig. 1). This is the operculum deciduae of Bryce (’24). There is uncoagulated blood in the intervillous spaces on the parietal surface of the chorion, but on the luminal surface beneath the operculum there are only the remnants of digested tissues which appear as formless masses of finely granular character. Occasional isolated trophoblast cells can be seen in the blood masses. These have been confused by some investigators With true decidual cells of maternal origin, but Mall ( ’15) asserts that they are of trophoblastic origin and have migrated from the tips of the villi.
The mesodermal cores of the villi are composed of mucoid connective tissue, some of the cells of which are rounded off as if in process of migration. In none of the villi are endothelium-lined spaces present; nor were blood islands observed in the Villi or in the wall of the chorion.
Opposite the caudal end of the embryo there is a fold of the chorion which is directed toward an evaginated duct-like portion of the amnion (fig. 11). The amniotic and chorionic components are not united, but from their positions it would seem that there was once a connection between them.
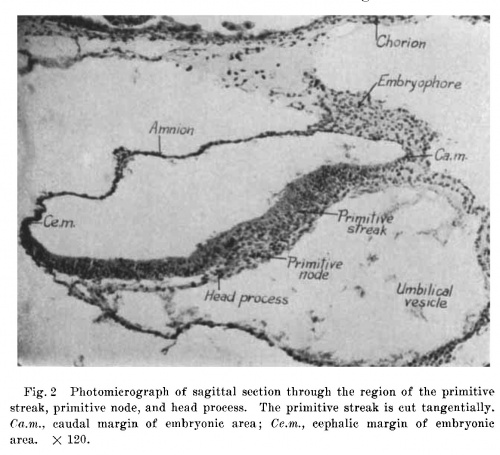
Fig. 2 Photomicrograph of sagittal section through the region of the primitive streak, primitive node, and head process. The primitive streak is cut tangentially. 0a.m., caudal margin of embryonic area; Ce./m., cephalic margin of embryonic area. X 120.
The lining mesoderm of the chorion is fairly regular in thickness, but there is no definite layer of mesothelium. Within the lumen of the chorion there is quite a bit of magma reticulé. This substance resembles fibrin in some places, and in others it is finely granular. In the region of the embryophore the lining of the chorion passes over into the substance of the embryophore.
The Embryophore
The embryophore is a short flat mass of mesoderm surmounting the amnion and extending down in the caudal region of the embryo to end on the umbilical vesicle at the site of the future allantoic diverticulum (figs. 13 to 26). It has a broad base about 0.2 mm. wide and is about 0.18 mm. long at its longest axis (fig. 18). Its area of connection with the chorion extends through thirty-eight sections which at 6 it per section make its width about 0.228 mm. (figs. 11 to 22). Hence it is roughly cylindrical. In the region of its continuity with the chorion, the embryophore has a Very loose gelatinous character with few cells (fig. 2). Where it joins the amnion and umbilical Vesicle it is densely cellular (figs. 18, 19, and 20). The nuclei of the cells are spherical and in some cells the cytoplasm has rounded off and withdrawn the processes characteristic of the mesoderm cells of the chorion. There are several clumps of cells in this region which suggest the primordia of blood islands, but no differentiation between blood cells and endothelium has taken place in these masses. No endothelium—lined spaces are present in the core of the embryophore. Its surface is covered with a layer of mesothelium which ends abruptly at the ohorion, but which is continuous caudad with the mesothelium of the umbilical Vesicle and cephalad with that of the amnion.
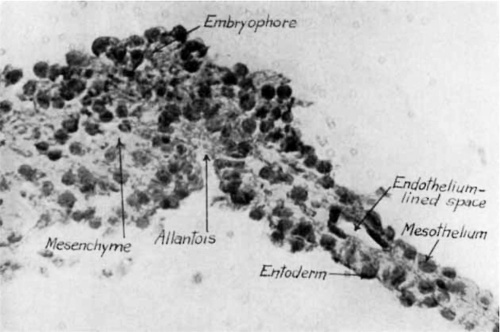
Fig. 3 Photomicrograph of sagittal section through the region of the allantois. X 190.
Fig. 4 Photomicrograph, showing details of allantoic region from section shown in figure 3. X 470.
At the site of the primordium of the allantoic duct the cells of the core of the embryophore mingle with the subepithelial mesoderm of the umbilical Vesicle (figs. 3 and 25). The alvlantoic duct is a small evagination of the epithelium of the umbilical vesicle into the embryophore. It is extremely narrow and extends through but two sections. Its sagittal dimension is 25 1.1, its depth about 100 u, and its diameter about 12 p. The lining cells are partly regularly cuboidal, but at the bottom of the diverticulum this regularity is lost (fig. 4).
