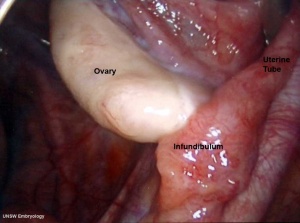
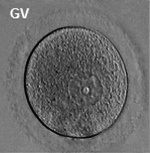
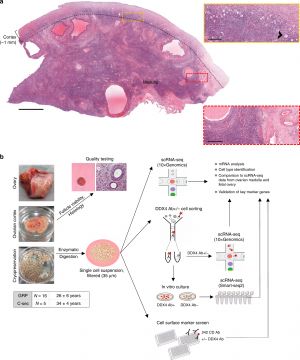
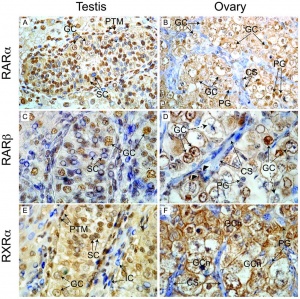
Ovary Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
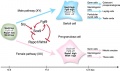
The female gonad is the ovary and is closely associated with female internal genital (reproductive) tract development. In humans, these laterally paired organs lie within the peritoneal cavity. Genes such as WNT-4 and DAX-1 necessary for initiation of female pathway ovary development, female gonad is not considered a default process.
Initial gonad development in females and males is virtually identical with germ cells migrating into an indifferent gonad. In females with XX, the ovary then begins to develop and the subsequent structure and timecourse of germ cell then differs between males and females. In the ovary oocytes proliferate prior to birth and arrest in meiosis 1.
| Historic Embryology |
| Regnier de Graaf (1641 – 1673) was a Dutch anatomist and physician who described the anatomy of the uterine tube and the development of follicles in the ovary. He was first to describe the "Graafian follicle" (preovulatory follicle) in the ovary of mammals, but erroneously believed the entire follicle to be the mammalian oocyte (egg). |
- Links: menstrual cycle | oocyte | X Chromosome | AMH | PCOS | ovarian reserve | Category:Ovary | Category:Oocyte
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Ovary Development | Ovarian Follicle Development | Folliculogenesis | Ovulation | Primordial Follicle | Graafian Follicle | Ovarian Reserve | Cumulus-Oocyte Complex |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Human Ovary Timeline
Approximate Timeline of human development listed below.
| Time | Carnegie Stage | Event |
|---|---|---|
| 24 days | 11 | intermediate mesoderm, pronephros primordium |
| 28 days | 12 | mesonephros and mesonephric duct |
| 35 days | 14 | uteric bud, metanephros, urogenital ridge |
| 42 days | 17 | cloacal divison, gonadal primordium (indifferent) |
| 49 days | 19 | paramesonephric duct, gonadal differentiation |
| 6-7 weeks (GA 8-9 weeks) | primordial germ cell mitosis proliferation commences | |
| 56 days | 23 | paramesonephric duct fusion (female) |
| 12-14 weeks (GA 14-16 weeks) | fetal | primordial germ cell meiosis germ cell differentiation, formation of syncitial clusters of oogonia |
| 15-18 weeks (GA 17–20 weeks) | fetal | breakdown of syncitial clusters and assembly of primordial follicles |
| Links: ovary | oocyte | timeline | Category:Timeline | ||
Movies
|
|
|
|
| Mouse Primordial Germ Cell Migration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
| |||||||||
Oogenesis
| The 2 human ovaries gradually lose follicles both before and after puberty (the beginning of ovulation); beginning with about several million before birth, maximum number at birth, 300-400,000 by puberty and finally by late 40’s have only a few follicles left. Recent studies suggest that the original calculations of ovary follicle numbers at birth were over-estimates and the actual figure should be about 2.5 million.[12] The number of antral follicles detected within the ovary also decreases with increasing maternal age.
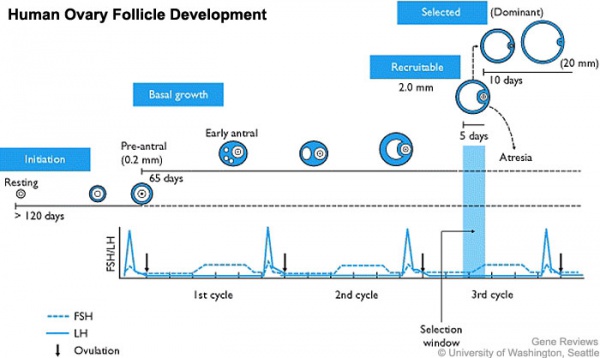
In humans, a primodial follicle take about 150 days to develop into a preantral follicle (primary) and another 120 days to form an antral follicle (secondary). A number of antral follicles will then "compete" for 14-15 days to become the dominant follicle, which will undergo ovulation. ovarian reserve - Clinical term for the number of oocytes (non-growing follicles) available for possible fertilization at the different times during female reproductive life. A blood test for Anti-Mullerian Hormone (AMH) levels is used clinically as a measure of the ovarian reserve. |

Human ovary non-growing follicle model[12] |
Cumulus-Oocyte Complex - (COC) Clinical term used in Assisted Reproductive Technology to describe the ovulated Graafian follicle consisting of the oocyte surrounded by a packed layers of cumulus cells.
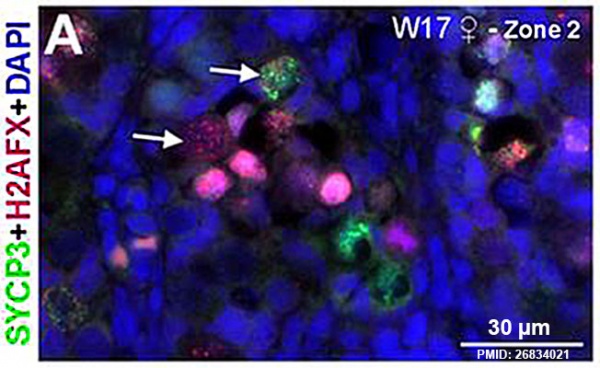
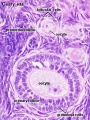
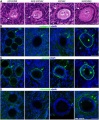
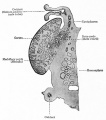
Fetal Ovary
Fetal human ovary meiosis (second trimester)[13] H2AFX and SYCP3 are meiotic markers
Infant Ovary
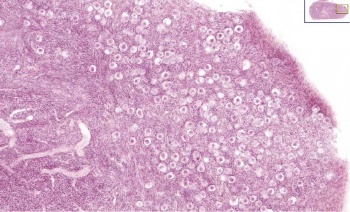
|
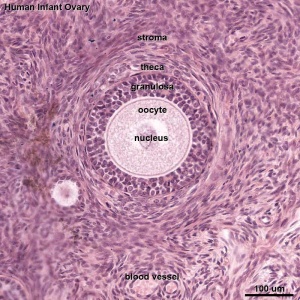
|
| Overview | Follicle |
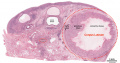
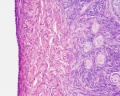
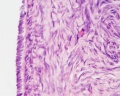
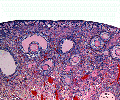
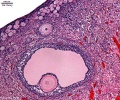
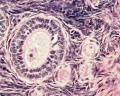
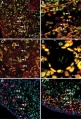
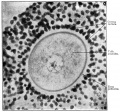
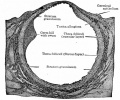
This image shows a region (see inset) of the infant ovary cortex.
There are a large number of developing oocytes which will eventually form a dense primordial germ layer at the ovary periphery.
Later stages of follicle development are completely absent and will begin to only appear just prior to puberty.
