Neural - Hippocampus Development
| Embryology - 9 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Recent studies have shown that secondary brain vesicle (telencephalon) dorsomedial region described as the "cortical hem" is involved with hippocampus development.[1] The cortical hem is the source for 2 patterning signals; Wingless-related (WNT) and bone morphogenetic protein (BMP).
The adult hippocampus is associated with memory (contextual and working), emotional reactivity and the hypothalamus-pituitary-adrenal (HPA) axis function.
Neural development is one of the earliest systems to begin and the last to be completed after birth. This development generates the most complex structure within the embryo and the long time period of development means in utero insult during pregnancy may have consequences to development of the nervous system.
The early central nervous system begins as a simple neural plate that folds to form a groove then tube, open initially at each end. Failure of these opening to close contributes a major class of neural abnormalities (neural tube defects).
Within the neural tube stem cells generate the 2 major classes of cells that make the majority of the nervous system : neurons and glia. Both these classes of cells differentiate into many different types generated with highly specialized functions and shapes. This section covers the establishment of neural populations, the inductive influences of surrounding tissues and the sequential generation of neurons establishing the layered structure seen in the brain and spinal cord.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Hippocampus Development | Hippocampus Embryology | dentate gyrus development | |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Development Overview
Neuralation begins at the trilaminar embryo with formation of the notochord and somites, both of which underly the ectoderm and do not contribute to the nervous system, but are involved with patterning its initial formation. The central portion of the ectoderm then forms the neural plate that folds to form the neural tube, that will eventually form the entire central nervous system.
- Early developmental sequence: Epiblast - Ectoderm - Neural Plate - Neural groove and Neural Crest - Neural Tube and Neural Crest
| Neural Tube | Primary Vesicles | Secondary Vesicles | Adult Structures |
|---|---|---|---|
| week 3 | week 4 | week 5 | adult |
| prosencephalon (forebrain) | telencephalon | Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles | |
| diencephalon | epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle | ||
| mesencephalon (midbrain) | mesencephalon | tectum, Cerebral peduncle, cerebral aqueduct, pons | |
| rhombencephalon (hindbrain) | metencephalon | cerebellum | |
| myelencephalon | medulla oblongata, isthmus | ||
| spinal cord, pyramidal decussation, central canal | |||
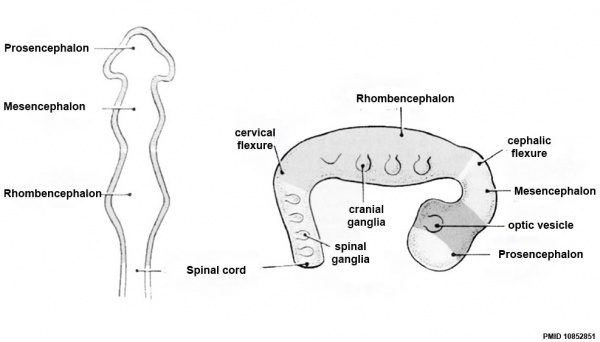
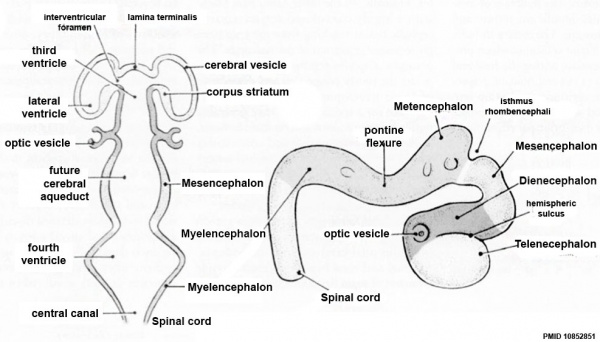
Early Brain Vesicles
Primary Vesicles