The cap of the embryophore extends onto the umbilical Vesicle in hook—shaped fashion. The stem of the hook is attached to the chorion and the hooked end extends caudad onto the surface of the umbilical Vesicle. For part of its extent the amnion projects deeply into the substance of the embryophore and approaches the chorion (figs. 11 to 14). There is, however, no sign of an epithelial cord in the embryophore which could be interpreted as the remnant of a connection between chorionic trophoderm and amniotic ectoderm.
Fig.5 Drawing of model of embryo. The amnion is cut at the region of junction with the embryonic area. Dorsal view. The model is 100 times the size of the embryo. The numbers on the line below the model refer to the planes of the sections shown in plate 1.
The embryonic area
Figure 5 is a dorsal View of the embryonic shield as modeled. Unfortunately, the left side of the shield Was folded in such a manner that in sectioning it was cut tangentially. The right side, however, is flat and indicates that the shield is in the form of a disc, slightly longer than wide. The longest section which passes through the region of the primitive streak measures 0.728 mm. (fig. 20). The embryonic shield extends through seventy—three sections at a thickness of 6 u per section, therefore, as cut, the disc measures 0.438 mm. in diameter. This measurement, however, does not represent the true diameter of the blastodisc, since the left side is folded. Therefore, if it is assumed that the region of the primitive streak represents the sagittal axis of the blastodisc, then the distance from the streak to the right edge (which is not folded) would represent one-half the diameter of the unfolded embryonic shield. Doubling this measurement gives the probable diameter of the Whole disc as about 0.588 mm.
From the appearance of the primitive node and the blastoderm cephalic and caudal to it, it is clear that the embryonic disc is cut parallel with its sagittal axis. Cephalic to the node the ectoderm is a flat, stratified, columnar epithelium; at the node the cells lose their stratified condition and form a clump (fig. 2). Caudal to the node, the cells are again arranged in a stratified manner, but mingle without demarcation with the underlying mesodermal cells. The difference between mesoderm and ectoderm can be readily seen, and inheres in the greater size of the nuclei of the mesodermal cells. This region is evidently the primitive streak. On the right side of the primitive streak the primitive fold can be seen on the surface of the blastoderm (fig. 5). Its height is exaggerated because of the concavity on the undersurface of the bastoderm in this region. The actual difference between the thickness of the ectoderm in the fold and that in the primitive streak is very slight. The primitive fold on the left side is cut tangentially and in the model appears as a steep fold to the left of the primitive streak.
The greatest longitudinal extent of the primitive streak is about 0.215 mm. There is a slight cephalic proliferation of cells from the primitive node, and a head process 0.078 mm. long is present (figs. 2, 20, 21). There are a few scattered mesodermal cells between the cephalic part of the ectoderm and the entoderm of the umbilical vesicle, and an abrupt transition occurs between the cephalic end of the node and the ectoderm immediately cephalic to it. This is well brought out in figure 2, which passes through the greatest thickness of the node. There is no sign of canalization of the node, so that the primordium of the chorda canal (archenteron of Bryce, ’24) is lacking.
At the caudal end of the primitive streak a layer of mesoderm lies between the ectoderm and entoderm, hence a definite cloacal membrane has not as yet appeared. No prochordal plate is present in the entoderm of the umbilical vesicle immediately in front of the head process (fig. 2). Examination of the figures near the median plane in plate 1 indicate that very little mesoderm is present between the ectoderm and entoderm and that the entoderm is a simple squamous layer.
Rostrally in the region of the future neural plate, the ectoderm is 0.032 mm. in thickness at about the middle part. Laterally, it is about the same thickness until it approaches the ectoderm of the amnion. As the amnio-embryonic margin is approached the Stratification of the embryonic ectoderm becomes progressively lower until it is simple cuboidal; at the amnion it becomes simple squamous. Near the cephalic end of the blastoderm is a transverse groove, in front of which the blastoderm makes a sharp dorsal tur11 (figs. 12, 13, 14). This may be the primordium of the cephalic fold which eventually delimits the head.
