Lecture - Fetal Development
| Embryology - 2 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
| <html5media height="480" width="400">File:fetal growth.mp4</html5media> |
The fetal period (9-36 weeks) is about continued differentiation of organs and tissues, most importantly this period is about growth both in size and weight. The long Fetal period (4x the embryonic period) is a time of extensive growth in size and mass as well as ongoing differentiation of organ systems established in the embryonic period and do so at different times. For example, the brain continues to grow and develop extensively during this period (and postnatally), the respiratory system differentiates (and completes only just before birth), the urogenital system further differentiates between male/female, endocrine and gastrointestinal tract begins to function.
Note - Some of the content of this fetal lecture has been discussed in earlier systems development lectures. |
Lecture Objectives
- Understanding of fetal growth - length and weight
- Understanding of fetal systems development/changes
- Understanding of fetal abnormalities
- First Trimester (1 - 12 weeks) - embryonic and early fetal
- Second Trimester (13 - 24 weeks) - organ development and function, growth
- Third Trimester (25 - 40 weeks) - organ function and rapid growth
Lecture Resources
| Movies | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
| |||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| Hill, M.A. (2020). UNSW Embryology (20th ed.) Retrieved March 2, 2026, from https://embryology.med.unsw.edu.au |
| |||||

|
Moore, K.L., Persaud, T.V.N. & Torchia, M.G. (2015). The developing human: clinically oriented embryology (10th ed.). Philadelphia: Saunders. (links only function with UNSW connection)
Chapter 6 Fetal Period Chapter 20 Human Birth Defects | |||||

|
Schoenwolf, G.C., Bleyl, S.B., Brauer, P.R., Francis-West, P.H. & Philippa H. (2015). Larsen's human embryology (5th ed.). New York; Edinburgh: Churchill Livingstone.(links only function with UNSW connection) |
| 2016 Lecture Video Recording |
|---|
| This 2016 lecture video recording is similar in content to the current 2017 online lecture.
<html5media height="600" width="800">File:2016Lecture-Fetal.mp4</html5media> Click to play new window - 2016 Lecture Video (51 MB) |
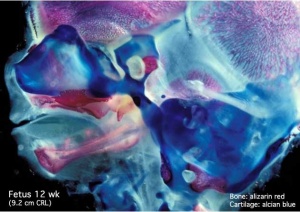
Musculoskeletal
- Ongoing process of ossification
- endochondral and intramembranous
- growth in long bone length
- Achondroplasia (common form of short limb dwarfism) third trimester ultrasound shortened long bones.
- increased limb length
- continues postnatally
- rapid growth during puberty
- relocation of haemopoietic stem cells to bone marrow
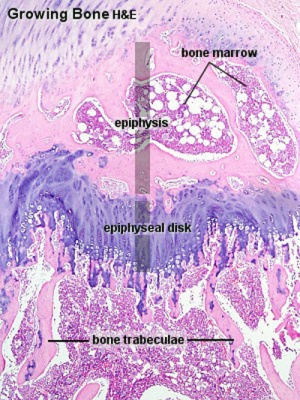
Endochondral ossification |
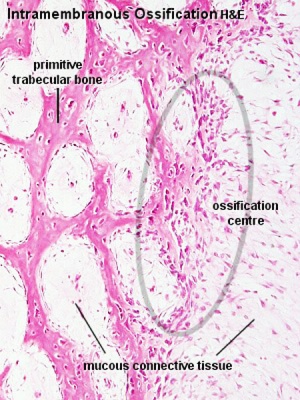
Intramembranous ossification |
The collapsed tables below are for information purposes and are not examinable.
| Table of Ossification of the Bones of the Superior Extremity | |||
|---|---|---|---|
| (Days and weeks refer to the prenatal, years to the postnatal period.) | |||
| Bone | Centres | Time of appearance of centre | Union of primary and secondary centres; remarks. |
| Clavicle | Diaphysis | 6th week | There are two centres in the shaft, a medial and a lateral. These blend on the 45th day (Mall). Shaft and epiphysis unite between the 20th and 25th years. |
| Sternal epiphysis | 18th to 20th year | ||
| Scapula | Primary centres: | The chief centre appears near the lateral angle. The subcoracoid centre appears at the base of the coracoid process and also gives rise to a part of the superior margin of the glenoid fossa. The coracoid process joins the body about the age of puberty. The acromial epiphysis centres (two or three in number) fuse with one another soon after their appearance and with the spine between the 22nd and 25th years (Quain); 20th year (Wilms). The subcoracoid and the epiphysis of the coracoid process, the glenoid fossa, the inferior angle, and the vertebral margin join between the 18th and 24th years in the order mentioned (Sappey). | |
| 1. That of the body, the spine, and the base of the glenoid cavity. | 8th week (Mall) 1 | ||
| 2. Goraooid process | 1st year | ||
| 3. Subcoracoid | 10th to 12th year | ||
| Epiphyses: | |||
| Acromial epiphyses | 15th to 18th year | ||
| Epiphysis of the inferior angle. | 16 to 18th year | ||
| Epiphyses of the vertebral border. | 18th to 20th year | ||
| Epiphyses of upper surface of coracoid. | 16th to 18th year. | ||
| Epiphysis of surface of glenoid fossa. | 16th to 18th year. | ||
| Humerus | Diaphysis | 6th to 7th week (Mall) | The epiphyses of the head, the tuberculum majus and the tuberculum minus (the last is inconstant) unite with one another in 4th-6th year and with the shaft in 20th-25th year. The epiphyses of the capitulum, lateral epicondyle, and trochlea unite with one another and then in the 16th-17th year join the shaft. The epiphysis of the medial epicondyle joins the shaft in the 18th year. |
| Epiphyses: | |||
| Head | 1st to 2d year | ||
| Tuberculum majus | 2d to 3d year | ||
| Tuberculum minus | 3d to 5th year | ||
| Capitulum | 2d to 3d year | ||
| Epioondylus med | 5th to 8th year | ||
| Lateral margin of trochlea | 11th to 12th year | ||
| Epicondylus lat | 12th to 14th year | ||