| Table showing the Growth of the Ovary | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vertex-breech length |
Greatest diam. of head |
Right Ovary | Left Ovary | Comparison between | |||||
| Breadth | Length | Breadth | Length | Length | Breadth | ||||
| R. | L. | R. | L. | ||||||
| 50.0 | 42.6 | 0.9 | 1.9 | 0.9 | 2.5 | .. | + | — | — |
| 125.0 | 123.0 | 1.2 | 5.9 | 1.5 | 4.1 | + | .. | .. | + |
| 138.0 | 115.0 | 1.6 | 5.0 | 2.0 | 5.0 | — | — | .. | + |
| 156.0 | 131.0 | 1.9 | 7.2 | 2.0 | 7.1 | + | .. | .. | + |
| 173.0 | 163.5 | 3.0 | 9.0 | ... | ... | .. | .. | .. | .. |
| 190.0 | 175.0 | 2.9 | 7.7 | 2.1 | 7.8 | .. | + | + | .. |
| 223.0 | 162.0 | 2.9 | 10.5 | 3.0 | 9.1 | + | .. | .. | + |
| 235.0 | 190.0 | 4.2 | 10.0 | 3.8 | 12.0 | .. | + | + | .. |
| 260.0 | 213.0 | 3.6 | 11.1 | 4.0 | 11.4 | .. | + | .. | + |
| 272.0 | 213.0 | 3.0 | 10.0 | 3.5 | 9.2 | + | .. | .. | + |
| 305.0 | 238.0 | 3.0 | 9.9 | 3.9 | 10.9 | .. | + | .. | + |
| 347.0 | ... | 3.5 | 10.8 | 4.9 | 8.5 | + | .. | .. | + |
| 355.0 | 273.0 | 4.0 | 14.0 | 5.2 | 9.9 | + | .. | .. | + |
| 386.0 | 324.0 | 5.1 | 11.5 | 3.0 | 9.9 | + | .. | + | .. |
| 402.0 | 301.0 | 5.05 | 10.5 | 3.0 | 12.0 | .. | + | + | .. |
| 3 weeks | ... | 5.0 | 17.0 | 5.0 | 14.0 | + | .. | — | — |
| 6 weeks | ... | 7.5 | 15.0 | 7.0 | 14.7 | + | .. | + | .. |
| 6 weeks | ... | 7.0 | 18.0 | 8.0 | 17.0 | + | .. | .. | + |
| 10 weeks | ... | ... | 14.0 | ... | 16.0 | + | .. | — | — |
| 2 months | ... | 6.0 | 14.5 | 4.0 | 13.0 | + | .. | + | .. |
| 3 months | ... | 6.0 | 15.5 | 5.0 | 14.7 | + | .. | + | .. |
| 7 months | ... | 5.9 | 15.5 | 4.5 | 18.1 | .. | + | + | .. |
| 15 months | ... | 9.0 | 18.0 | 9.0 | 19.5 | + | .. | — | — |
| I.75 years | ... | 7.0 | 20.0 | 8.5 | 15.0 | + | .. | .. | + |
| 4 years | ... | 10.0 | 27.0 | 12.7 | 23.2 | + | .. | .. | + |
| 5.5 years | ... | 11.1 | 29.0 | 9.1 | 26.1 | + | .. | + | .. |
| 14 years | ... | 11.9 | 26.5 | 12.0 | 29.5 | .. | + | .. | + |
| The measurements are all given in millimeters, breech length is measured along the nape and the back. | |||||||||
| Reference: Felix W. The development of the urinogenital organs. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp 752-979. | |||||||||
| Links: 1912 Ovary Growth | Table Ovary Growth Table | Collapsible Table | Ovary Development | |||||||||
Ovarian Reserve

Ovarian Reserve - Clinical term for the number of oocytes (non-growing follicles) available for possible fertilization at the different times during female reproductive life. A blood test for Anti-Mullerian Hormone (AMH) levels is used clinically as a measure of the ovarian reserve. A negative finding has been described as Diminished Ovarian Reserve, or an ovarian insufficiency or premature ovarian failure and may be seen in adult childhood cancer survivors and adult patients undergoing a number of therapies.
The dogma in human (mammalian) development is that new oocyte and follicle production does not occur during postnatal life. A recently published human ovary molecular study has reinforced this dogma.[1] There is also substantial data that shows human ovarian changes postnatally are loss by apoptosis of prenatal oocytes.
| Alternative Theories |
|---|
| Postnatal Generation - A research group (Tilly JL, Johnson J. 2004, 2007) has published experiments using mice, showing potentially other sources/sites (bone marrow) of oocyte (putative germ cell) generation. They recently stated that the argument should be based upon "experimental approaches than simply an absence of evidence, especially from gene expression analyses". Several other research groups (Eggan K etal. 2004 and Veitia etal. 2007) have argued against these findings. |
- Links: Image - human follicle number | Assisted Reproductive Technology | oocyte | Search PubMed - Ovarian Reserve | Search PubMed - Diminished Ovarian Reserve
Adult Follicle Structure
A follicle usually contains a single oocyte (egg, ovum, female gamete) and a series of supporting cells and a single fluid-filled space in layers surrounding this cell. The 3 layers below are arranged in layers outward from the oocyte.
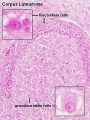
Granulosa Cells
- A specific cell type that proliferates in association with the oocyte within the developing follicles of the ovary. These cells form the follicle stratum granulosa and are also given specific names based upon their position within the follicle.
- With development of the antral follicle, there are two populations of granulosa cells with distinct characteristics and functions: mural granulosa cells and cumulus cells.[14]
- mural granulosa cells - an endocrine role by producing steroid hormones and various other ligands
- cumulus cells - play a support role for oocyte development
Alternate Histological Terms
- The membrana granulosa cells sit on the follicular basal lamina and line the antrum as a stratified epitelium. Following ovulation, these granulosa cells contribute to corpus luteum.
- The cumulus oophorus is a column of granulosa cells that attaches the oocyte to the follicle wall. At ovulation, this column of cells is broken or separates to release the oocyte from its follicle attachment.
- The corona radiata are the granulosa cells that directly surround the oocyte, and are released along with it at ovulation. Following ovulation, the corona radiata provide physical protection to the oocyte and are the initial structural barrier that spermatazoa must penetrate during fertilization.
- Links: granulosa cell
Follicular Fluid
- The antrum is a fluid-filled space in the secondary (antral) follicle
- At ovulation, fluid is released along with the oocyte
- Thought to "carry" the oocyte out of the follicle (like a boat on a wave)
- Aids entry into the uterine tube
Theca Interna
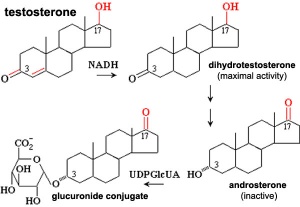
(Greek, thek = box) The ovarian follicle endocrine cells forming the inner layer of the theca folliculi surrounding the developing follicle within the ovary. This vascularized layer of cells respond to leutenizing hormone (LH) synthesizing and secreting androgens (androstendione) transported to glomerulosa cells which process initially into testosterone and then by aromatase into estrogen (estradiol). Theca cells do not begin hormonal functions until puberty.
Theca Externa
(Greek, thek = box) The ovarian follicle stromal cells forming the outer layer of the theca folliculi surrounding the developing follicle within the ovary. Consisting of connective tissue cells, smooth muscle and collagen fibers.
Follicle Classification
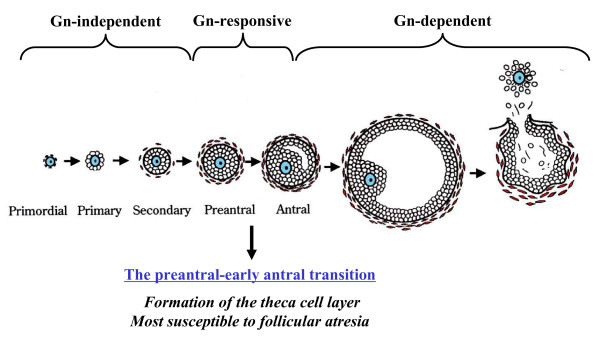
There are several different nomenclatures for the stages of follicle maturation. It probably does not matter which naming system you use, as long as you are consistent and use the same set of terminology for all stages. Early stages of follicle development appear to be gonadotropin (Gn) independent and with development become gonadotropin "sensitive" and then "dependent" . (UK spelling is gonadotrophin).