The details of the nuclei and cytoplasm of the ectodermal cells cannot be clearly distinguished. This unfortunate condition is probably due to the death of the cells before fixation. The nuclei are a homogeneous blue and the cytoplasm is foamy with a very coarse reticulum. Only two mitoses were found in the whole blastoderm. Both of these are at the caudal end in the region of the left margin of the embryophore. In one cell, the spindle is at right angles to the surface of the ectoderm and in the other, parallel with it. These planes of division indicate that the ectoderm was growing in both thickness and width at this stage.
In brief, the embryonic area is very simple in organization. It is ovoid in shape, its long axis is not very much greater than its width. The only evidence of differentiation is the presence of a primitive streak and primitive folds, primitive node, and a short head process. Neurenteric canal, prochordal plate, cloacal membrane, and neural plate are lacking.
The Amnion
The amnion is composed of two layers of simple squamous epithelium, an inner layer of ectodermal origin and an outer layer of mesothelial origin (fig. 2). The mesothelial cells are continuous with the mesothelium of the embryophore above and with that of the umbilical vesicle below. In most regions the two epithelial layers of the amnion are closely applied to each other.
The right end of the amnion has two protrusions. One of these projects from the extreme right end in the form of a tube (figs. 8 to 10). Opposite the free end of this projection there is a projection of the chorion. These relations suggest an original continuity. The amniotic duct, which is present in other embryos as a prolongation of the amniotic cavity into the embryophore uniting at its distal end with the chorion (Bryce, ’24), is represented in the Goodwin ovum by a deep cavity in the embryophore, but there is no continuity between the cells lining this cavity and the trophoblast cells of the chorion (figs. 11, 12). Of the two amniotic diverticula, the one lying in the embryophore is without doubt the remnant of the amniotic duct and the one which ends freely is an accessory structure which may be interpreted as a variation. Another diverticulum of the amnionic vesicle extends from its left caudal margin and ends blindly on the surface of the umbilical vesicle in the vicinity of the primordium of the allantoic duct (figs. 24, 25). This diverticulum probably represents the amnio-allantoic duct. The dimensions of the amnio-embryonic vesicle correspond in length and breadth to those of the embryonic shield. In its deepest region it measures 0.250 mm.
The Umbilical Vesicle
The illustration of the small model of the ovum with its adnexa shows the general shape of the umbilical Vesicle (fig. 5). The greater part of it is swung to the left of the embryonic area. Below the embryonic area it is pear—shaped with a narrow cephalic portion and a wide caudal portion (figs. 18 to 22). It projects for about 0.252 mm. beyond the left margin of the embryo. The right side is much flattened and lies beneath the right side of the embryonic area, but is separated from it by a scattered layer of mesodermal cells (figs. 13 to 15). Its greatest depth is 0.3 mm., its length is 0.704 mm., and its width is 0.588 mm. The caudal end projects up under the blastodisc which is concave in this region (figs. 21 to 24).
The character of the epithelium lining varies; beneath the blastodisc it is simple squamous and often discontinuous (fig. 2). The squamous epithelium is characteristic of its cephalo—Ventral portion, but in the caudal region the epithelium is low cuboidal. The outer layer of mesoderm is Very irregular in character; in some places the cells are flat and closely applied to the entoderm; in other places, particularly on the caudal and left wall of the vesicle, there are nodules of cells possibly representing the mesothelial buds which, accarding to Bremer (’14), are the primordia of the Vasa. In one place near the apical end of the vesicle there is a submesothelial syncytium of mesodermal cells which suggests the beginning of a blood island. This is the only one on the vesicle. Here a.nd there among the mesodermal cells are endothelium—lined spaces devoid of content. One of these is clearly shown in figure 4.
As mentioned above, the allantoic duct is just beginning to form as a diverticulum from the caudal surface of the umbilical Vesicle in the region of termination of the embryophore (figs. 3 and 4).
A small convoluted diverticulum extends from the apex of the umbilical Vesicle and doubles upon it (figs. 6 and 7). This diverticulum is lined with more regular cuboidal epithelium than is found elsewhere in the vesicle (fig. 7). Externally, it is covered with mesothelium beneath Which there are no traces of blood island formation. The diverticulum ends blindly ‘and its mesothelium has no continuity with the mesoderm of the chorion across the lumen of the eXtra—embryonic celom.
Fig.6 Photomicrograph of section passing through the apical end of the umbilical vesicle, showing it relation to the diverticulum. X 190.