| Radius | Diaphysis | 7th week (Mall) | The superior epiphysis and shaft unite between the 17th and 20th years. The inferior epiphysis and shaft about the 21st year (Pryor); M 21st year, F 21st-25th year (Sappey). Sometimes an epiphysis is found m the tuberosity (R. and K.) and in the styloid process (Sappey). |
| Epiphyses: | |||
| Carpal end | F 8th month - M 15th month (Pryor) | ||
| Humeral end | 6th-7th year | ||
| Ulna | Diaphysis | 7th week | The centre for the shaft of the ulna arises a few days later than that for the radius. The proximal epiphysis is united to the shaft about the 17th year; the inferior epiphysis between the 18th and 20th years; F 20th - 21st years, M 21st - 24th years (Sappey). There is sometimes an epiphysis in the styloid process (Sohwegel) and in the tip of the olecranon process (Sappey). |
| Epiphyses: | |||
| Carpal end | F 6th-7th year - M 7th-8th year (Pryor) | ||
| Humeral end | 10th year | ||
| Carpus | Os capitatum | F 3d-6th month M 4th-10th month | The navicular sometimes has two centres of ossification (Serres. Rambaud and Renault). Serres and Pryor have described two centres of ossification in the lunatum. Debierre has described two centres in the pisiform, one in a girl of eleven, the other in a boy of twelve. The OS hamatum may have a special centre for the hamular process. Pryor has found two centres in the triquetrum. Pryor (1908), describes the centres of ossification of the carpal bones as assuming shapes characteristic of each bone at an early period. |
| Os hamatum | F 5th-10th month M 6th-12th month | ||
| Os triquetrum | F 2d-3d year M about 3 years | ||
| Os lunatum | F 3rd-4th year M about 4 years | ||
| Os naviculare | F at 4 years, or early in 5th year M about 5 years | ||
| Os mult. maj. | F 4th-5th year M 5th-6th year | ||
| Osmult. min. | F 4th-5th year M 6th-6th year | ||
| Os pisiforme | F 9th-10th year M 12th-3th year | ||
| Metacarpals | Diaphyses | 9th week (Mall) | The centres for the shafts of the second and third metacarpals are the first to appear. There may be a distal epiphysis for the first metacarpal and a proximal epiphysis for the second. Pryor (1906). found the distal epiphysis of the first metacarpal in about 6 per cent, of cases. It is a family characteristic. It arises before the 4th year and unites later. Pryor found the proximal epiphysis of the second metacarpal in six out of two hundred families. It unites with the shaft between the 4th and 6th-7th year; sometimes, however, not until the 14th year. In the seal and some other animals all the metacarpals have proximal and distal epiphyses (Quain). The epiphyses join the shafts between the 15th and 20th years. There may bean independent epiphysis for the styloid process of the 5th metacarpal. The epiphysis of the metacarpal of the index finger appears first. This is followed by those of the 3d, 4th, 5th, and 1st digits. |
| Proximal epiphysis of the first metacarpal | 3d year | ||
| Distal epiphyses of the metacarpals | 2d year | ||
| Phalanges | Diaphyses | 9th week (Mall) | |
| First row | Proximal epiphyses | 1st-3rd year (Pryor) | The shafts of the phalanges of the second and third fingers are the first to show centres of ossification. The phalanges of the little finger are the last, the epiphysis in the middle finger is the first to appear. This is followed by those of the 4th, 2d, 5th, and 1st digits. |
| Middle row | Diaphyses | 11th-12th week (Mall) | The centres in the shafts of this row are the last to appear. The epiphysis of the phalanx of the middle finger is the first to appear. This is followed by those of the ring, index, and little finger (Pryor). |
| Proximal epiphyses | 2nd-3rd year | ||
| Terminal row | Diaphyses | 7th-8th week | The terminal phalanx of the thumb is the first to show a centre of ossification in the shaft. This is the first centre of ossification in the hand. It is developed in connective tissue while the centres of the other phalanges are developed in cartilage (Mall). The epiphysis of the ungual phalanx of the thumb is followed by those of the middle, ring, index, and little fingers. The fusion of the epiphyses of the phalanges with the diaphyses takes place in the 18th-20th year. |
| Proximal epiphyses | 2nd-3rd year | ||
| Sesamoid bones | Ossification begins generally in the 13th - 14th years, and may not take place until after middle life (Thilenius). For table of relative frequency in the embryo and adult see p. 385. | ||
M = male F = female. | |||
| Reference: Manual of Human Embryology by Franz Keibel and Franklin P. Mall (1910) Table - Upper Limb | |||
| Table of Ossification of the Bones of the Inferior Extremity | |||
|---|---|---|---|
| (Days and weeks refer to the prenatal, years to the postnatal period.) | |||
| Bone | Centres | Time of appearance of centre | Time of fusion: general remarks |
| Os coxae | Os ilium | 56th day (Mall) | The rami of the ischium and the pubis are united by bone in the 7th or 8th year (Quain) ( 12-14 year Sappey). In the acetabulum the three hip bones are separated by a Y-shaped cartilage until after puberty. In this cartilage between the ilium and pubis the "os acetabuli" appears between the ninth and twelfth years. This bone, variable in size, forms a greater or less part of the pubic portion of the articular cavity. Leche (1884). Krause (1885), and many others consider it primarily an independent bone. About puberty between the ilium and ischium and over the acetabular surfaces of these bones small irregular epiphyseal centres appear. The os acetabuli becomes imited to the pubic bone about puberty and soon afterwards the acetabular portions of the ilium and ischium and the ischium and pubis begin to become united by bone. The acetabular portions of the pubis and ilium are unite a little later. Osseous union takes place earlier on the pelvic than on the articular surface of the acetabulum. The union of the several primary centres and the epiphyses is usually completed about the twentieth year. |