Secondary Vesicles
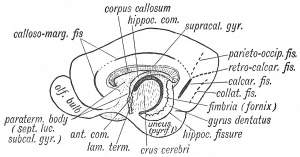
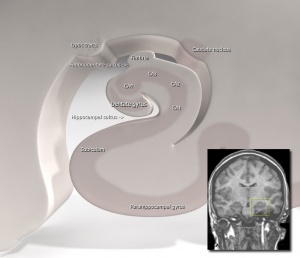
Human Hippocampus Development
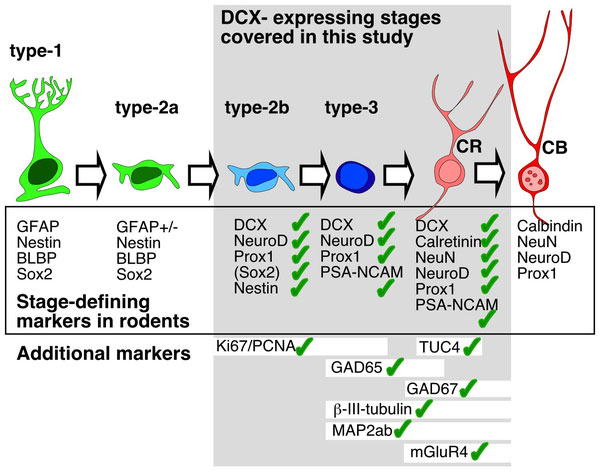
Human and rodent hippocampus developmental markers.[10]
Fetal
The following data (GA gestational age weeks) is from an imaging study of the human fetal hippocampus.[11]
- 13 to 14 weeks - unfolded hippocampus, on the medial surface of the temporal lobe, surrounds a widely open hippocampal sulcus (hippocampal fissure)
- 15 to 16 weeks - dentate gyrus and cornu ammonis have started to infold. The hippocampal sulcus remains open. The parahippocampal gyrus is larger and more medially positioned. The CA1, CA2, and CA3 fields of the cornu ammonis are arranged linearly. The dentate gyrus has a narrow U shape.
- 18 to 20 weeks - fetal hippocampus begins to resemble the adult hippocampus. The dentate gyrus and cornu ammonis have folded into the temporal lobe. The hippocampus and subiculum approximate each other across a narrow hippocampal sulcus. The CA1-3 fields form an arc and the CA4 field has increased in size within the widened arch of the dentate gyrus.
Childhood
The following data is from a postnatal magnetic resonance imaging study.[12]
- 3 months - a longitudinal fasciculus of high signal intensity was seen in the white matter beneath the subiculum
- birth to 2 years - volume increases rapidly
- after 2 years - volume increases slowly thereafter
- hippocampal formations on the right side were larger than those on the left in 38 cases (91%)
- anterior temporal lobes on the right were larger than those on the left in 32 cases (76%)
- right-left asymmetry of the hippocampal formations and anterior temporal lobes was observed from early infancy
Adult
The following data is from an magnetic resonance imaging study of adult Chinese aged 6 to 82 years.[13] Other studies have described a reduction in the hippocampal volume during ageing.
- volume of right hippocampus was larger than that of the left side (p<0.001)
- right side volume of hippocampus - 2.204 to 2.944 cm3
- left side volume of hippocampus - 2.068 to 2.700 cm3
- no statistically significant differences among different age and gender groups (p>0.05)
Adult hippocampal neurogenesis is influenced by both external stimuli and internal growth factors (BDNF, VEGF and IGF-1).[14]
Neuronal Development
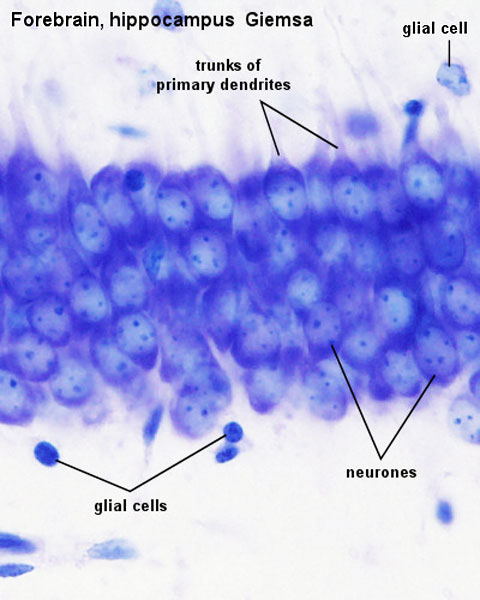
The following data is from a histological study of postmortem hippocampi neurons.[15]
- Bilateral coronal sections from postmortem hippocampus, 24 to 76 weeks postmenstrual age (gestational age plus postnatal age), were studied.
- Cell body (soma) size correlated positively and significantly with age in CA2 and CA3, bilaterally.
- CA2 somata were significantly larger (left, 34%; right, 32%) than adjacent CA3 somata.
- Variability in soma form or size increased appreciably with age, in both subfields, bilaterally
- Variability in soma orientation was weakly correlated with brain growth.