Discussion
The Goodwin embryo has certain features which warrant interpreting it as morphologically similar to the embryos included by Streeter (’20) iii group 2 of his classification of young human embryos and to the older embryos included in group D of Bryce (’24). In this group Streeter includes the ‘v.H.’ ovum of Graf von Spee, The Minot embryo of Lewis, and the embryos described by Debeyre, van Houkelon and Gviacomini, and his own Mateer embryo. Regarding this group of embryos, Streeter says (p. 414) :
Fig. 7 Photomierograph showing details of diverticulum of umbilical vesicle from section shown in figure 6. X 470.
A summary of the features occurring in all of the embryos of this group would include the presence of a primitive groove and an allantois, with evidences of blood formation in the Wall of the yolk—sac (in most of t.hem) also in the body stalk and chorionic membrane. Furthermore, in all of them the yolksac is considerably larger than the amniotic cavity. The chorion is covered with freely branching villi and its ectoderm is clearly differentiated into two layers. The mesoderm lining the chorion forms a Well defined supporting membrane, from which processes extend into the cores of the villi. The internal diameter of the chorion of such an ovum is from 4 to 6 mm.
The Goodwin embryo conforms in most of its features to the classification given above. The chief differences lie in the extremely small size of the allantoic rudiment and the small dimensions of the chorion. It has all of the other features and in addition has the beginning of a head process, or, as Waldeyer (’29) calls it, the primordium of the secondary mesoderm as contrasted with the primary mesoderm of the adnexa.
The small size of the chorion of the Goodwin embryo may be explained by the site of embedding. The ovum was originally embedded in the uterine tube before being aborted into the peritoneal cavity. The sections show that it has been compressed in its growth. The greater chance for expansion of the growing chorion which is offered by the uterus is lacking in the tube. Consequently, the small size of the chorion of the Goodwin ovum as compared with the size of the chorions included in group 2 of Streeter, is probably the result of the retarding influence of its tubal environment.
The diverticulum of the umbilical vesicle described above and illustrated in figures 6 and 7 seems to be identical with the apical yolk-sac diverticulum described by Bryce (’24) in the embryo Teacher-Bryce II. In both embryos the duct is lined with a low columnar epithelium and is in luminal continuity with the umbilical vesicle. Its outer surface is covered with mesothelium continuous with the mesothelium of the umbilical vesicle. In the Teacher—Bryce II embryo the mesothelium is continuous distally with the mesoderm of the chorion, but in the Goodwin embryo the distal end lies free in the extra—embryonic celom. Blood islands and endothelium—lined spaces are present in the mesoderm of the diverticulum of the Teacher—Bryce II embryo, but in the Goodwin embryo only a few endothelium-lined spaces are present.
Bryce states that there is evidence that such a diverticulum is normally persent in young human embryos of his group D, but in none is it so well developed as in the Teacher-Bryce II embryo. The only older embryo which contains a remnant of this diverticulum is that described by Grosser (’13), in which there is a mesodermal strand between the umbilical vesicle and the chorion and in which there are cyst—like masses of entodermal cells. Since the Goodwin embryo is much further developed than the Teacher—Bryce II embryo in all respects, except the size of the chorion, and is not as far advanced as the Grosser embryo, it may be concluded that it represents a stage between the conditions in these two embryos. It cannot be assumed, however, that the separation of the umbilical diverticulum from the chorion is the result of growth of the chorion, since the chorion of the Goodwin embryo is much smaller than that of other embryos of this group and even of the Teacher-Bryce II embryo. The assumption that the diverticulum represents a remnant of an earlier condition in which the umbilical vesicle was coextensive with the chorion, as in the early stages of the ungulates, may possibly be the correct interpretation, but more material must be found before full acceptance of this view.
The primordium of the Vascular system in the Goodwin embryo is very limited in its extent as compared with other embryos of Streeter’s group 2. There is no extensive development of blood islands or cords of vasculogenic tissues such as are described for the Mateer embryo (Streeter, ’20). The endothelium-lined spaces which are present are limited to the caudal and apical regions of the umbilical vesicle and are particularly prominent in the region of the primordium of the allantois. None of them contains free cells. Several masses of mesenchymal cells suggestive of blood islands are present. Two of these are at the junction of the embryophore and the dextrocaudal surface of the amnion; and the third lies between the entoderm and mesothelium of the apex of the umbilical vesicle. Aside from these regions, the embryophore, chorion, and villi contain no angioblastic cords or hemogenic mesenchymal masses. From this evidence it would seem that the endothelium-lined spaces may develop independently of the later appearing corpuscular contents.