Follicle development stages and the relationship to gonadotropin (Gn)[15]]]
Human ovary follicle development
| Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) | Size ultrasound (mm) |
|---|---|---|---|---|---|
| primordial follicle | small | 1, 2, 3 | 25 | less than 50 | |
| primary follicle | preantral | 4 5 |
26 - 100 101 - 300 |
up to 200 | |
| secondary follicle | antral small antral large antral |
6 7 |
3001 - 500 501 - 1000 |
500 1000 - 6000 |
less than 18 |
| preovulatory follicle | Graafian | 8 | greater than 1000 | greater than 6000 | 18 – 28 |
| Links: ovary | oocyte | menstrual cycle | |||||
Primordial Follicle
| Human Follicle Classification | ||||
|---|---|---|---|---|
| Follicle Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) |
| Primordial | small | 1, 2, 3 | 25 | less than 50 |
| Human Ovarian Follicle Classification | |||||
| Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) | Size ultrasound (mm) |
|---|---|---|---|---|---|
| primordial follicle | small | 1, 2, 3 | 25 | less than 50 | |
| primary follicle | preantral | 4 5 |
26 - 100 101 - 300 |
up to 200 | |
| secondary follicle | antral small antral large antral |
6 7 |
3001 - 500 501 - 1000 |
500 1000 - 6000 |
less than 18 |
| preovulatory follicle | Graafian | 8 | greater than 1000 | greater than 6000 | 18 – 28 |
| Links: ovary | oocyte | menstrual cycle | |||||
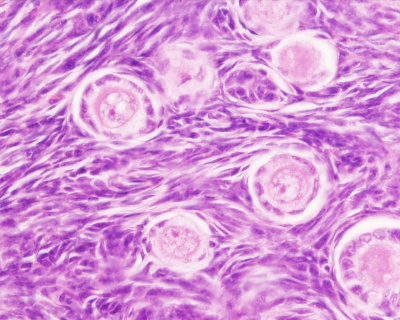
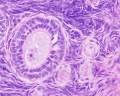
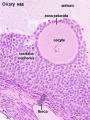
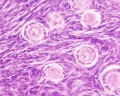
Primate primordial follicles (Stain - Haematoxylin Eosin). Note the single layer of follicle cells surrounding the oocyte. |
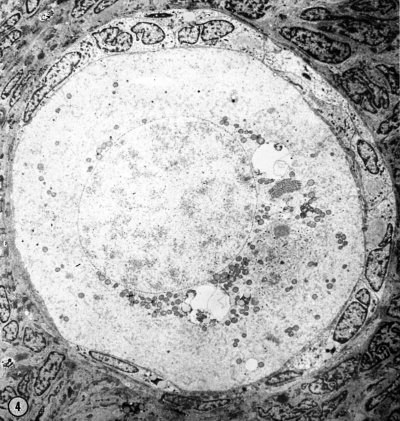
Electron Micrograph of a human oocyte in a primordial follicle. Most of the organelles are concentrated in one pole of the oocyte.[16] |
Primary Follicle
| Human Follicle Classification | ||||
|---|---|---|---|---|
| Follicle Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) |
| Primary | preantral | 4 5 |
26 - 100 101 - 300 |
up to 200 |
| Human Ovarian Follicle Classification | |||||
| Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) | Size ultrasound (mm) |
|---|---|---|---|---|---|
| primordial follicle | small | 1, 2, 3 | 25 | less than 50 | |
| primary follicle | preantral | 4 5 |
26 - 100 101 - 300 |
up to 200 | |
| secondary follicle | antral small antral large antral |
6 7 |
3001 - 500 501 - 1000 |
500 1000 - 6000 |
less than 18 |
| preovulatory follicle | Graafian | 8 | greater than 1000 | greater than 6000 | 18 – 28 |
| Links: ovary | oocyte | menstrual cycle | |||||
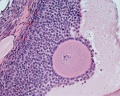
Histological image of a primary follicle.
Secondary Follicle
| Human Follicle Classification | ||||
|---|---|---|---|---|
| Follicle Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) |
| Secondary | small antral large antral |
6 7 |
3001 - 500 501 - 1000 |
500 1000 - 6000 |
| Human Ovarian Follicle Classification | |||||
| Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) | Size ultrasound (mm) |
|---|---|---|---|---|---|
| primordial follicle | small | 1, 2, 3 | 25 | less than 50 | |
| primary follicle | preantral | 4 5 |
26 - 100 101 - 300 |
up to 200 | |
| secondary follicle | antral small antral large antral |
6 7 |
3001 - 500 501 - 1000 |
500 1000 - 6000 |
less than 18 |
| preovulatory follicle | Graafian | 8 | greater than 1000 | greater than 6000 | 18 – 28 |
| Links: ovary | oocyte | menstrual cycle | |||||
Preovulatory Follicle
From the cohort of secondary follicles, in the late luteal phase of the previous menstrual cycle, a dominant follicle now grows, the "Graafian follicle". The follicle growth rate is about 2 mm per day, achieving a preovulatory diameter between 18 to 28 mm as measured by ultrasound. Ovulation is triggered by a surge of pituitary luteinizing hormone (LH) about 36 hours before follicle release.
| Human Follicle Classification | ||||
|---|---|---|---|---|
| Follicle Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) |
| Preovulatory | Graafian | 8 | greater than 1000 | greater than 6000 |
| Human Ovarian Follicle Classification | |||||
| Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) | Size ultrasound (mm) |
|---|---|---|---|---|---|
| primordial follicle | small | 1, 2, 3 | 25 | less than 50 | |
| primary follicle | preantral | 4 5 |
26 - 100 101 - 300 |
up to 200 | |
| secondary follicle | antral small antral large antral |
6 7 |
3001 - 500 501 - 1000 |
500 1000 - 6000 |
less than 18 |
| preovulatory follicle | Graafian | 8 | greater than 1000 | greater than 6000 | 18 – 28 |
| Links: ovary | oocyte | menstrual cycle | |||||
Atresia
At any one time the majority of follicles are destined not to complete maturation and at any stage (from type 4-7) degeneration of the follicle can occur. Cells die by apoptosis.
Follicle Growth
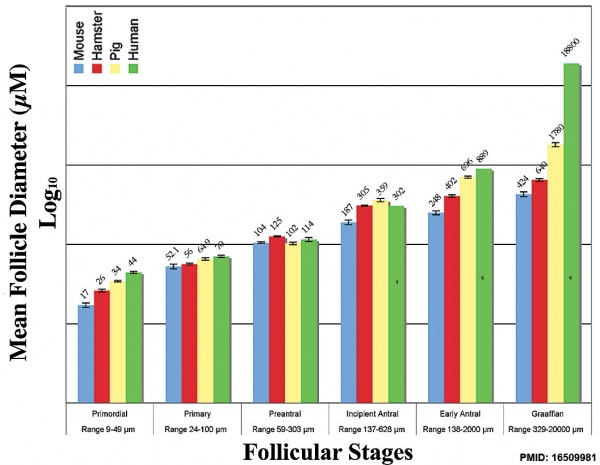
Graph shows species comparison in follicle size growth (diameter) at different stages of follicle development.[17] (See also Oocyte size graph)
| Human Ovarian Follicle Classification | |||||
| Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) | Size ultrasound (mm) |
|---|---|---|---|---|---|
| primordial follicle | small | 1, 2, 3 | 25 | less than 50 | |
| primary follicle | preantral | 4 5 |
26 - 100 101 - 300 |
up to 200 | |
| secondary follicle | antral small antral large antral |
6 7 |
3001 - 500 501 - 1000 |
500 1000 - 6000 |
less than 18 |
| preovulatory follicle | Graafian | 8 | greater than 1000 | greater than 6000 | 18 – 28 |
| Links: ovary | oocyte | menstrual cycle | |||||
Follicle Factors
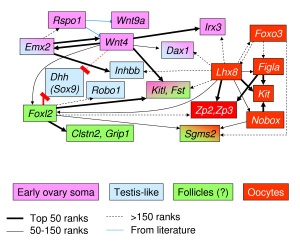
There are both external endocrine factors and internal follicle factors that can influence the development and atresia of ovarian follicles.
External Factors
Leutenizing Hormone (LH)
- from the anterior pituitary
- stimulate the theca interna to synthesize and secrete androgens (androstendione) transported to granulosa cells
- granulosa cells process initially into testosterone and then by aromatase into estrogen (estradiol)
Follicle-stimulating hormone (FSH)
- from the anterior pituitary
- initiates follicle growth through the granulosa cells
- involved in selecting the most advanced (sensitive) follicle to proceed to ovulation
Internal Factors
Oocyte Factors
- Growth Differentiation Factor-9 (GDF-9) - involved in the differentiation of theca cells during this early stage of follicular development OMIM 601918
- Bone morphogenetic protein 15 (BMP15)
- Fibroblast growth factor 8B (FGF8B)
Granulosal Factor(s)
- stimulates the recruitment of theca cells from cortical stromal cells
Thecal Factor(s)
- appear to be several inhibitors of apoptotic cell death
- Epidermal growth factor (EGF)
- Transforming growth factor alpha (TGF-α)
- keratinocyte growth factor (KGF)
- hepatocyte growth factor (HGF)
- Bone morphogenetic protein 7 (BMP-7) also known as osteogenic protein-1 or OP-1
Molecular
| Gene (OMIM) | Protein Function | Gonad Phenotype of Null Mice | Human Syndrome | |
| Ovary-determining pathway | ||||
| Wnt4 | Signaling molecule | Müllerian duct agenesis, testosterone synthesis, and coelomic vessel formation | XY female (GOF) | |
| FoxL2 | Transcription factor | Premature ovarian failure | BPES | |
| Dax1 | Nuclear receptor | XY sex reversal (GOF) | XY sex reversal (GOF) | |
| RSPO1 | Signaling molecule | XX sex reversal (LOF) | XX sex reversal (LOF) | |
| Table Legend | ||||
|
a No mutations in human sexual disorders identified to date.