Mouse Hippocampus
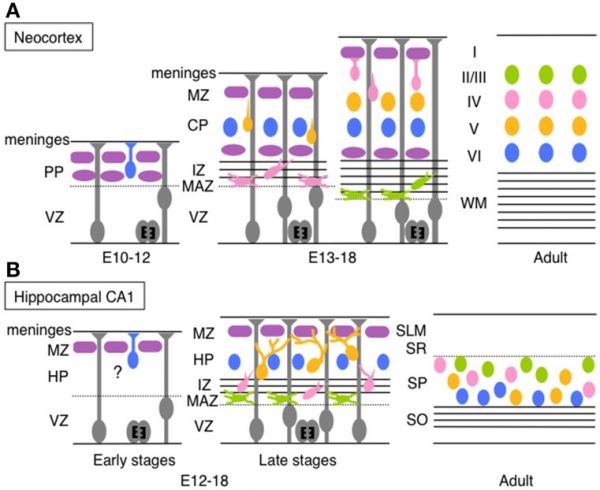
| Timeline comparison of the migration and layer arrangement in neocortex and hippocampal CA1 during cortical development[16] | |
|
(A) Neocortical neurons born between E10 and E12 radially migrate using the somal translocation mode. In contrast, late-born neurons transform their migration mode sequentially to multipolar migration, locomotion mode, and terminal translocation mode during their radial migration. These neurons form neocortical layers in a birthdate-dependent inside-out manner.
PP, preplate; VZ, ventricular zone; MZ, marginal zone; CP, cortical plate; IZ, intermediated zone; MAZ, multipolar cell accumulation zone; WM, white matter; HP, hippocampal plate; SLM, stratum lacunosum-moleculare; SR, stratum radiatum; SP, stratum pyramidale; SO, stratum oriens. (text modified from original figure legend) |
The developmental changes of GFP+ newborn mouse dentate granule cells.[17]
References
- ↑ Caronia-Brown G, Yoshida M, Gulden F, Assimacopoulos S & Grove EA. (2014). The cortical hem regulates the size and patterning of neocortex. Development , 141, 2855-65. PMID: 24948604 DOI.
- ↑ Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z & Alvarez-Buylla A. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature , 555, 377-381. PMID: 29513649 DOI.
- ↑ Feng S, Shi T, Qiu J, Yang H, Wu Y, Zhou W, Wang W & Wu H. (2017). Notch1 deficiency in postnatal neural progenitor cells in the dentate gyrus leads to emotional and cognitive impairment. FASEB J. , 31, 4347-4358. PMID: 28611114 DOI.
- ↑ Hevner RF. (2016). Evolution of the mammalian dentate gyrus. J. Comp. Neurol. , 524, 578-94. PMID: 26179319 DOI.
- ↑ Montiel JF, Vasistha NA, Garcia-Moreno F & Molnár Z. (2016). From sauropsids to mammals and back: New approaches to comparative cortical development. J. Comp. Neurol. , 524, 630-45. PMID: 26234252 DOI.
- ↑ Striedter GF. (2016). Evolution of the hippocampus in reptiles and birds. J. Comp. Neurol. , 524, 496-517. PMID: 25982694 DOI.
- ↑ Li D, Takeda N, Jain R, Manderfield LJ, Liu F, Li L, Anderson SA & Epstein JA. (2015). Hopx distinguishes hippocampal from lateral ventricle neural stem cells. Stem Cell Res , 15, 522-529. PMID: 26451648 DOI.
- ↑ Bekiari C, Giannakopoulou A, Siskos N, Grivas I, Tsingotjidou A, Michaloudi H & Papadopoulos GC. (2015). Neurogenesis in the septal and temporal part of the adult rat dentate gyrus. Hippocampus , 25, 511-23. PMID: 25394554 DOI.
- ↑ Zhang Z, Liu S, Lin X, Teng G, Yu T, Fang F & Zang F. (2011). Development of laminar organization of the fetal cerebrum at 3.0T and 7.0T: a postmortem MRI study. Neuroradiology , 53, 177-84. PMID: 20981415 DOI.
- ↑ Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B & Kempermann G. (2010). Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE , 5, e8809. PMID: 20126454 DOI.