| Os ischii | 105th day (Mall) | ||
| Os pubis | 4th to 5th fetal month | ||
| Os acetabuli. | 9th to 12th year | ||
| Epiphyses:
Those of the acetabulum |
Soon after puberty | ||
| Crest of ilium | Soon after puberty | Fuses with main bone 20th to 25th year | |
| Tuberosity of ischium | Soon after puberty | Fusion begins in the 17th year and is completed between the 20th and 24th years (Sappey) | |
| Ischial spine | Soon after puberty | 18th to 20th year (Poirier). | |
| Ant. inf. spine of ilium | Soon after puberty | 18th to 20th year (Poirier) | |
| Symphysis end of os pubis (1 or 2 centres) | 18th to 20th year (Sappey) | After the 20th year | |
| Femur | Diaphysis | 43d day (Mall) | |
| Epiphyses:
Distal end |
Shortly before birth1 | 20th to 24th year | |
| Head | 1st year | 18th to 19th year | |
| Great trochanter | 3d to 4th year (Osseous granules soon after birth, (Poirier) | 18th year | |
| Small trochanter | 13th to 14th year
8th year (Sappey) |
17th year (Quain)
Proximal epiphysis 18th to 22d year (Poirier) | |
| Patella | 3d to 5th year | The osseous patella reaches its definitive form soon before puberty | |
| Tibia | Diaphysis | 44th day (Mall) | |
| Epiphyses:
Proximal end |
About birth | 19th to 24th year (Sappey) | |
| Distal end | 2d year | 16th to 19th year | |
| Tubercle (occas.) | 13th year | Fuses with epiphysis of the proximal end and then with this to the diaphysis | |
| Fibula | Diaphysis | 55th day (Mall). | |
| Epiphyses:
Distal end |
2d year | 20th to 22d year | |
| Proximal end | 3d to 5th year | 22d to 24th year | |
| Calcaneus | Chief centre | 6th fetal month | The chief nucleus is endochondral. A periosteal nucleus appears frequently in the 4-5 fetal month (Hasselwander) |
| Epiphysis (distal end) | 10th year (Quain)
7th-8th year ( Sappey) |
15th-16th year (Quain)
16th-18th year (Poirier) M 17-21, average 20 years F 13-17, average 16 years (Hasselwander) | |
| Talus | 6th fetal month (Hasselwander) | In the 7th-8th year the posterior part of the talus, the os trigonum, is frequently ossified from a special centre (v. Bardeleben). It fuses about the 18th year. | |
| Cuboid | About birth | ||
| Cuneiform III | 1st year | ||
| Cuneiform I | 2d-3d year | ||
| Cuneiform II | 3d-4th year | ||
| Navicular | 4th-5th year | ||
| Metatarsals | Diaphyses | 8th-10th week | According to v. Bardeleben a second centre of ossincation appears much later than the primary in the navicular, and finally about the time of puberty a medial epiphyseal centre arises. |
| Epiphyses | 3d-8th year | The centre for the 2d metatarsal usually appears first, then come the 3rd, 4th, 1st and 5th. The epiphysis of the 1st metatarsal appears at the proximal end of the bone: the other epiphyses arise at the distal ends of the metatarsals. There may be a distal epiphysis in the first metatarsal also.2 In some instances a proximal epiphysis is formed on the tuberosity of the fifth metatarsal (Gruber). The epiphyses unite with the shafts in the 17-21 year in males and in the 14-19 year in females. (Hasselwander). | |
| Phalanges: | |||
| Terminal row | Diaphyses | 58th day (Mall) | |
| Epiphyses (distal) | 4th year | M 13-23, average 16-21 year.
F 13-17, average 14-17 year (Hasselwander). | |
| Middle row | Diaphyses | 4th-10th fetal month | |
| Epiphyses | 3d year | M 15-19 year
F 13-16 year (Hasselwander) | |
| Proximal row | Diaphyses | 3d fetal month | |
| Epiphyses | 3d year | M 15-17 year.
F 14-15 year (Hasselwander) The centres for the shafts of the phalanges often appear double, one for the dorsal and one for the plantar surface. The centres for the medial phalanges in each row usually appear before the more laterally placed centres. The centre for the 5th terminal phalanx appears much later than the other centres in this row (Mall). According to Rambaud and Renault the epiphyses arise each from two centres which fuse together. In the terminal phalanx of the great toe the ossification centre of the epiphysis often appears as early as the second or even the first year. (Hasselwander) | |
| Sesamoid bones of the great toe | M 14th year
F 12th-13th year |
Ossification may begin in the 8th year in females, in the 11th in males (Hasselwander). | |
M = male F = female. | |||
| Manual of Human Embryology by Franz Keibel and Franklin P. Mall (1910) Table - Lower Limb | |||
- Links: Bone Development Timeline | Bone Histology
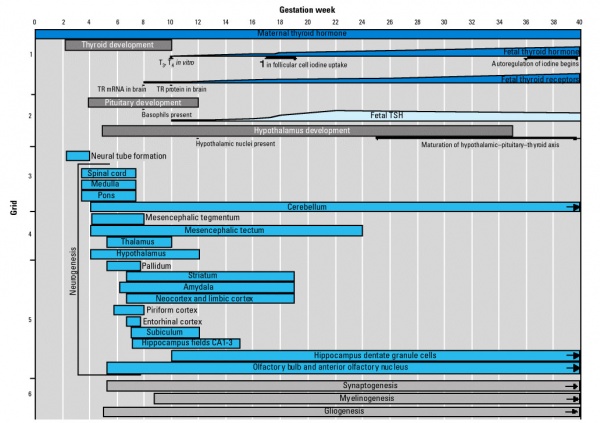
Fetal Neural
- 19 weeks (GA 21 weeks) neuronal migration ends and the radial glial cells that aided the migration now become transformed into astrocytes and astrocytic precursors.[2]
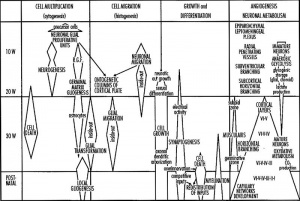
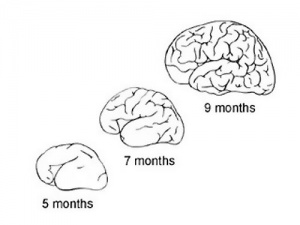
During the fetal period there is ongoing growth in size, weight and surface area of the brain and spinal cord. Microscopically there is ongoing: cell migration, extension of processes, cell death and glial cell development.