b Candidate gene for 9p deletion, XY sex reversal. | |||
| Table modified from [19] | ||||
Ovary Growth
Table below is from historic data (1912) from measurement of human histological materials.[20]
| Table showing the Growth of the Ovary | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vertex-breech length |
Greatest diam. of head |
Right Ovary | Left Ovary | Comparison between | |||||
| Breadth | Length | Breadth | Length | Length | Breadth | ||||
| R. | L. | R. | L. | ||||||
| 50.0 | 42.6 | 0.9 | 1.9 | 0.9 | 2.5 | .. | + | — | — |
| 125.0 | 123.0 | 1.2 | 5.9 | 1.5 | 4.1 | + | .. | .. | + |
| 138.0 | 115.0 | 1.6 | 5.0 | 2.0 | 5.0 | — | — | .. | + |
| 156.0 | 131.0 | 1.9 | 7.2 | 2.0 | 7.1 | + | .. | .. | + |
| 173.0 | 163.5 | 3.0 | 9.0 | ... | ... | .. | .. | .. | .. |
| 190.0 | 175.0 | 2.9 | 7.7 | 2.1 | 7.8 | .. | + | + | .. |
| 223.0 | 162.0 | 2.9 | 10.5 | 3.0 | 9.1 | + | .. | .. | + |
| 235.0 | 190.0 | 4.2 | 10.0 | 3.8 | 12.0 | .. | + | + | .. |
| 260.0 | 213.0 | 3.6 | 11.1 | 4.0 | 11.4 | .. | + | .. | + |
| 272.0 | 213.0 | 3.0 | 10.0 | 3.5 | 9.2 | + | .. | .. | + |
| 305.0 | 238.0 | 3.0 | 9.9 | 3.9 | 10.9 | .. | + | .. | + |
| 347.0 | ... | 3.5 | 10.8 | 4.9 | 8.5 | + | .. | .. | + |
| 355.0 | 273.0 | 4.0 | 14.0 | 5.2 | 9.9 | + | .. | .. | + |
| 386.0 | 324.0 | 5.1 | 11.5 | 3.0 | 9.9 | + | .. | + | .. |
| 402.0 | 301.0 | 5.05 | 10.5 | 3.0 | 12.0 | .. | + | + | .. |
| 3 weeks | ... | 5.0 | 17.0 | 5.0 | 14.0 | + | .. | — | — |
| 6 weeks | ... | 7.5 | 15.0 | 7.0 | 14.7 | + | .. | + | .. |
| 6 weeks | ... | 7.0 | 18.0 | 8.0 | 17.0 | + | .. | .. | + |
| 10 weeks | ... | ... | 14.0 | ... | 16.0 | + | .. | — | — |
| 2 months | ... | 6.0 | 14.5 | 4.0 | 13.0 | + | .. | + | .. |
| 3 months | ... | 6.0 | 15.5 | 5.0 | 14.7 | + | .. | + | .. |
| 7 months | ... | 5.9 | 15.5 | 4.5 | 18.1 | .. | + | + | .. |
| 15 months | ... | 9.0 | 18.0 | 9.0 | 19.5 | + | .. | — | — |
| I.75 years | ... | 7.0 | 20.0 | 8.5 | 15.0 | + | .. | .. | + |
| 4 years | ... | 10.0 | 27.0 | 12.7 | 23.2 | + | .. | .. | + |
| 5.5 years | ... | 11.1 | 29.0 | 9.1 | 26.1 | + | .. | + | .. |
| 14 years | ... | 11.9 | 26.5 | 12.0 | 29.5 | .. | + | .. | + |
| The measurements are all given in millimeters, breech length is measured along the nape and the back. | |||||||||
| Links: 1912 Ovary Growth | Table Ovary Growth Table | Collapsible Table | Ovary Development | |||||||||
| Table showing the Growth of the Ovary | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vertex-breech length |
Greatest diam. of head |
Right Ovary | Left Ovary | Comparison between | |||||
| Breadth | Length | Breadth | Length | Length | Breadth | ||||
| R. | L. | R. | L. | ||||||
| 50.0 | 42.6 | 0.9 | 1.9 | 0.9 | 2.5 | .. | + | — | — |
| 125.0 | 123.0 | 1.2 | 5.9 | 1.5 | 4.1 | + | .. | .. | + |
| 138.0 | 115.0 | 1.6 | 5.0 | 2.0 | 5.0 | — | — | .. | + |
| 156.0 | 131.0 | 1.9 | 7.2 | 2.0 | 7.1 | + | .. | .. | + |
| 173.0 | 163.5 | 3.0 | 9.0 | ... | ... | .. | .. | .. | .. |
| 190.0 | 175.0 | 2.9 | 7.7 | 2.1 | 7.8 | .. | + | + | .. |
| 223.0 | 162.0 | 2.9 | 10.5 | 3.0 | 9.1 | + | .. | .. | + |
| 235.0 | 190.0 | 4.2 | 10.0 | 3.8 | 12.0 | .. | + | + | .. |
| 260.0 | 213.0 | 3.6 | 11.1 | 4.0 | 11.4 | .. | + | .. | + |
| 272.0 | 213.0 | 3.0 | 10.0 | 3.5 | 9.2 | + | .. | .. | + |
| 305.0 | 238.0 | 3.0 | 9.9 | 3.9 | 10.9 | .. | + | .. | + |
| 347.0 | ... | 3.5 | 10.8 | 4.9 | 8.5 | + | .. | .. | + |
| 355.0 | 273.0 | 4.0 | 14.0 | 5.2 | 9.9 | + | .. | .. | + |
| 386.0 | 324.0 | 5.1 | 11.5 | 3.0 | 9.9 | + | .. | + | .. |
| 402.0 | 301.0 | 5.05 | 10.5 | 3.0 | 12.0 | .. | + | + | .. |
| 3 weeks | ... | 5.0 | 17.0 | 5.0 | 14.0 | + | .. | — | — |
| 6 weeks | ... | 7.5 | 15.0 | 7.0 | 14.7 | + | .. | + | .. |
| 6 weeks | ... | 7.0 | 18.0 | 8.0 | 17.0 | + | .. | .. | + |
| 10 weeks | ... | ... | 14.0 | ... | 16.0 | + | .. | — | — |
| 2 months | ... | 6.0 | 14.5 | 4.0 | 13.0 | + | .. | + | .. |
| 3 months | ... | 6.0 | 15.5 | 5.0 | 14.7 | + | .. | + | .. |
| 7 months | ... | 5.9 | 15.5 | 4.5 | 18.1 | .. | + | + | .. |
| 15 months | ... | 9.0 | 18.0 | 9.0 | 19.5 | + | .. | — | — |
| I.75 years | ... | 7.0 | 20.0 | 8.5 | 15.0 | + | .. | .. | + |
| 4 years | ... | 10.0 | 27.0 | 12.7 | 23.2 | + | .. | .. | + |
| 5.5 years | ... | 11.1 | 29.0 | 9.1 | 26.1 | + | .. | + | .. |
| 14 years | ... | 11.9 | 26.5 | 12.0 | 29.5 | .. | + | .. | + |
| The measurements are all given in millimeters, breech length is measured along the nape and the back. | |||||||||
| Reference: Felix W. The development of the urinogenital organs. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp 752-979. | |||||||||
| Links: 1912 Ovary Growth | Table Ovary Growth Table | Collapsible Table | Ovary Development | |||||||||
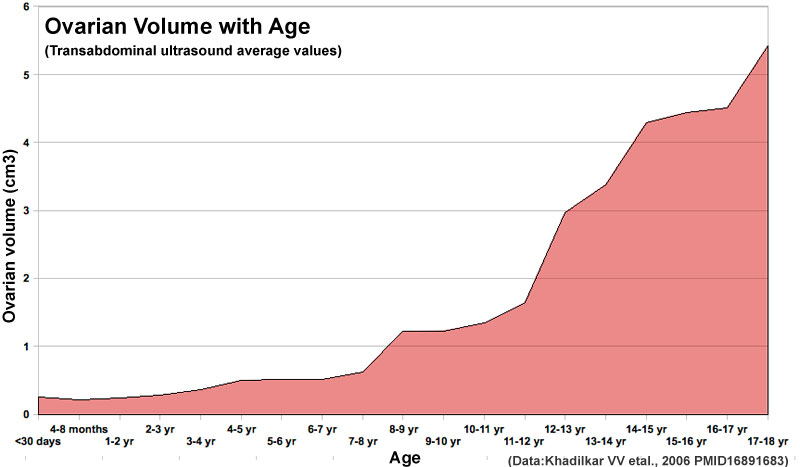
Postnatal Growth
Human ovary postnatal volume growth[21]
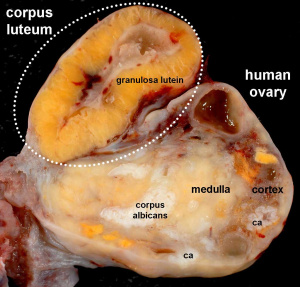
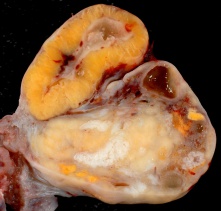
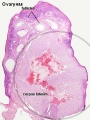
Corpus Luteum
The corpus luteum (Latin, corpus = body, luteum = yellow) develops from the remains of Graffian follicle after ovulation. Functions as an endocrine organ (produce progesterone and oestrogens) supporting pregnancy and preventing menstruation (loss of the endometrial lining). Formed during the luteal phase (secretory phase) of the menstrual cycle by proliferation of both follicular granulosa cells (granulosa lutein cells) and thecal cells (theca lutein cells), which together interact to produce progesterone and oestrogens.