- ↑ Kier EL, Kim JH, Fulbright RK & Bronen RA. (1997). Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. AJNR Am J Neuroradiol , 18, 525-32. PMID: 9090416
- ↑ Utsunomiya H, Takano K, Okazaki M & Mitsudome A. (1999). Development of the temporal lobe in infants and children: analysis by MR-based volumetry. AJNR Am J Neuroradiol , 20, 717-23. PMID: 10319988
- ↑ Li YJ, Ga SN, Huo Y, Li SY & Gao XG. (2007). Characteristics of hippocampal volumes in healthy Chinese from MRI. Neurol. Res. , 29, 803-6. PMID: 17601367 DOI.
- ↑ Lee E & Son H. (2009). Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep , 42, 239-44. PMID: 19470236
- ↑ Zaidel DW. (1999). Quantitative morphology of human hippocampus early neuron development. Anat. Rec. , 254, 87-91. PMID: 9892421
- ↑ Hayashi K, Kubo K, Kitazawa A & Nakajima K. (2015). Cellular dynamics of neuronal migration in the hippocampus. Front Neurosci , 9, 135. PMID: 25964735 DOI.
- ↑ Zhao S, Zhou Y, Gross J, Miao P, Qiu L, Wang D, Chen Q & Feng G. (2010). Fluorescent labeling of newborn dentate granule cells in GAD67-GFP transgenic mice: a genetic tool for the study of adult neurogenesis. PLoS ONE , 5, . PMID: 20824075 DOI.
Journals
Hippocampus - "source for investigators seeking the latest research on the hippocampal formation and related structures and is widely read by neuroscientists, anatomists, behavioral scientists, physiologists, biochemists, and cellular, developmental, and molecular biologists." Wiley
http://www.ncbi.nlm.nih.gov/pubmed?term=%22Hippocampus%22[jour]
Reviews
Gilchrist C, Cumberland A, Walker D & Tolcos M. (2018). Intrauterine growth restriction and development of the hippocampus: implications for learning and memory in children and adolescents. Lancet Child Adolesc Health , 2, 755-764. PMID: 30236384 DOI.
Salazar P, Cisternas P, Martinez M & Inestrosa NC. (2018). Hypothyroidism and Cognitive Disorders during Development and Adulthood: Implications in the Central Nervous System. Mol. Neurobiol. , , . PMID: 30073507 DOI.
Zeid D, Kutlu MG & Gould TJ. (2018). Differential Effects of Nicotine Exposure on the Hippocampus Across Lifespan. Curr Neuropharmacol , 16, 388-402. PMID: 28714396 DOI.
Greene ND & Copp AJ. (2009). Development of the vertebrate central nervous system: formation of the neural tube. Prenat. Diagn. , 29, 303-11. PMID: 19206138 DOI.
Li Y, Mu Y & Gage FH. (2009). Development of neural circuits in the adult hippocampus. Curr. Top. Dev. Biol. , 87, 149-74. PMID: 19427519 DOI.
Georgieff MK. (2008). The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem. Soc. Trans. , 36, 1267-71. PMID: 19021538 DOI.
Faulkner RL, Low LK & Cheng HJ. (2007). Axon pruning in the developing vertebrate hippocampus. Dev. Neurosci. , 29, 6-13. PMID: 17148945 DOI.
Parnavelas JG. (2000). The origin and migration of cortical neurones: new vistas. Trends Neurosci. , 23, 126-31. PMID: 10675917
Sitoh YY & Tien RD. (1997). The limbic system. An overview of the anatomy and its development. Neuroimaging Clin. N. Am. , 7, 1-10. PMID: 9100228
Articles
Bachevalier J. (2019). Nonhuman primate models of hippocampal development and dysfunction. Proc. Natl. Acad. Sci. U.S.A. , , . PMID: 31871159 DOI.
Anstey KJ, Maller JJ, Meslin C, Christensen H, Jorm AF, Wen W & Sachdev P. (2004). Hippocampal and amygdalar volumes in relation to handedness in adults aged 60-64. Neuroreport , 15, 2825-9. PMID: 15597062
Jack CR, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC & Cascino GD. (1989). Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology , 172, 549-54. PMID: 2748838 DOI.
Bhatia S, Bookheimer SY, Gaillard WD & Theodore WH. (1993). Measurement of whole temporal lobe and hippocampus for MR volumetry: normative data. Neurology , 43, 2006-10. PMID: 8413958
Search PubMed
Search Pubmed: Hippocampus Embryology | Hippocampus Development | Limbic Development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 9) Embryology Neural - Hippocampus Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Hippocampus_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G