Monkey Development
| Embryology - 28 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The monkey is often used as a primate model for human development. Another historic model of primate development was the smaller tarsius[1]
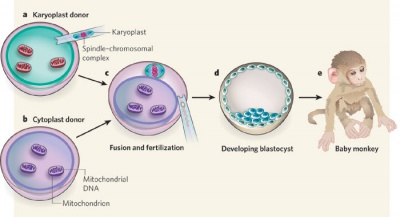
Swapping mitochondrial DNA mammalian oocytes[2]
- Links: Category:Monkey
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Primate development | | Monkey development | Monkey embryology | Monkey embryo |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Macaque Timeline
| Day | Event |
|---|---|
| 9 | Ovulation beginning contact. Endometrial proliferation to form epithelial plaque. |
| 10 | Invasion. Erosion of strorna, gland and vessels. Lacunae beginning. Transition of cytotrophoblast to syncytium. |
| 12 | Trabeculae and cascades of trophoblast cells. Mesoblast, yolk sac. Ovum walls collapsed, no sinusoids. |
| 13 | Sinusoids communicate with lacunae. Villi begin. Ovum walls dilated, abembryonic wall hypertrophies preparatory to formation of second implantation site. |
| 15 | Maternal epithelium reaches end of proliferative period. |
| 17 | Necrotic border zone. Blood in glands. Primitive body mesoderm. |
| 19 | Villi with three zones established, and branching. Pinocytosis (imbibing of liquid substances}. |
| 22.5 | Villi vascular. Oedema at junction zone. |
| Data from [8] Links: monkey | placenta | timeline | |
Historic Papers
Gatenby JB. Some notes on recent primate embryology. (1945) Irish J. Med. Sci. 177-187.
Ramsey EM. Corner GW. Jr. Donner MW. and Stran HM. Radioangiographic studies of circulation in the maternal placenta of the rhesus monkey: preliminary report. (1960) Proc. Natl. Acad. Sci. U.S.A., 46(7): 1003-8 PMID 16590693
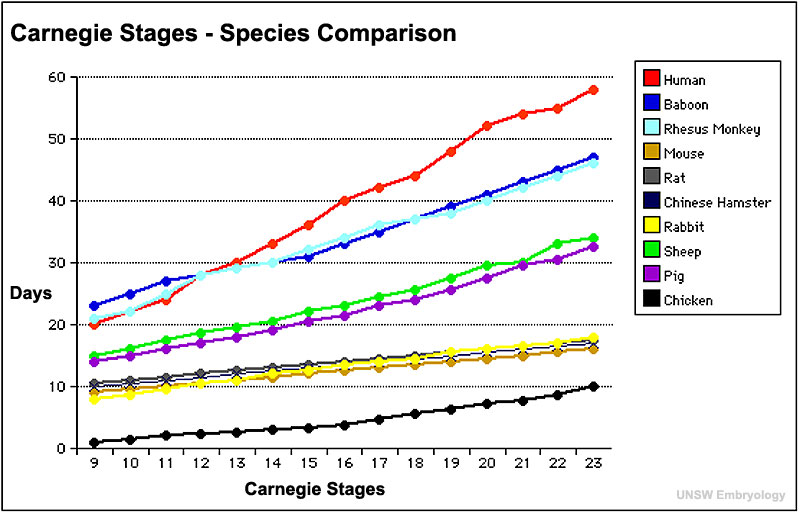
Carnegie Stages
Monkey Data - Hendrickx and Sawey Embryology of the rhesus monkey. The Rhesus Monkey, Bourne (ed), Academic Press, NY (1975)
- Links: [[Carnegie Stages}}
Fetal Brain Development
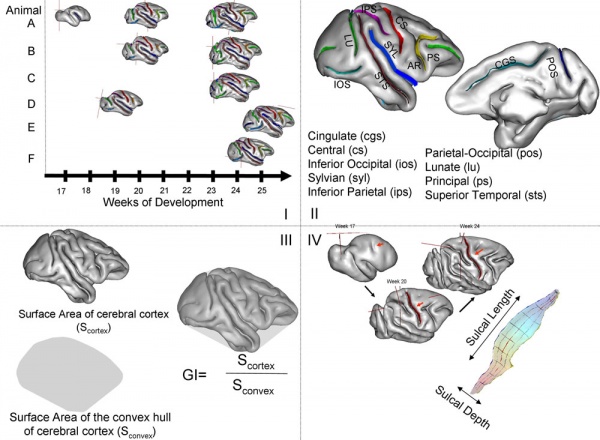
Mapping primary gyrogenesis during fetal development in primate brains: high-resolution in utero structural MRI of fetal brain development in pregnant baboons[9]
- "The global and regional changes in the fetal cerebral cortex in primates were mapped during primary gyrification (PG; weeks 17-25 of 26 weeks total gestation). Studying pregnant baboons using high-resolution MRI in utero, measurements included cerebral volume, cortical surface area, gyrification index and length and depth of 10 primary cortical sulci. Seven normally developing fetuses were imaged in two animals longitudinally and sequentially. We compared these results to those on PG that from the ferret studies and analyzed them in the context of our recent studies of phylogenetics of cerebral gyrification. We observed that in both primates and non-primates, the cerebrum undergoes a very rapid transformation into the gyrencephalic state, subsequently accompanied by an accelerated growth in brain volume and cortical surface area. However, PG trends in baboons exhibited some critical differences from those observed in ferrets. For example, in baboons, the growth along the long (length) axis of cortical sulci was unrelated to the growth along the short (depth) axis and far outpaced it. Additionally, the