Peak luteal function during the menstrual cycle, determined by maximum luteal area, progesterone concentration and estradiol concentration, is observed about 6 days following ovulation.[22]
If fertilization and pregnancy does not occur, the corpus luteum degenerates to form the corpus albicans.
History
- Regnier de Graaf (1641 – 1673) was the first observer in the ovary of a cow as a yellow structure, the yellow colour was caused by accumulation of steroidal hormones.
- Ludwig Fraenkel (1870 - 1951) first identified the endocrine function of the corpus luteum.[23]
Embryo Virtual Slide
| Human Ovary and Corpus Luteum |
| Ovary | Embryo Slides |
| Corpus Luteum Links: anatomy overview | image - histology overview | image - Layers granulosa and theca | image - Layers detail granulosa and theca | image - low power label | image - high power label | image - low power | image - high power | image - corpus albicans | theca and granulosa lutein cells | Granulosa cell | corpus luteum | granulosa lutein cells | theca lutein cells | corpus albicans | ovary | menstrual cycle |
| Historic Papers: 1969 corpus luteum ultrastructure 1 | 1969 corpus luteum ultrastructure 2 |
Corpus Albicans
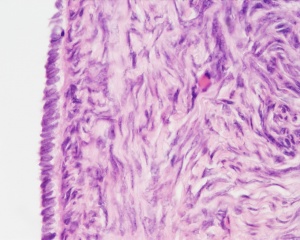
Corpus albicans histology
(corpora albicantia) (Latin, corpus = body, albicans = whitish) The histological structure formed by luteolysis of the corpus luteum in the ovary. If implantation does not occur and the hormone hCG is not released the corpus luteum degenerates and the structure is white, not yellow, because of the absence of steroid hormone synthesis/accumulation.
| Corpus Luteum Links: anatomy overview | image - histology overview | image - Layers granulosa and theca | image - Layers detail granulosa and theca | image - low power label | image - high power label | image - low power | image - high power | image - corpus albicans | theca and granulosa lutein cells | Granulosa cell | corpus luteum | granulosa lutein cells | theca lutein cells | corpus albicans | ovary | menstrual cycle |
| Historic Papers: 1969 corpus luteum ultrastructure 1 | 1969 corpus luteum ultrastructure 2 |
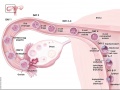
Animal Models
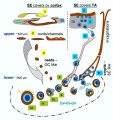
Bovine Ovary
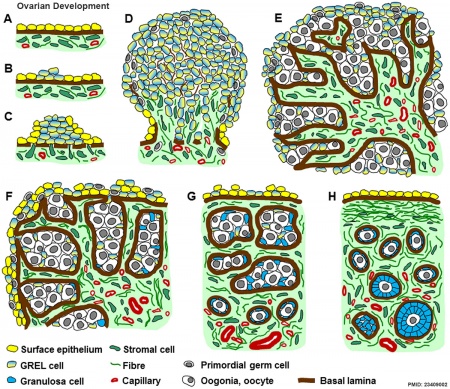
|
Ovarian Development Model[24]
|
Histology
Ovary histology: Tunica Albuginea x20 | Tunica albuginea, Germinal epithelium x40 | Primary follicle, primordial follicle, oocyte, x40 | Secondary follicle, cumulus oophorus, zona pelucida, granulosa cells, oocyte x20 | Corpus luteum, theca lutein cells, granulosa lutein cells, Loupe | Corpus luteum, theca lutein cells, granulosa lutein cells, x10 | Corpus luteum, theca lutein cells, granulosa lutein cells, x40 | Corpus albicans, primary follicle, primordial follicle, granulosa cells, oocyte x20 | Menstrual Cycle | Ovary Development
Germinal Epithelium
(germinal epithelium of Waldeyer) A layer of simple cuboidal epithelium covering the ovary. Arising from mesoderm and related to the mesothelium of the peritoneum.
This is a historic misnomer, based upon Waldeyer's (1870) hypothesis that germ cells arise from this coelomic epithelium (More? Gray Fig 1113). This epithelium has no role in the formation of the oocyte, formed developmentally from primordial germ cells migrating into the ovary.
In contrast, a similar named "epithelial" tissue layer is found in male genital system within the seminiferous tubules. This is the layer where the spermatozoa develop.
Tunica Albuginea
A dense connective tissue layer lying near the ovary surface, that is covered by a layer of simple cuboidal epithelium. A similar connective tissue layer is found in male genital system, as fibrous envelope covering the penis corpora cavernosa and corpus spongiosum.
Abnormalities
See also Genital System - Abnormalities
Female Infertility Genes
| Gene abbreviation | Name | Gene Location | Online Mendelian Inheritance in Man (OMIM) |
HUGO Gene Nomenclature Committee (HGNC) |
GeneCards (GCID) | Diagnosis |
|---|---|---|---|---|---|---|
| BMP15 | Bone morphogenetic protein 15 | Xp11.22 | 300247 | 1068 | GC0XP050910 | Primary ovarian insufficiency |
| CLPP | Caseinolytic mitochondrial matrix peptidase proteolytic subunit | 19p13.3 | 601119 | 2084 | GC19P006369 | Primary ovarian insufficiency |
| EIF2B2 | Eukaryotic translation initiation factor 2B subunit beta | 14q24.3 | 606454 | 3258 | GC14P075002 | Primary ovarian insufficiency |
| FIGLA | Folliculogenesis-specific BHLH transcription factor | 2p13.3 | 608697 | 24669 | GC02M070741 | Primary ovarian insufficiency |
| FMR1 | Fragile X mental retardation 1 | Xq27.3 | 309550 | 3775 | GC0XP147912 | Primary ovarian insufficiency |
| FOXL2 | Forkhead box L2 | 3q22.3 | 605597 | 1092 | GC03M138944 | Primary ovarian insufficiency |
| FSHR | Follicle stimulating hormone receptor | 2p16.3 | 136435 | 3969 | GC02M048866 | Primary ovarian insufficiency |
| GALT | Galactose-1-phosphate uridylyltransferase | 9p13.3 | 606999 | 4135 | GC09P034636 | Primary ovarian insufficiency |
| GFD9 | Growth differentiation factor 9 | 5q31.1 | 601918 | 4224 | GC05M132861 | Primary ovarian insufficiency |
| HARS2 | Histidyl-TRNA synthetase 2, mitochondrial | 5q31.3 | 600783 | 4817 | GC05P141975 | Primary ovarian insufficiency |
| HFM1 | HFM1, ATP-dependent DNA helicase homolog | 1p22.2 | 615684 | 20193 | GC01M091260 | Primary ovarian insufficiency |
| HSD17B4 | Hydroxysteroid 17-beta dehydrogenase 4 | 5q23.1 | 601860 | 5213 | GC05P119452 | Primary ovarian insufficiency |
| LARS2 | Leucyl-TRNA synthetase 2, mitochondrial | 3p21.31 | 604544 | 17095 | GC03P045405 | Primary ovarian insufficiency |
| LHCGR | Luteinizing hormone/choriogonadotropin receptor | 2p16.3 | 152790 | 6585 | GC02M048647 | Primary ovarian insufficiency |
| LHX8 | LIM homeobox 8 | 1p31.1 | 604425 | 28838 | GC01P075128 | Primary ovarian insufficiency |
| MCM8 | Minichromosome maintenance 8 homologous recombination repair factor | 20p12.3 | 608187 | 16147 | GC20P005926 | Primary ovarian insufficiency |
| MCM9 | Minichromosome maintenance 9 homologous recombination repair factor | 6q22.31 | 610098 | 21484 | GC06M118813 | Primary ovarian insufficiency |
| NOBOX | NOBOX oogenesis homeobox | 7q35 | 610934 | 22448 | GC07M144397 | Primary ovarian insufficiency |
| NOG | Noggin | 17q22 | 602991 | 7866 | GC17P056593 | Primary ovarian insufficiency |
| PMM2 | Phosphomannomutase 2 | 16p13.2 | 601785 | 9115 | GC16P008788 | Primary ovarian insufficiency |
| POLG | DNA polymerase gamma, catalytic subunit | 15q26.1 | 174763 | 9179 | GC15M089316 | Primary ovarian insufficiency |
| REC8 | REC8 meiotic recombination protein | 14q12 | 608193 | 16879 | GC14P024171 | Primary ovarian insufficiency |
| SMC1B | Structural maintenance of chromosomes 1B | 22q13.31 | 608685 | 11112 | GC22M045344 | Primary ovarian insufficiency |
| SOHLH1 | Spermatogenesis and oogenesis-specific basic helix–loop–helix 1 | 9q34.3 | 610224 | 27845 | GC09M135693 | Primary ovarian insufficiency |
| STAG3 | Stromal antigen 3 | 7q22.1 | {{Chr 608489 | 11356 | GC07P100177 | Primary ovarian insufficiency |
| SYCE1 | Synaptonemal Complex Central Element Protein 1 | 10q26.3 | 611486 | 28852 | GC10M133553 | Primary ovarian insufficiency |
| TLE6 | Transducin-like enhancer of split 6 | 19p13.3 | 612399 | 30788 | GC19P002976 | Embryonic lethalithy |
| TUBB8 | Tubulin beta 8 Class VIII | 10p15.3 | 616768 | 20773 | GC10M000048 | Oocyte maturation arrest |
| TWNK | Twinkle MtDNA helicase | 10q24.31 | 606075 | 1160 | GC10P100991 | Primary ovarian insufficiency |
| Table data source[25] (table 1) Links: fertilization | oocyte | ovary | | Female Infertility Genes | spermatozoa | testis | Male Infertility Genes | Genetic Abnormalities | ART Primary ovarian insufficiency - depletion or dysfunction of ovarian follicles with cessation of menses before age 40 years. | ||||||
International Classification of Diseases
| ICD-11 5A80 Ovarian dysfunction |
|---|
|
Polycystic Ovary Syndrome
ICD-11 5A80.1 Polycystic ovary syndrome | 5A80.2 Polycystic ovary - Ovary with increased size (> 7 mL) and stromal volume, and with increased number of follicles (12 or more measuring 2-0 mm in diameter), that may be present in women with PCOS, but also in women with normal ovulatory function and normal fertility (unilaterally or bilaterally).