The clinical data associated with the Goodwin embryo show two possible fruitful sexual acts. According to the first one (April 26th), allowing 24 hours for fertilization, and reckoning to recovery of the ovum (May 19th), the fertilization age of the Goodwin embryo would be 21 days. If the second coitus were the fruitful one (May 5th), again allowing 24 hours for fertilization, the fertilization age would be 12 days. Since, however, the Goodwin embryo had already been aborted and was found in the peritoneal cavity, and, furthermore, since the histologic appearance of the cells of the embryo show that it was dead before fixation, at least 2 days must have elapsed between death and removal. If these 2 days are subtracted from the fertilization age estimated from the data of the first coitus, the maximum fertilization age would be 19 days; and if the data from the second coitus were similarly treated, the minimum fertilization age would be 10 days. Obviously, from the accurate data associated with the BryceTeacher embryo I (Bryce and Teacher, ’08) which serves as. a standard for estimation of the age of young human embryos, the minimum age estimated for the Goodwin embryo is not correct, since the morphologic features are much too far advanced for comparison with the Bryce-Teacher embryo I.
Further argument for the assumption that the coitus of April 26th was the fruitful act rests on the evidence presented by Allen et al (’28) on the relationship between theonset of menstruation and the recovery of ova from theuterine tubes. In their report of the recovery of seven ova from the uterine tubes, one ovum was recovered on the twelfth, four on the fifteenth, and two on the sixteenth day of the menstrual cycle. The last two showed evidences of degeneration. Applying these data to the history of the patient from whom the Goodwin embryo was obtained, it is to be noted that menstruation began on April 13th and hence ovulation could have occurred between the 25th and 29th of April, during which time (April 26th) coitus took place. Therefore, making such allowances as have been stated above, it is concluded that the Goodwin embryo has a fertilization age of about 19 days and has the morphologic features characteristic of Streeter’s group 2.
Summary
The Goodwin embryo is a young human embryo of the presomite stage obtained from an intratubal abortion. It was recovered in a blood clot found in the peritoneal cavity following operation for appendicitis and salpingitis. The chorion measurements are over all: 5.8 mm. in length, 2.72 mm. in width, and 2.25 mm. in depth. The dimensions of the lumen of the chorion are: 2.25 mm. X 2.44 mm. X 0.8 mm. The other dimensions are: amnion, 0.728 mm. X 0.6 mm. X 0.240 mm.; umbilical vesicle, 0.704 mm. X 0.588 mm. X 0.33 mm.; embryonic area, 0.728 mm. X 0.588 mm.; length of primitive streak, 0.215 mm.; length of primitive node, 0.078 mm., thickness 0.082 mm.; length of head process, 0.078 mm.; thickness of rostral ectoderm, 0.032 mm.
The trophoblast is two layered a11d well preserved. The villi are large and branched with some evidence of degeneration. The cells of the embryonic shield, amnion, and umbilical vesicle were dead before the embryo was fixed. The primitive streak and node are well developed. A head process is present, but a prochordal plate is absent. The allantois is very rudimentary. Angiogenesis is just beginning.
The Goodwin embryo agrees morphologically with the human embryos of group 2 of Streeter’s classification in all respects, except in the dimensions of the chorion; this structure is much smaller than is usual for embryos of this group. The fertilization age of the Goodwin embryo is estimated at about 19 days, but this is not as accurate as it could be, because the length of time elapsing between the death of the embryo and its recovery is assumed to be only 2 days. The discrepancy between morphologic and fertilization age may be the result of a retarding action of the environment in the uterine tube.
Literature Cited
ALLEN, E., J. P. PRATT, Q. U. NEWELL, AND L. BLAND 1928 Recovery of human ova from the uterine tubes. Time of ovulation in the menstrual cycle. J. Am. Med. Assoc., vol. 91, pp. 1018-1020.
Bremer JL. The earliest blood-vessels in man. (1914) Amer. J Anat. 16(4): 447-475.
BRYCE, T. H., AND J. H. TEACHER 1908 Contributions to the study of the early development and embedding of the human ovum. Glasgow.
BRYCE, T. H. 1924 Observations on the early development of the human embryo. Trans. Roy. Soc. Edinburgh, vol. 53, pp. 533-567.
GROSSER, O. 1913 Ein menschlieher Embryo mit Chordacanal. Anat. Hefte, 1 Abth., Bd. 47, S. 649-686.
Mall FP. On the fate of the human embryo in tubal pregnancy. (1915) Contrib. Embryol., Carnegie Inst. Wash. Publ. 221, 1: 1-104.