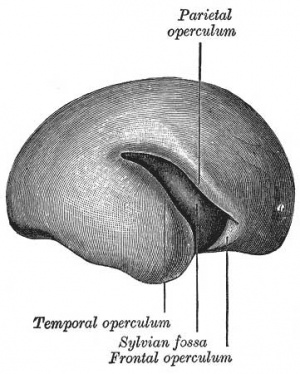
Cortical maturation (sulcation and gyration) and vascularization of the lateral surface of the brain starts with the insular cortex (insula, insulary cortex or insular lobe) region during the fetal period. This cerebral cortex region in the adult brain lies deep within the lateral sulcus between the temporal lobe and the parietal lobe.
- sulcation - The process of brain growth in the second to third trimester which forms sulci, grooves or folds visible on fetal brain surface as gyri grow (gyration). Abnormalities of these processes can lead to a smooth brain (lissencephaly).
- gyration - The development of surface folds on the brain (singular, gyrus)
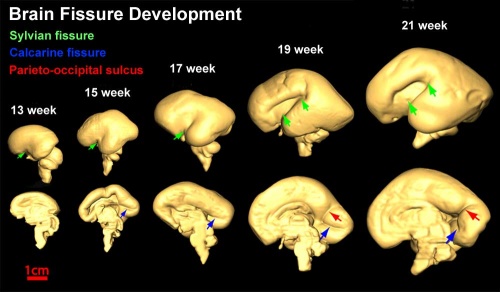
Insular Gyral and Sulcal Development[3]
- 13-17 gestational weeks - appearance of the first sulcus
- 18-19 gestational weeks - development of the periinsular sulci
- 20-22 gestational weeks - central sulci and opercularization of the insula
- 24-26 gestational weeks - covering of the posterior insula
- 27-28 gestational weeks - closure of the laeteral sulcus (Sylvian fissure or lateral fissure)
- Between 29-41 weeks volumes of: total brain, cerebral gray matter, unmyelinated white matter, myelinated, and cerebrospinal fluid (from MRI)
- grey matter- mainly neuronal cell bodies; white matter- mainly neural processes and glia.
- total brain tissue volume increased linearly over this period at a rate of 22 ml/week.
- Total grey matter also showed a linear increase in relative intracranial volume of approximately 1.4% or 15 ml/week.
- The rapid increase in total grey matter is mainly due to a fourfold increase in cortical grey matter.
- Quantification of extracerebral and intraventricular CSF was found to change only minimally.
(Text - modified from Huppi etal., (1998) Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol 43(2):224-235.)
Neural development will continue after birth with substantial growth, death and reorganization occuring during the postnatally (MH - postnatal not described in this current lecture)
Fetal Cardiovascular
MH - covered in lecture Late Vascular Development.
Blood Cells
|
|
Immune System
- maternal placenta transfer of IgG - not other immunoglobulin isotypes.
- fetal lymphocytes (mature T and B cells) - produced but not activated.
MH - see postnatal lecture - maternal milk IgG and IgA antibodies, leukocytes, secretory IgA, lactoferrin, lysozyme, and oligosaccharides and glycoconjugates that are receptor analogs for microbial adhesins and toxins.
Fetal Respiratory
MH - covered also in Lecture - Respiratory Development.
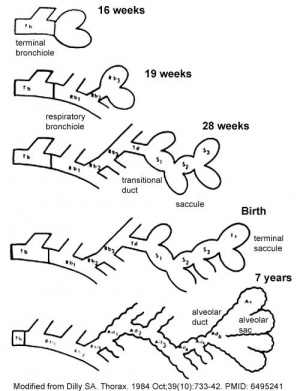
Month 3 to 6 - lungs appear glandular, end month 6 alveolar cells type 2 appear and begin to secrete surfactant.
Month 7 - respiratory bronchioles proliferate and end in alveolar ducts and sacs.
Lung Stages
- week 4 - 5 embryonic
- week 5 - 17 pseudoglandular
- week 16 - 25 canalicular
- week 24 - 40 terminal sac
- late fetal - 8 years alveolar
Pulmonary Neuroendocrine Cells
- develop in late embryonic to early fetal period.[4][5]
- first cell type to differentiate in the airway epithelium.
- later in mid-fetal period clusters of these cells form neuroepithelial bodies (NEBs) located in the fetal lung at bronchiole branching points.