Polycystic ovary syndrome (PCOS) or Stein-Leventhal syndrome (1930s researchers) clinical term for a metabolic hormone syndrome leading to anovulation and with many other symptoms (hyperandrogenism, insulin resistance) and is one of the most common forms of female infertility, see reviews.[27][28] Using the European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine criteria, about 15% - 20% of women suffer from this disease. Ovarian cysts arise through incomplete follicular development or failure of ovulation (anovulation). A range of drugs has been used to induce ovulation in these women and more recently in vitro maturation (IVM) has also been suggested as a possible future technique. Androgen has been recently identified as a key mediator in the development of polycystic ovary syndrome.[29]
Recently in 2018 an Australian-led PCOS international evidence-based guideline for the diagnosis and management of polycystic ovary syndrome (PCOS) was developed for health professionals and consumers.[30]
In December 2012, the NIH held a workshop on "Evidence-based Methodology on Polycystic Ovary Syndrome". PCOS is a common disorder affecting 5 million women of reproductive-age in the United States. Symptoms include: irregular or no menstrual periods in women of reproductive age (ovulatory dysfunction), acne, weight gain, excess hair growth on the face and body (hirsutism), thinning scalp hair, ovarian cysts (polycystic ovarian morphology) and mental health problems.
One key workshop finding was that the name "Polycystic Ovary Syndrome" is inappropriate for defining the condition and should be renamed to reflect the complex metabolic, hypothalamic, pituitary, ovarian, and adrenal interactions that characterize the syndrome and their reproductive implications.
- Androgen Excess + Ovulatory Dysfunction
- Androgen Excess + Polycystic Ovarian Morphology
- Ovulatory Dysfunction + Polycystic Ovarian Morphology
- Androgen Excess + Ovulatory Dysfunction + Polycystic Ovarian Morphology
Recurrent pregnancy loss also occurs in about 50% of total pregnancies with polycystic ovary syndrome.[31]
A recent study in the Han Chinese population[32] identified associations between PCOS and three genetic loci: 2p16.3, 2p21 and 9q33.3.
Introduction to PCOS
<html5media width="480" height="360">https://www.youtube.com/embed/NhyYZCBq5A8</html5media>
- Links: menstrual cycle | genital abnormalities | endocrine abnormalities | 2012 NIH Workshop on PCOS | 2011 Australia Guideline assessment and management PCOS | OMIM 184700]
Premature Ovarian Failure
Premature Ovarian Failure (POF)[33] a clinical term describes the absence of normal ovarian function due to the depletion of the primordial follicle pool before 40 years of age a range of factors (autoimmune, iatrogenic, infections, genetic defects). Primary ovarian insufficiency (POI) occurs in approximately 1% of women below 40 years of age.
Premature Ovarian Failure (POF) can be primary or secondary based on puberty.
- primary - absence of puberty, development and primary amenorrhea, generally caused by ovarian dysgenesis (45XO, Turner syndrome, Monosomy X).
- secondary - normal puberty, usually present with the later disappearance of menstrual cycles.
Search PubMed: Premature Ovarian Failure
Ovarian Cancer
Ovarian cancer (Template:ICD–10 C56) is a major cause of death in the postnatal female population.[34][35][36] Both BRCA1 and BRCA2 genes are high-risk genes of ovarian cancer. A recent mouse study has also identified a population of stem cells located at the hilum region of the ovary that are prone to epithelial ovarian cancer.[37]
In Australia, ovarian cancer was in 2014 the 8th most commonly diagnosed cancer among females and 2018 estimate is that it will become the 10th most commonly diagnosed cancer among females.[38]
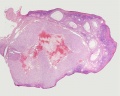
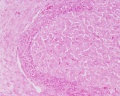
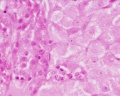
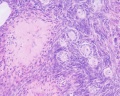
Luteoma of Pregnancy
Luteoma of pregnancy is a rare non-neoplastic tumor-like mass of the ovary that emerges during pregnancy and regresses spontaneously after delivery. Luteomas can be hormonally active producing androgens that can result in maternal and fetal hirsutism and virilization.
Image: Dr Ed Uthman (Houston, Texas) - other pathology images
References
- ↑ 1.0 1.1 1.2 1.3 Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S, Hovatta O, Kere J, Lanner F & Damdimopoulou P. (2020). Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun , 11, 1147. PMID: 32123174 DOI.
- ↑ McKey J, Bunce C, Batchvarov IS, Ornitz DM & Capel B. (2019). Neural crest-derived neurons invade the ovary but not the testis during mouse gonad development. Proc. Natl. Acad. Sci. U.S.A. , 116, 5570-5575. PMID: 30819894 DOI.
- ↑ Dalman A, Totonchi M & Valojerdi MR. (2018). Establishment and characterization of human theca stem cells and their differentiation into theca progenitor cells. J. Cell. Biochem. , , . PMID: 30132968 DOI.
- ↑ Meseke M, Pröls F, Schmahl C, Seebo K, Kruse C, Brandt N, Fester L, Zhou L, Bender R & Rune GM. (2018). Reelin and aromatase cooperate in ovarian follicle development. Sci Rep , 8, 8722. PMID: 29880879 DOI.
- ↑ Escobar-Morreale HF. (2018). Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol , 14, 270-284. PMID: 29569621 DOI.
- ↑ Bothun A, Gao Y, Takai Y, Ishihara O, Seki H, Karger B, Tilly J & Woods DC. (2018). Quantitative proteomic profiling of the human ovary from early to mid-gestation reveals protein expression dynamics of oogenesis and folliculogenesis. Stem Cells Dev. , , . PMID: 29631484 DOI.
- ↑ Walters KA & Handelsman DJ. (2017). Role of androgens in the ovary. Mol. Cell. Endocrinol. , , . PMID: 28687450 DOI.
- ↑ Müller A, Keller K, Wacker J, Dittrich R, Keck G, Montag M, Van der Ven H, Wachter D, Beckmann MW & Distler W. (2012). Retransplantation of cryopreserved ovarian tissue: the first live birth in Germany. Dtsch Arztebl Int , 109, 8-13. PMID: 22282711 DOI.
- ↑ Baillet A & Mandon-Pepin B. (2012). Mammalian ovary differentiation - a focus on female meiosis. Mol. Cell. Endocrinol. , 356, 13-23. PMID: 21964319 DOI.
- ↑ Tomaselli S, Megiorni F, Lin L, Mazzilli MC, Gerrelli D, Majore S, Grammatico P & Achermann JC. (2011). Human RSPO1/R-spondin1 is expressed during early ovary development and augments β-catenin signaling. PLoS ONE , 6, e16366. PMID: 21297984 DOI.
- ↑ Childs AJ, Cowan G, Kinnell HL, Anderson RA & Saunders PT. (2011). Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS ONE , 6, e20249. PMID: 21674038 DOI.
- ↑ 12.0 12.1 Wallace WH & Kelsey TW. (2010). Human ovarian reserve from conception to the menopause. PLoS ONE , 5, e8772. PMID: 20111701 DOI.
- ↑ Heeren AM, He N, de Souza AF, Goercharn-Ramlal A, van Iperen L, Roost MS, Gomes Fernandes MM, van der Westerlaken LA & Chuva de Sousa Lopes SM. (2016). On the development of extragonadal and gonadal human germ cells. Biol Open , 5, 185-94. PMID: 26834021 DOI.