correlation between the rate of growth along the short sulcal axis and heritability of sulcal depth was negative and approached significance (r = -0.60; p < 0.10), while the same trend for long axis was positive and not significant (p = 0.3; p = 0.40). These findings, in an animal that shares a highly orchestrated pattern of PG with humans, suggest that ontogenic processes that influence changes in sulcal length and depth are diverse and possibly driven by different factors in primates than in non-primates."
Thalidomide Model
Crab-eating Macaque (Macaca fascicularis, Cynomolgus Monkey, Philippine Monkey, Long-tailed Macaque)[7] "Cynomolgus monkeys were orally administered thalidomide at 15 or 20mg/kg-d on days 26-28 of gestation, and fetuses were examined on day 100-102 of gestation. Limb defects such as micromelia/amelia, paw/foot hyperflexion, polydactyly, syndactyly, and brachydactyly were observed in seven of eight fetuses."
Infections
Herpes B
Macaques are native to Asia and North Africa. They are also housed in research facilities, zoos, and wildlife or amusement parks and are kept as pets in private homes throughout the world. Monkey bites occasionally occur in certain urban sites, such as temples in Nepal or India, and can transmit herpes B virus.
Herpes B virus is related to the herpes simplex viruses that cause oral and genital ulcers. Herpes B infection is rare in humans. The virus was discovered in 1933, and since that time approximately 50 cases have been reported in humans, with an 80% case-fatality ratio. No cases of herpes B infection have been reported in people exposed to monkeys in the wild. Most documented cases have resulted from occupational exposures. However, travelers to areas where macaques range freely should be aware of the potential risk. A monkey infected with herpes B may appear completely healthy.
Documented routes of human infection with herpes B virus include animal bites and scratches, exposure to infected tissue or body fluids from splashes, and in one instance, human-to-human transmission. Even minor scratches or bites should be considered potential exposures, because, experimentally, herpes B virus has been isolated from surfaces for up to 2 weeks after it was applied. The incubation period ranges from <1 week to a month or longer. Neurologic symptoms develop as the virus infects the central nervous system and may lead to ascending paralysis and respiratory failure. Increased public and clinician awareness about the risks associated with an injury from a macaque, improved first aid after exposure, the availability of better diagnostic tests, and improved antiviral therapeutics have decreased the case-fatality ratio to 20% in treated people. As a result, from 1987 through 2004 only 5 infections were fatal.
Although only macaque bites pose a herpes B virus threat, any monkey bite may pose a threat for rabies.
(Text CDC)
West Nile Virus
Plasmodium vivax
References
- ↑ Template:Ref-Hubrecht1907
- ↑ Shoubridge EA. (2009). Developmental biology: Asexual healing. Nature , 461, 354-5. PMID: 19759608 DOI.
- ↑ Ma H, Zhai J, Wan H, Jiang X, Wang X, Wang L, Xiang Y, He X, Zhao ZA, Zhao B, Zheng P, Li L & Wang H. (2019). In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science , 366, . PMID: 31672918 DOI.