- may stimulate mitosis to increase branching. secrete 2 peptides - gastrin-releasing peptide (GRP) and calcitonin gene related peptide (CGRP)
| Neonatal Human | Fetal Rabbit |
|---|---|
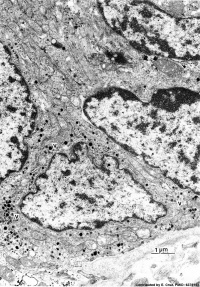
|
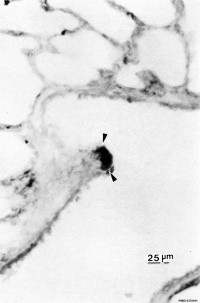
|
| Pulmonary neuroendocrine cell (EM)[6] | Neuroepithelial body[6] |
Gastrointestinal Tract
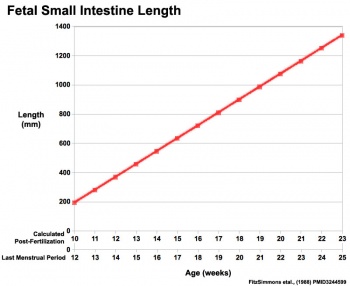
|
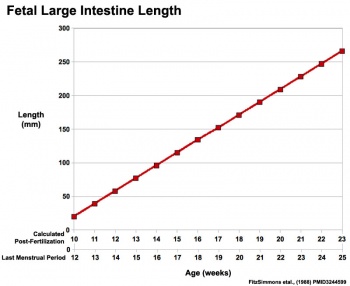
|
Fetal developmental features include:
- initially herniated outside the ventral body wall
- the growth and rotation of intestines
- changes in mesenteries
- development of the blood supply and tract wall.
Initial functions:
- amionic fluid swallowing - absorbed through the gastrointestinal tract and respiratory tract epithelium
- meconium - accumulation of both secretions and swallowed components within the large intestine
Renal
- Nephron differentiation occurs mainly during the fetal period.
- Human total nephron number ranges between 617,000 and 1,075,000 (mean 850,000 nephrons).
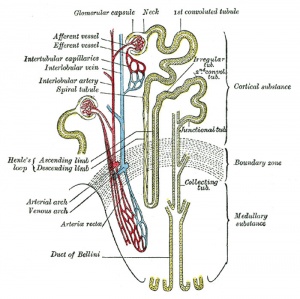
|
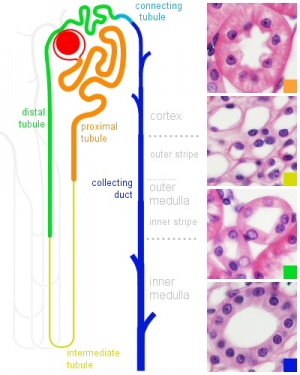
|
| Adult nephron structure | Nephron histology |
Development has four developmental stages:
- vesicle (V) stage (13-19 weeks)
- S-shaped body (S) stage ( 20-24 weeks)
- capillary loop (C) stage (25-29 weeks)
- maturation (M) stage (infants aged 1-6 months)
Genital
MH - introduced in the Genital Development lecture.
Gamete Development
- Female - oogenesis occurs through the fetal period with all primordial oocytes formed before birth and arrested in meiosis I. Meiosis recommences at puberty.
- Male - spermatogenesis does not occur until puberty, gametes are present only as diploid spermatogonia. Mitosis and meiosis commences at puberty.
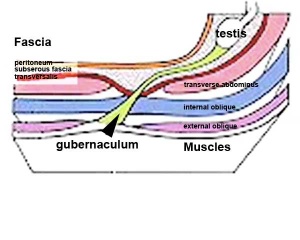
Gonad Descent
- Both kidney and gonads develop retroperitoneally, with the gonads moving into the abdomen or eventually into the scrotal sacs.
- During fetal development the gubernaculum and fetal growth in both male and female, changes the gonads’ relative positions finally reaching their adult locations.
Both female and male gonads undergo anatomical descent.
- Ovaries ‐ undergo caudal and lateral shifts to be suspended in the broad ligament of the uterus, gubernaculum does not shorten, it attaches to paramesonephric ducts, causing medial movement into the pelvis.
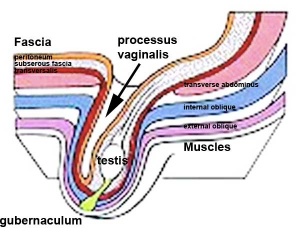
- Testes ‐ two anatomical phases in descent, transabdominal and transinguinal, under the influence of the shortening gubernaculum.
| <html5media height="300" width="360">File:Testis_Descent_001.mp4</html5media> | Beginning
End | |||
|
The testis (white) lies in the subserous fascia (spotted) a cavity processus vaginalis evaginates into the scrotum, and the gubernaculum (green) attached to the testis shortens drawing it into the scotal sac. As it descends it passes through the inguinal canal which extends from the deep ring (transversalis fascia) to the superficial ring (external oblique muscle). Descent of the testes into the scrotal sac begins generally during week 26 and may take several days. The animation shows the path of a single testis.
Gubernaculum - mesenchymal structure occurring associated with gonad development and involved in testes descent. Two factors - insulin-like peptide hormone 3 (INSL3) and androgen, have been shown to be involved with gubernaculum development. |
Incomplete or failed descent can occur unilaterally or bilaterally, is more common in premature births, and can be completed postnatally.
Data from a recent study of male human fetal (between 10 and 35 weeks) gonad position.
- 10 to 23 weeks - (9.45%) had migrated from the abdomen and were situated in the inguinal canal
- 24 to 26 weeks - (57.9%) had migrated from the abdomen
- 27 to 29 weeks - (16.7%) had not descended to the scrotum
Postnatal Genital Development
- Not completed until puberty.
- Development of secondary sexual characteristics.
- Humans - earlier in female than male.
- Female - commencing menstrual cycle, follicle development, recommence meiosis.
- Male - decrease in MIS, Leydig cell testosterone, activation of spermatogenesis, begin meiosis.
Endocrine
The fetal endocrine glands function throughout 2nd and 3rd trimester and are important for normal fetal development. Note that some maternal hormones can cross the placental barrier and that the placenta also acts as a multifunctional endocrine organ.