- ↑ Hultén MA, Patel S, Jonasson J & Iwarsson E. (2010). On the origin of the maternal age effect in trisomy 21 Down syndrome: the Oocyte Mosaicism Selection model. Reproduction , 139, 1-9. PMID: 19755486 DOI.
- ↑ Orisaka M, Tajima K, Tsang BK & Kotsuji F. (2009). Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res , 2, 9. PMID: 19589134 DOI.
- ↑ Hertig AT. and Adams EC. Studies on the human oocyte and its follicle. I. Ultrastructural and histochemical observations on the primordial follicle stage. (1967) J Cell Biol. 34(2):647-75. PMID 4292010
- ↑ Griffin J, Emery BR, Huang I, Peterson CM & Carrell DT. (2006). Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J. Exp. Clin. Assist. Reprod. , 3, 2. PMID: 16509981 DOI.
- ↑ Garcia-Ortiz JE, Pelosi E, Omari S, Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole SW, Forabosco A, Schlessinger D & Ottolenghi C. (2009). Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC Dev. Biol. , 9, 36. PMID: 19538736 DOI.
- ↑ Wilhelm D, Palmer S & Koopman P. (2007). Sex determination and gonadal development in mammals. Physiol. Rev. , 87, 1-28. PMID: 17237341 DOI.
- ↑ Felix W. The development of the urinogenital organs. In Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia. pp 752-979.
- ↑ Khadilkar VV, Khadilkar AV, Kinare AS, Tapasvi HS, Deshpande SS & Maskati GB. (2006). Ovarian and uterine ultrasonography in healthy girls between birth to 18 years. Indian Pediatr , 43, 625-30. PMID: 16891683
- ↑ Baerwald AR, Adams GP & Pierson RA. (2005). Form and function of the corpus luteum during the human menstrual cycle. Ultrasound Obstet Gynecol , 25, 498-507. PMID: 15846762 DOI.
- ↑ Simmer HH. (1971). The first experiments to demonstrate an endocrine function of the corpus luteum. On the occasion of the 100th birthday of Ludwig Fraenkel (1870-1951). Sudhoffs Arch , 55, 392-417. PMID: 4261581
- ↑ Hummitzsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier L, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D & Rodgers RJ. (2013). A new model of development of the mammalian ovary and follicles. PLoS ONE , 8, e55578. PMID: 23409002 DOI.
- ↑ Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I, Lundin K, Mertes H, Morris M, Pennings G, Sermon K, Spits C, Soini S, van Montfoort APA, Veiga A, Vermeesch JR, Viville S & Macek M. (2018). Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur. J. Hum. Genet. , 26, 12-33. PMID: 29199274 DOI.
- ↑ Yaba A & Demir N. (2012). The mechanism of mTOR (mammalian target of rapamycin) in a mouse model of polycystic ovary syndrome (PCOS). J Ovarian Res , 5, 38. PMID: 23185989 DOI.
- ↑ Sirmans SM & Pate KA. (2013). Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol , 6, 1-13. PMID: 24379699 DOI.
- ↑ Azziz R & Adashi EY. (2016). Stein and Leventhal: 80 years on. Am. J. Obstet. Gynecol. , 214, 247.e1-247.e11. PMID: 26704896 DOI.
- ↑ Caldwell ASL, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ & Walters KA. (2017). Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc. Natl. Acad. Sci. U.S.A. , 114, E3334-E3343. PMID: 28320971 DOI.
- ↑ Teede HJ, Misso ML, Boyle JA, Garad RM, McAllister V, Downes L, Gibson-Helm M, Hart RJ, Rombauts L, Moran L, Dokras A, Laven J, Piltonen T, Rodgers RJ, Thondan M, Costello MF & Norman RJ. (2018). Translation and implementation of the Australian-led PCOS guideline: clinical summary and translation resources from the International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Med. J. Aust. , 209 Suppl 7, S3-S8. PMID: 31210359 DOI.
- ↑ Chakraborty P, Goswami SK, Rajani S, Sharma S, Kabir SN, Chakravarty B & Jana K. (2013). Recurrent pregnancy loss in polycystic ovary syndrome: role of hyperhomocysteinemia and insulin resistance. PLoS ONE , 8, e64446. PMID: 23700477 DOI.
- ↑ Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Zhao J, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Li Y, Ma J & Zhao Y. (2011). Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat. Genet. , 43, 55-9. PMID: 21151128 DOI.
- ↑ Beck-Peccoz P & Persani L. (2006). Premature ovarian failure. Orphanet J Rare Dis , 1, 9. PMID: 16722528 DOI.
- ↑ Muinao T, Pal M & Deka Boruah HP. (2018). Origins based clinical and molecular complexities of epithelial ovarian cancer. Int. J. Biol. Macromol. , 118, 1326-1345. PMID: 29890249 DOI.
- ↑ Wilson MK, Mercieca-Bebber R & Friedlander M. (2018). A practical guide to understanding, using and including patient reported outcomes in clinical trials in ovarian cancer. J Gynecol Oncol , 29, e81. PMID: 30022641 DOI.
- ↑ Tajik P, van de Vrie R, Zafarmand MH, Coens C, Buist MR, Vergote I, Bossuyt PMM & Kenter GG. (2018). The FIGO Stage IVA Versus IVB of Ovarian Cancer: Prognostic Value and Predictive Value for Neoadjuvant Chemotherapy. Int. J. Gynecol. Cancer , 28, 453-458. PMID: 29324537 DOI.
- ↑ Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G & Nikitin AY. (2013). Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature , 495, 241-5. PMID: 23467088 DOI.
- ↑ AIHW Web report - Cancer compendium: information and trends by cancer type (2018). ovarian cancer
Reviews
Cox E & Whitten R. (2018). Embryology, Ovarian Follicle Development. , , . PMID: 30335333
Escobar-Morreale HF. (2018). Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol , 14, 270-284. PMID: 29569621 DOI.
Nef S & Vassalli JD. (2009). Complementary pathways in mammalian female sex determination. J. Biol. , 8, 74. PMID: 19735582 DOI.
Hultén MA, Patel S, Jonasson J & Iwarsson E. (2010). On the origin of the maternal age effect in trisomy 21 Down syndrome: the Oocyte Mosaicism Selection model. Reproduction , 139, 1-9. PMID: 19755486 DOI.
Articles
Lin HA, Dutta R, Mandal S, Kind A, Schnieke A & Razansky D. (2016). Advancing ovarian folliculometry with selective plane illumination microscopy. Sci Rep , 6, 38057. PMID: 27905503 DOI.
Geber S, Megale R, Vale F, Lanna AM & Cabral AC. (2012). Variation in ovarian follicle density during human fetal development. J. Assist. Reprod. Genet. , 29, 969-72. PMID: 22710858 DOI.
Duffin K, Bayne RA, Childs AJ, Collins C & Anderson RA. (2009). The forkhead transcription factor FOXL2 is expressed in somatic cells of the human ovary prior to follicle formation. Mol. Hum. Reprod. , 15, 771-7. PMID: 19706741 DOI.
Dontchev N. (1971). [The prenatal development of the human ovary]. Arch Anat Histol Embryol , 54, 183-90. PMID: 4950043
Search Pubmed
Search Pubmed: Ovary Development | Follicle Development | Follicle Atresia
Books
Leung P. and Adashi E. The Ovary (2019) 3rd Edn eBook ISBN: 9780128132104 Hardcover ISBN: 9780128132098 Academic Press.
- The Ovarian Follicular Apparatus: Operational Characteristics
- Oocyte Maturation and Ovulation
- The Corpus Luteum
- Novel Experimental Models
- Human Ovarian Pathophysiology: Select Aspects
- Human Ovarian Cancer
Gilbert SF. Developmental Biology. (2000) 6th edn. Sunderland (MA): Sinauer Associates.
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Terms
Note there are additional specific term glossaries available listed at bottom of this table.
- antral follicle - (secondary) the stage following preantral in the decription of the sequence ovarian follicle development.
- antrum - (L. a cave), cavity; a nearly-closed cavity or bulge. In the ovary this refers to the follicular fluid-filled space within the follicle.
- atretic follicle - An ovarian follicle that fails to mature and degenerates. Also called "atresia" refering to the process of degeneration of the ovarian follicle. This process can occur at any stage of follicle development (folliculogenesis).
- clomiphene citrate - drug taken orally to promote the process of follicle/egg maturation.
- COCs - (cumulus-oocyte complexes) term used in Assisted Reproductive Technology to describe the ovulated Graafian follicle consisting of the oocyte surrounded by a packed layers of cumulus cells.
- corona radiata - Layer of follicle cells of cumulus oophorus remaining directly attached to zona pellucida of the oocyte. These cells communicate with the oocyte through the zone pellucida, also called granulosa cells.
- corpus albicans - (L. corpus = body, L. albicans = whitish); a degenerating corpus luteum in ovary.