Streeter GL. A human embryo (Mateer) of the pre-somite period. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. 272, 9: 389-424.
WALDEYER, A. 1929 Mesodermbildung bei einem jungen menschlichen Embryo (Soho). Anat. Anz., Bd. 35, S. 145-151.
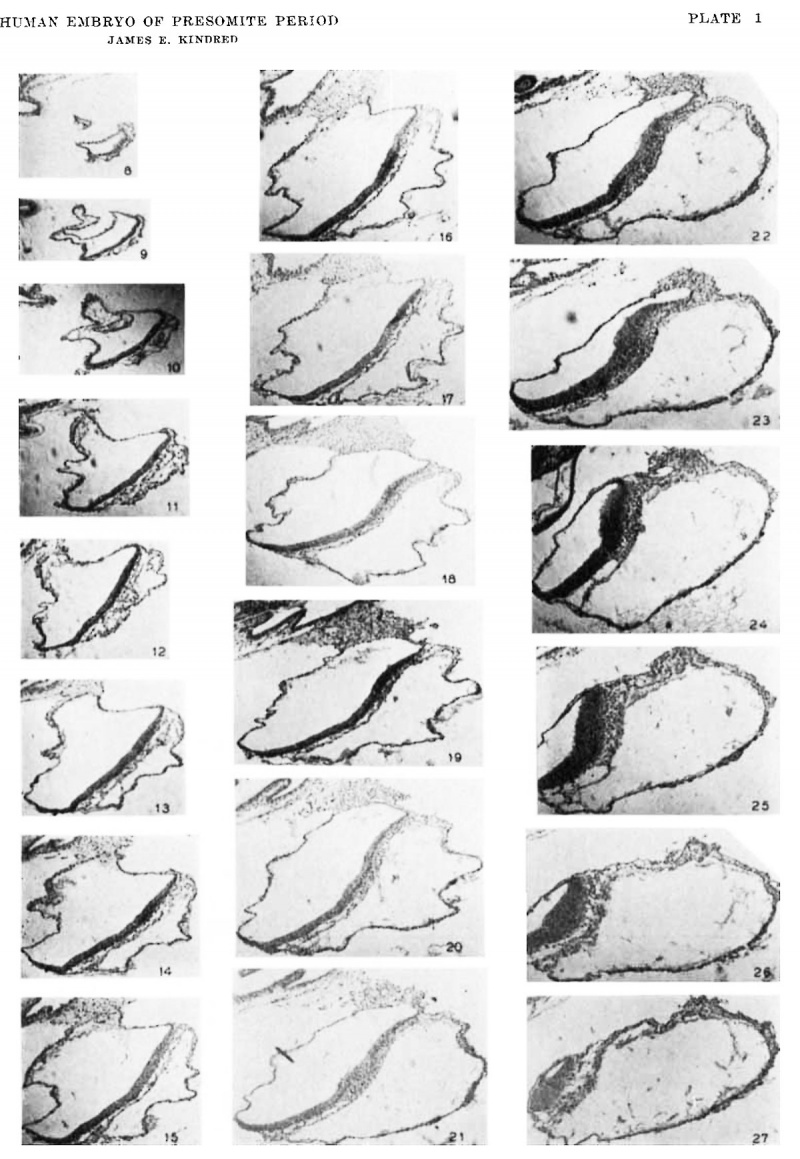
Plate 1
8 to 27 Photomierographs of parasagittal sections through the embryo and its immediate adnexa. The progression of the sections is from the right side of the embryonic area to the left. The planes through which the sections pass are shown on the model (fig. 5). The levels indicated by the planes are only approximate, because of the inherent error in modeling and drawing.
The actual distances between sections photographed are as follows: 8-9, 12 ,u; 9-10, 12 M; 10-11, 12 ,u.; 11-12, 18 M; 12-13, 1811.; 13-14, 18 M; 14-15, 18M; 15-16, 12 ,u; 16-17, 18 11.; 17-18, 18 p; 18-19, 362; 19-20, 12/1; 20-21, 182; 21-22, 18 u; 22-23, 18 2; 23-24, 18 2; 24-25, 182; and 26-27, 18 p. x 50.
Cite this page: Hill, M.A. (2024, May 8) Embryology Paper - A human embryo of the pre-somite period from the uterine tube. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_human_embryo_of_the_pre-somite_period_from_the_uterine_tube
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G