Pituitary Hormones
- HPA axis established by week 20
- Pituitary functional throughout fetal development
Thyroid Hormone
- required for metabolic activity, also in the newborn
- important for neural development
- placenta inactivates most of the maternal T4 to reverse T3 (rT3)
- Fetal to term up to 30% of the fetal thyroid hormones are of maternal origin.
Parathyroid Hormone
- newborn has total calcium levels (approx 20 grams) accumulated mainly in the 3rd trimester (weeks 28–40)
- fetal parathyroid hormone (PTH) potentially available from 10–12 weeks and PTH does not cross the placenta
- fetus relatively hypercalcemic, active transplacental transport of Ca2+ to fetus
- maternal serum - calcium ions (Ca2+), inorganic phosphate (Pi) and PTH concentrations are within the non-pregnant normal range throughout pregnancy.
- maternal bone turnover increases in the 3rd trimester.
(Based on Endocrinology - Materno—fetal calcium balance)
Pancreatic Hormones
- maternal diabetes can affect fetal pancreas development (increase in fetal islet beta cells).
Adrenal Hormones
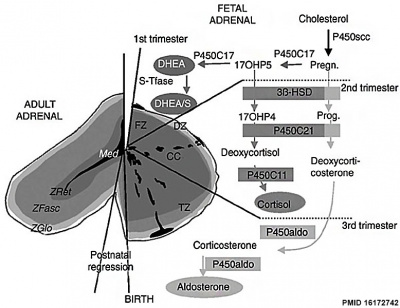
|
|
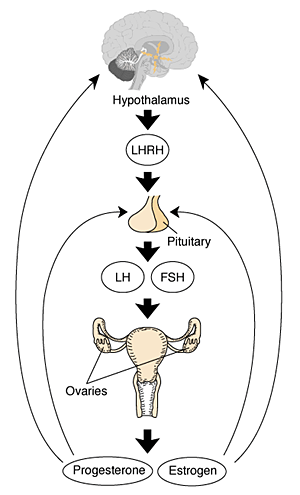
Gonadal Hormones
- testosterone - required during fetal development for external genital development and internal genital tract in male.
- estrogens - secreted inactive precursor converted to active form by placenta.
- Links: Endocrinology - Control of steroid production in the fetal gonads | Neuroscience - The Effect of Sex Hormones on Neural Circuitry
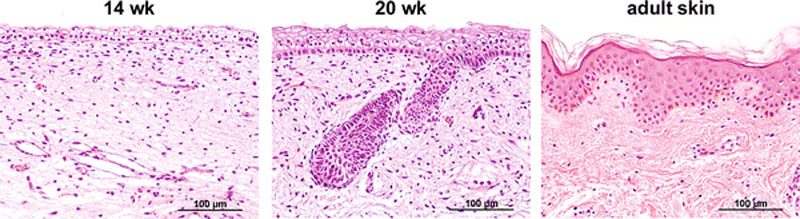
Skin Development
- 4 weeks
- simple ectoderm epithelium over mesenchyme.
- 1 - 3 months
- ectoderm - germinative (basal) cell repeated division of generates stratified epithelium.
- mesoderm - somite dermatome spreads out under the epithelium, differentiates into connective tissue and blood vessels.
- 4 months
- basal cell - proliferation generates folds in basement membrane.
- neural crest cells - (melanocytes) migrate into epithelium. These are the pigment cell of the skin.
- embryonic connective tissue - differentiates into dermis, a loose ct layer over a dense ct layer. Beneath the dense ct layer is another loose ct layer that will form the subcutaneous layer.
- Ectoderm contributes to nails, hair follictles and glands.
- Nails form as thickening of ectoderm epidermis at the tips of fingers and toes. These form germinative cells of nail field.
- Cords of these cells extend into mesoderm forming epithelial columns. These form hair follicles, sebaceous and sweat glands.
- 5 months
- Hair growth initiated at base of cord, lateral outgrowths form associated sebaceous glands.
- Other cords elongate and coil to form sweat glands.
- Cords in mammary region branch as they elongate to form mammary glands. These glands will complete development in females at puberty. Functional maturity only occurs in late pregnancy.
- Vernix cases begins to form and cover the skin (arms an intact visible coating in the third trimester)
Sensory Development
|
|
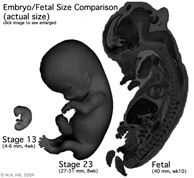
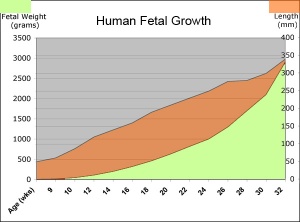
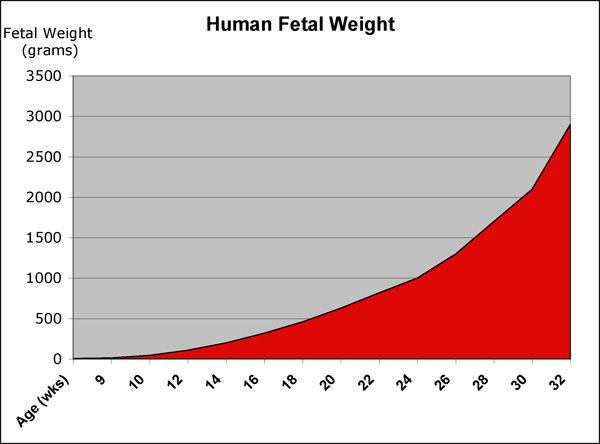
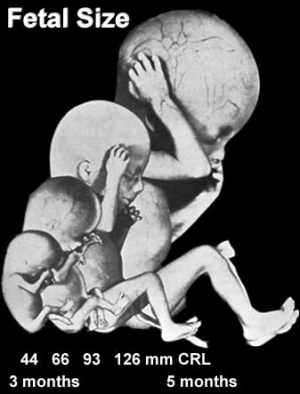
Fetal Size
- Fetal length increases through both the second and third trimesters.