- corpus luteum - (L. corpus = body, L. luteum = yellow) The remains of the ovulating ovarian follicle after ovulation, that acts as the initial endocrine organ supporting pregnancy and preventing menstruation (loss of the endometrial lining). de Graaf first observed it in the ovary of a cow as a yellow structure.
- cortical - (L. corticalis) at the outside (like the bark of a tree), usually combined with medulla meaning the core.
- cumulus oophorus - (L. cumulus = a little mound G. oon = egg + phorus = bearing); part of the wall of an ovarian follicle surrounding and carrying the ovum (oocyte).
- dictyate arrest - (prophase arrest) the oocyte meiosis state before puberty resumed with a surge of pituitary luteinizing hormone.
- first polar body - a small cytoplasmic exclusion body contains the excess DNA from the oocyte meiosis formed during meiosis 1.
- follicle - (L. folliculus = little bag,dim. of L. follis). A structure which develops in the ovary and contains a developing egg (oocyte).
- follicle stimulating hormone - (FSH, gonadotropin) A glycoprotein hormone secreted by anterior pituitary (adenohypophysis gonadotrophs, a subgroup of basophilic cells) and acts on gametogenesis and other systems in both males and females. Females, FSH acts on the ovary to stimulate follicle development. Males, acts on the testis Sertoli cells to increase androgen-binding protein (ABP) that binds androgens and has a role in spermatogenesis. pituitary
- follicular fluid - the fluid found in the antrum of a secondary follicle. Secreted by cells in the wall of the follicle. This fluid is released along with the oocyte at ovulation.
- germinal epithelium - cellular component covering surface of ovary, it is continuous with mesothelium covering mesovarium. Note that it is a historical misnomer, as it is not the actual site of germ cell formation.
- Graafian follicle - named after Regnier de Graaf (1641-1673), an historic Dutch physician embryologist who studied pregnancy using rabbits.
- granulosa cells - the supporting cells that surround the developing egg within the follicle thecal layers.
- homologs - maternal and paternal homologous chromosomes.
- Izumo1 - a protein located on the equatorial segment of acrosome-reacted spermatozoa recognizes its receptor Juno, on the oocyte surface, for plasma membrane binding and fusion. Named for a Japanese shrine dedicated to marriage. OMIM609278
- Juno - (folate receptor-δ; FOLR-δ) a glycophosphatidylinositol (GPI)-anchored, cysteine-rich glycoprotein on the oocyte surface for fertilisation that is the receptor of Izumo1 on the spermatozoa, for plasma membrane binding and fusion. OMIM615737
- luteinizing hormone - (LH, gonadotropin, lutropin, Interstitial Cell Stimulating Hormone, ICSH) glycoprotein hormone releasd from anterior pituitary hormone that acts on the gonad and has a role in male and female reproduction. Female, LH triggers ovulation (release of the oocyte). Male, LH stimulates testis interstital cell (Leydig cell) production of testosterone. Have been used clinically in humans for the treatment of female infertility.
- meiosis - oocyte reductive (diploid to haploid) cell division, with 1 round of DNA replication is followed by 2 rounds of chromosome segregation. The process beginning in the fetus and only completed at fertilization.
- mesovarium - mesentry of the ovary formed from a fold of the broad ligament that attaches the ovary.
- medullary - (L. medius = in the middle) relating to the medulla; pith, marrow, inner portion of an organ. Usually combined with cortex (cortical) meaning the outer layer.
- oocyte - (Greek, oo = egg, ovum) The term used to describe the haploid egg or ovum formed within the ovary (female gonad) and released to enter the uterine tube and be transported to the uterus. The mature oocyte is the cell released from the ovary during ovulation.
- oocyte retrieval - (egg retrieval) A clinical in vitro fertilization (IVF) procedure to collect the eggs contained in the ovarian follicles.
- oogenesis - (Greek, oo = egg + genesis = origin, creation, generation) process of diploid oogonia division and differentiation into an haploid oocyte (egg) within the ovary (female gonad). Mammalian meiosis will only be completed within the oocyte if fertilization occurs.
- oogonia - (Greek, oo = egg) diploid germ cells within the ovary (female gonad) which provide the primary oocytes for oocyte (egg) formation. In humans, all oogonia form primary oocytes within the ovary before birth.
- oolemma - (zona pellucida, vitelline membrane).
- oophorus - (Greek, oo = egg + phorus = carrying, egg-bearing) cumulus oophorus, used to describe the granulosa cells within the follicle that tether or link the oocyte to the wall of the follicle.
- ovarian reserve - Clinical term for the number of oocytes (non-growing follicles) available for possible fertilization at the different times during female reproductive life. A blood test for Anti-Mullerian Hormone (AMH) levels is used clinically as a measure of the ovarian reserve. human graph
- ovastacin - an oocyte released enzyme following fertilization that cleaves ZP2 protein to prevent polyspermy.
- ovulation - release of the oocyte from the mature follicle. In humans generally a single oocyte is released from a cohort of several maturing follicles.
- ovum - oocyte, note that historically this same term was also used to describe the early stages following fertilisation.
- polar body - small cytoplasmic exclusion body contains the excess DNA from the oocyte meiosis reductive division. The first polar body formed during meiosis 1, the second and sometimes third polar bodies are formed from meiosis 2 at fertilization.
- polyspermy - abnormal fertilization by more that a single spermatozoa, may generate a hydatidiform mole.
- preantral follicle - (primary) the stage following primordial in the description of the sequence ovarian follicle development.
- primary follicle - (preantral) the stage following primordial in the description of the sequence ovarian follicle development.
- primordial follicle - the first stage in the description of the sequence ovarian follicle development. Present in the ovary from birth, located in the stroma of the ovary cortex beneath the tunica albuginea. The primordial follicle is the oocyte and the surrounding follicular cells.
- primordial germ cell - oocyte present in the primordial follicle ovary from birth, located in the stroma of the ovary cortex beneath the tunica albuginea. The primordial follicle is the oocyte and the surrounding follicular cells.
- second polar body - a small cytoplasmic exclusion body contains the excess DNA from the oocyte formed during meiosis 2 at fertilization.
- secondary follicles - the stage following primary in the description of the sequence ovarian follicle development.
- stromal cells - in the ovary, cells surrounding the developing follicle that form a connective tissue sheath (theca folliculi). This layer then differentiates into 2 layers (theca interna, theca externa). This region is richly vascularized and involved in hormone secretion.
- superovulation therapy - a fertility drug treatement (oral clomiphene citrate and/or injectable FSH with or without LH) aimed at stimulating development/release of more than one follicle during a single menstrual cycle.
- tertiary follicle - (preovulatory, Graffian) the stage following secondary in the description of the sequence ovarian follicle development.
- theca folliculi - stromal cells in the ovary, cells surrounding the developing follicle that form a connective tissue sheath. This layer then differentiates into 2 layers (theca interna, theca externa). This region is vascularized and involved in hormone secretion.
- theca externa - stromal cells forming the outer layer of the theca folliculi surrounding the developing follicle. Consisting of connective tissue cells, smooth muscle and collagen fibers.
- theca interna - stromal cells forming the inner layer of the theca folliculi surrounding the developing follicle. This vascularized layer of cells respond to LH (leutenizing hormone) synthesizing and secreting androgens which are processed into estrogen.
- transzonal projection - (TZP) ovarian follicle term describing the cellular membraneous extension from the granulosa cell through the zona pellucida to the oocyte cell membrane where it forms gap junctions or adherens junctions allowing signalling and adhesion between the two cells.
- tunica albuginea - dense connective tissue layer lying near the ovary surface, a layer of simple squamous to cuboidal epithelial covers this layer. A similar named structure is found in male genital system.
- uterus - site of embryo implantation and development. Uterine wall has 3 major layers: endometrium, myometrium, and perimetrium. Endometrium can be further divided into the functional layer (shed/lost during menstruation) and basal layer (not lost during menstruation).
- zinc sparks following fertilization the oocyte releases a burst of zinc atoms in brief bursts (zinc sparks) has a role in zonal pellucida induced structural changes (hardening) along with ovastacin cleavage of ZP2 protein.
- zona hardening - following fertilization the structural changes that occur to the zona pellucida to prevents further spermatozoa binding acting as a block to polyspermy.
- zona pellucida - extracellular layer lying directly around the oocyte underneath follicular cells. Has an important role in egg development, fertilization and blastocyst development. This thick extracellular matrix consists of glcosaminoglycans and 3 glycoproteins (ZP1, ZP2, ZP3). (More? Zona pellucida)
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Genital Links: genital | Lecture - Medicine | Lecture - Science | Lecture Movie | Medicine - Practical | primordial germ cell | meiosis | endocrine gonad | Genital Movies | genital abnormalities | Assisted Reproductive Technology | puberty | Category:Genital
| ||||
|
| Menstrual Cycle | X Chromosome
Cite this page: Hill, M.A. (2024, April 27) Embryology Ovary Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Ovary_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G