- ↑ Matsumoto M, Sawada M, García-González D, Herranz-Pérez V, Ogino T, Bang Nguyen H, Quynh Thai T, Narita K, Kumamoto N, Ugawa S, Saito Y, Takeda S, Kaneko N, Khodosevich K, Monyer H, Manuel García-Verdugo J, Ohno N & Sawamoto K. (2019). Dynamic changes in ultrastructure of the primary cilium in migrating neuroblasts in the postnatal brain. J. Neurosci. , , . PMID: 31685650 DOI.
- ↑ Kwon D, Koo OJ, Kim MJ, Jang G & Lee BC. (2016). Nuclear-mitochondrial incompatibility in interorder rhesus monkey-cow embryos derived from somatic cell nuclear transfer. Primates , 57, 471-8. PMID: 27165688 DOI.
- ↑ Simerly CR, Castro CA, Jacoby E, Grund K, Turpin J, McFarland D, Champagne J, Jimenez JB, Frost P, Bauer C, Hewitson L & Schatten G. (2010). Assisted Reproductive Technologies (ART) with baboons generate live offspring: a nonhuman primate model for ART and reproductive sciences. Reprod Sci , 17, 917-30. PMID: 20631291 DOI.
- ↑ 7.0 7.1 Ema M, Ise R, Kato H, Oneda S, Hirose A, Hirata-Koizumi M, Singh AV, Knudsen TB & Ihara T. (2010). Fetal malformations and early embryonic gene expression response in cynomolgus monkeys maternally exposed to thalidomide. Reprod. Toxicol. , 29, 49-56. PMID: 19751816 DOI.
- ↑ Gatenby JB. Some notes on recent primate embryology. (1945) Irish J. Med. Sci. 177-187.
- ↑ Kochunov P, Castro C, Davis D, Dudley D, Brewer J, Zhang Y, Kroenke CD, Purdy D, Fox PT, Simerly C & Schatten G. (2010). Mapping primary gyrogenesis during fetal development in primate brains: high-resolution in utero structural MRI of fetal brain development in pregnant baboons. Front Neurosci , 4, 20. PMID: 20631812 DOI.
Articles
Enders AC, Schlafke S & Hendrickx AG. (1986). Differentiation of the embryonic disc, amnion, and yolk sac in the rhesus monkey. Am. J. Anat. , 177, 161-85. PMID: 3788819 DOI.
Search Pubmed
Search Pubmed: Monkey development | rhesus monkey development
Historic
Bartelmez GW. The form and the functions of the uterine blood vessels in the rhesus monkey. (1957) Contrib. Embryol., Carnegie Inst. Wash. 36:153—182.
Enders AC. Hendrickx AG. and Schlafke S. Implantation in the rhesus monkey: initial penetration of endometrium. (1983) Amer.J. Anat., 167: 275-298.
Hart DB. and Gulland GL. The Anatomy of Advanced Pregnancy in Macacus rhesus studied by Frozen Sections, by Casts and Microscopically. (1893) J Anat Physiol. 27(3): 360.1-376. PMID 17232038
Heuser CH. An intrachorionic mesothelial membrane in young stages of the monkey (Macacus rhesus). (1932) Anat. Rec, 52, Suppl., 15-16.
Heuser CH. Early development of the primitive mesoblast in embryos of the rhesus monkey. (1938) Carnegie Inst. (Publ. 501), Cooperation in Research 383-88.
Heuser CH. The chimpanzee ovum in the early stages of implantation (about 10.5 days). (1940) J Morphol. : 155- .
Heuser CH. and Streeter GL. Development of the macaque embryo. (1941) Contrib. Embryol., Carnegie Inst. Wash. Publ. 525, 29: 15-55.
Luckett WP. The origin of extraembyronic mesoderm in the early human and rhesus monkey embryos. (1971) Anat. Rec, 169: 369-370.
Luckett WP. Amniogenesis in the early human and rhesus monkey embryos. (1973) Anat. Rec, 175: 375.
Luckett WP. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. (1978) Amer. J Anat., 152: 59-97. PMID 98035
Ramsey EM. Corner GW. Jr. Donner MW. and Stran HM. Radioangiographic studies of circulation in the maternal placenta of the rhesus monkey: preliminary report. (1960) Proc. Natl. Acad. Sci. U.S.A., 46(7): 1003-8 PMID 16590693
Wislocki GE. and Bennett SB. The histology and cytology of the human and monkey placenta, with special reference to the trophoblast. (1943) Amer. J Anat. 337-448.
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Primate Portal USA Colonies
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 28) Embryology Monkey Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Monkey_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G