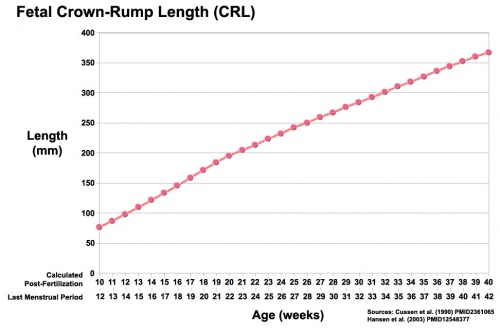
- Fetal growth parameters can now be accurately measured by ultrasound Crown-Rump Length (CRL): biparietal diameter (BPD), abdominal circumference (AC) and femoral diaphysis length (FDL).
| Fetal Development - Length - Weight | |||
|---|---|---|---|
| Gestational age | Fertilization age | Length | Mass |
| (LMP) (GA weeks) | (weeks) | (cm) | (g) |
| 8 (embryonic) | 6 | 1.6 (crown to rump) | 1 |
| 9 | 7 | 2.3 | 2 |
| 10 | 8 | 3.1 | 4 |
| 11 (fetal) | 9 | 4.1 | 7 |
| 12 | 10 | 5.4 | 14 |
| 13 (second trimester) | 11 | 7.4 | 23 |
| 14 | 12 | 8.7 | 43 |
| 15 | 13 | 10.1 | 70 |
| 16 | 14 | 11.6 | 100 |
| 17 | 15 | 13 | 140 |
| 18 | 16 | 14.2 | 190 |
| 19 | 17 | 15.3 | 240 |
| 20 | 18 | 16.4 25.6 (crown to heel) |
300 |
| 21 | 19 | 26.7 | 360 |
| 22 | 20 | 27.8 | 430 |
| 23 | 21 | 28.9 | 501 |
| 24 | 22 | 30 | 600 |
| 25 | 23 | 34.6 | 660 |
| 26 | 24 | 35.6 | 760 |
| 27 | 25 | 36.6 | 875 |
| 28 (third trimester) | 26 | 37.6 | 1005 |
| 29 | 27 | 38.6 | 1153 |
| 30 | 28 | 39.9 | 1319 |
| 31 | 29 | 41.1 | 1502 |
| 32 | 30 | 42.4 | 1702 |
| 33 | 31 | 43.7 | 1918 |
| 34 | 32 | 45 | 2146 |
| 35 | 33 | 46.2 | 2383 |
| 36 | 34 | 47.4 | 2622 |
| 37 | 35 | 48.6 | 2859 |
| 38 | 36 | 49.8 | 3083 |
| 39 | 37 | 50.7 | 3288 |
| 40 | 38 | 51.2 | 3462 |
| 41 | 39 | 51.7 | 3597 |
| 42 | 40 | 51.5 | 3685 |
| References [7][8][9] | |||
|
|
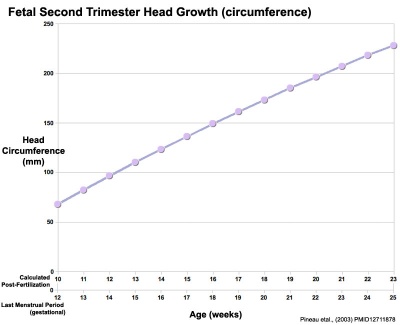
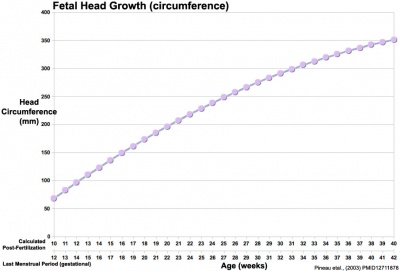
Head Size
- Fetal Graphs: Crown-Rump Length (CRL) | Third trimester CRL | Head Circumference | Head Circumference 2nd Trimester | Liver Weight | Pancreas Weight | Thymus Weight | Small Intestine Length | Large Intestine Length | Length and Weight Changes | Fetal Development
Fetal Weight
See also Fetal origins hypothesis and Low Birth Weight.
Birth Weight
| Birth weight (grams) | less 500 | 500 – 999 | 1,000 – 1,499 | 1,500 – 1,999 | 2,000 – 2,499 | 2,500 – 2,999 | 3,000 – 3,499 | 3,500 – 3,999 | 4,000 – 4,499 | 4,500 – 4,999 | 5,000 or more |
| Classification | Extremely Low Birth Weight | Very Low Birth Weight | Low Birth Weight | Normal Birth Weight | High Birth Weight | ||||||
The primary causes of VLBW are premature birth (born <37 weeks gestation, and often <30 weeks) and intrauterine growth restriction (IUGR), usually due to problems with placenta, maternal health, or to birth defects. Many VLBW babies with IUGR are preterm and thus are both physically small and physiologically immature.
Fetal Origins Hypothesis
- Maternal derived abnormalities relate to lifestyle, environment and nutrition and while some of these directly effect development.
- Growing evidence that some effects are more subtle and relate to later life health events.
- Original theory based on the early statistical analysis carried out by Barker of low birth weight data collected in the early 1900's in the south east of England
- He then compared with these same babies later health outcomes.
- Theory was therefore originally called the "Barker Hypothesis"
- recently been renamed as "developmental origins of health and disease" (DOHAD).
- Links: Fetal Origins Hypothesis
Premature Birth
| Year | < 34 weeks % | 34-36 weeks % | total preterm % |
| 1990 | 3.3 | 7.3 | 10.6 |
| 1995 | 3.3 | 7.7 | 11 |
| 2000 | 3.4 | 8.2 | 11.6 |
| 2005 | 3.6 | 9.1 | 12.7 |
Table data from: Prevention of preterm birth: a renewed national priority.[10]
Australia Recommendations
Perinatal care at the borderlines of viability: a consensus statement based on a NSW and ACT consensus workshop (February 2005) published in The Medical Journal of Australia 2006.[11]
- < 23 weeks survival is minimal and the risk of major morbidity is so high that initiation of resuscitation is not appropriate.
- 23 weeks active treatment may be discussed, but would be discouraged in NSW/ACT neonatal intensive care units.
- 23 to 25 weeks otherwise normal infant, there is an increasing obligation to treat. However, it is acceptable medical practice not to initiate intensive care if parents so wish, following appropriate counselling.
- 24 weeks antenatal transfer to a tertiary centre for fetal reasons is indicated. The option of non-initiation of intensive care/resuscitation should be offered.
- 25 weeks active treatment is usually offered, but the option of non-initiation of intensive care/resuscitation (presence of adverse fetal factors such as twin-to-twin transfusion, intrauterine growth restriction or chorioamnionitis) should also be discussed.
- 26 weeks + otherwise normal infant the obligation to treat is very high, and treatment should generally be initiated unless there are exceptional circumstances.
Abnormalities
Teratology
How different environmental effects during the pregnancy may influence outcomes. A teratogen (Greek, teraton = monster) is defined as any agent that causes a structural abnormality (congenital abnormalities) following fetal exposure during pregnancy. The overall effect depends on dosage and time of exposure (see critical periods below).
Absolute risk - the rate of occurrence of an abnormal phenotype among individuals exposed to the agent. (e.g. fetal alcohol syndrome)
Relative risk - the ratio of the rate of the condition among the exposed and the nonexposed. (e.g. smokers risk of having a low birth weight baby compared to non-smokers) A high relative risk may indicate a low absolute risk if the condition is rare.
Mutagen - a chemical or agent that can cause permanent damage to the deoxyribonucleic acid (DNA) in a cell. DNA damage in the human egg or sperm may lead to reduced fertility, spontaneous abortion (miscarriage), birth defects and heritable diseases.
Fetotoxicant - is a chemical that adversely affects the developing fetus, resulting in low birth weight, symptoms of poisoning at birth or stillbirth (fetus dies before it is born).
Synergism - when the combined effect of exposure to more than one chemical at one time, or to a chemical in combination with other hazards (heat, radiation, infection) results in effects of such exposure to be greater than the sum of the individual effects of each hazard by itself.
Toxicogenomics - the interaction between the genome, chemicals in the environment, and disease. Cells exposed to a stress, drug or toxicant respond by altering the pattern of expression of genes within their chromosomes. Based on new genetic and microarray technologies.
Critical Periods
Critical periods of development refer to times when genetic or materal effects can impact upon the developmental process. The timing of these effects will impact on different systems at different times.
Links: Embryonic Development | Timeline human development | Movie - Human Development annotated cartoon | Human - critical periods | Human Abnormal Development
Systems with long periods of development or complex developmental origins are more susceptible to developmental abnormalities.
- Which systems will take a long time to develop?
- Which systems are complex in origin?
References
- ↑ Streeter GL. Developmental horizons in human embryos (fourth issue). A review of the histogenesis of cartilage and bone. (1949) Carnegie Instn. Wash. Publ. 583, Contrib. Embryol., 33: 149-169. PMID: 18144445
- ↑ Kadhim HJ, Gadisseux JF & Evrard P. (1988). Topographical and cytological evolution of the glial phase during prenatal development of the human brain: histochemical and electron microscopic study. J. Neuropathol. Exp. Neurol. , 47, 166-88. PMID: 3339373
- ↑ Afif A, Bouvier R, Buenerd A, Trouillas J & Mertens P. (2007). Development of the human fetal insular cortex: study of the gyration from 13 to 28 gestational weeks. Brain Struct Funct , 212, 335-46. PMID: 17962979 DOI.
- ↑ Cutz E. (1982). Neuroendocrine cells of the lung. An overview of morphologic characteristics and development. Exp. Lung Res. , 3, 185-208. PMID: 6188605
- ↑ Cutz E, Gillan JE & Bryan AC. (1985). Neuroendocrine cells in the developing human lung: morphologic and functional considerations. Pediatr. Pulmonol. , 1, S21-9. PMID: 3906540
- ↑ 6.0 6.1 DiAugustine RP & Sonstegard KS. (1984). Neuroendocrinelike (small granule) epithelial cells of the lung. Environ. Health Perspect. , 55, 271-95. PMID: 6376101
- ↑ Cussen L, Scurry J, Mitropoulos G, McTigue C & Gross J. (1990). Mean organ weights of an Australian population of fetuses and infants. J Paediatr Child Health , 26, 101-3. PMID: 2361065
- ↑ Hansen K, Sung CJ, Huang C, Pinar H, Singer DB & Oyer CE. (2003). Reference values for second trimester fetal and neonatal organ weights and measurements. Pediatr. Dev. Pathol. , 6, 160-7. PMID: 12548377 DOI.
- ↑ Archie JG, Collins JS & Lebel RR. (2006). Quantitative standards for fetal and neonatal autopsy. Am. J. Clin. Pathol. , 126, 256-65. PMID: 16891202 DOI.
- ↑ Damus K. (2008). Prevention of preterm birth: a renewed national priority. Curr. Opin. Obstet. Gynecol. , 20, 590-6. PMID: 18989136 DOI.
- ↑ Lui K, Bajuk B, Foster K, Gaston A, Kent A, Sinn J, Spence K, Fischer W & Henderson-Smart D. (2006). Perinatal care at the borderlines of viability: a consensus statement based on a NSW and ACT consensus workshop. Med. J. Aust. , 185, 495-500. PMID: 17137454