Gastrointestinal Tract - Gallbladder Development
| Embryology - 1 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
This section of notes gives an overview of gallbladder (gall bladder, gall-bladder) and billary tree development, histology and abnormalities associated with the biliary system. In the adult, the gallbladder is a site of bile salt storage and concentration, to then be released into the duodenum where they act to solubilize dietary lipids by their detergent effect. Bile salts are a cholesterol derivative (breakdown product).
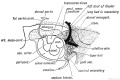
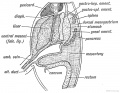
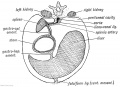
The transverse septum differentiates to form the hepatic diverticulum and the hepatic primordium, these two structures together will go on to form different components of the mature liver and gallbladder.
The hepatic diverticulum divides into two parts: pars hepatica (larger cranial part, primordium of the liver) and pars cystica (smaller ventral invagination, primordium of gallbladder).
The pars cystica vacuolates and expands, the stalk becoming the cystic duct. This structure is initially hollow, then solid (by proliferation of epithelial lining), and then recanalized occurs by vacuolation of this expanded epithelium. There are several opinions as to whether the duct has a solid phase or remains patent throughout development.[1][2]
Note that in some animals, for example horse and elephant, the gall bladder is normally absent.
See also: Gallbladder Histology.
Historic: Halpert B. and Lee H. The gall bladder and the extrahepatic biliary passages in late embryonic and early fetal life. (1932) Anat. Rec. 54(1): 29-42.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Gallbladder Embryology | Gallbladder Development | Bile Embryology |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
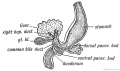
Embryonic Development
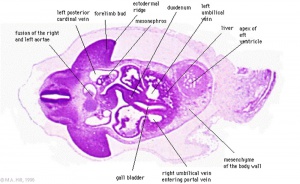
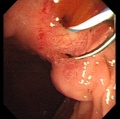
Stage 13
Early embryonic gallbladder (Carnegie stage 13, Week 4)
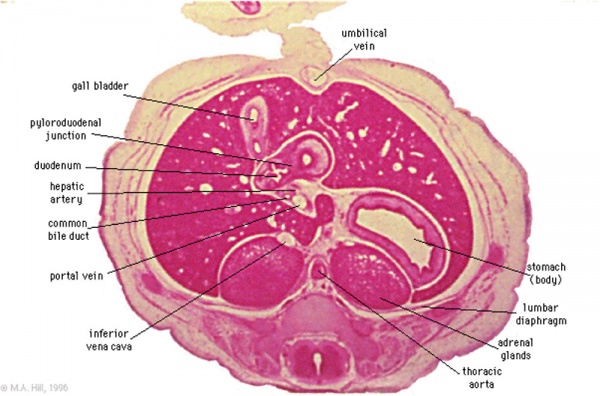
Stage 22
Late embryonic gallbladder (Carnegie stage 22, Week 8)
Historic
Grosser O. Lewis FT. and McMurrich JP. The Development of the Digestive Tract and of the Organs of Respiration. (1912) chapter 17, vol. 2, in Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
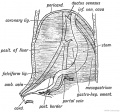
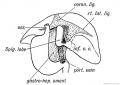
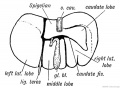
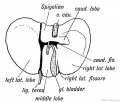
Gall-bladder Human Embryo (CRL)
- 7.5 mm - epithelium is surrounded by a layer of mesenchyma, and the entire structure is so imbedded in the under surface of the liver that it causes only a slight swelling of the peritoneal surface. Above and on the sides the mesenchyma is in direct relation with the hepatic trabecular, and it receives a few prolongations of the venous capillaries. Below it is covered by the peritoneal epithelium except on the left, where that layer is reflected to the abdominal walls in connection with the falciform ligament. In later stages the gall-bladder is separated from the hepatic trabecular on either side, and is attached to the liver only along its upper surface.
- 16 mm mesenchyma surrounding the gall-bladder is still undifferentiated.
- 22.8 mm forms two broad concentric zones, of which the inner is darker and more compact than the outer.
- 29 mm certain cells in the peripheral part of the dark zone form a third layer, which is thin and somewhat interrupted. As seen in later stages these cells are myoblasts, so that at 29 mm all three layers of the adult gall-bladder are indicated. These are the mucosa, muscularis, and serosa. The layers become gradually less distinct toward the hepatic duct.
Abnormalities
LB20.1 Structural developmental anomalies of gallbladder
LB20.10 Agenesis, aplasia or hypoplasia of gallbladder
LB20.10 Agenesis, aplasia or hypoplasia of gallbladder
Bile Ducts
LB20.2 Structural developmental anomalies of bile ducts
LB20.21 Biliary atresia
LB20.21 Biliary atresia - Biliary atresia is a rare disease characterised by an inflammatory biliary obstruction of unknown origin that presents in the neonatal period. It is the most frequent surgical cause of cholestatic jaundice in this age group. Untreated, this condition leads to cirrhosis and death within the first years of life.
LB20.22 Congenital stenosis or stricture of bile ducts (Congenital hypoplasia of bile ducts)
LB20.23 Structural developmental anomalies of cystic duct
Infections
These mainly relate to postnatal infections. Recent studies in the mouse have identified that gastrointestinal tract listeria infections can relocate to the gallbladder and reside there, leading to later reinfection of the gastrointestinal tract.
- Links: Bacterial Infection
References
- ↑ Crawford JM. (2002). Development of the intrahepatic biliary tree. Semin. Liver Dis. , 22, 213-26. PMID: 12360416 DOI.
- ↑ 2.0 2.1 Ando H. (2010). Embryology of the biliary tract. Dig Surg , 27, 87-9. PMID: 20551648 DOI.
- ↑ Kruepunga N, Hakvoort TBM, Hikspoors JPJM, Köhler SE & Lamers WH. (2018). Anatomy of rodent and human livers: What are the differences?. Biochim Biophys Acta Mol Basis Dis , , . PMID: 29842921 DOI.
- ↑ Hikspoors JPJM, Peeters MMJP, Mekonen HK, Kruepunga N, Mommen GMC, Cornillie P, Köhler SE & Lamers WH. (2017). The fate of the vitelline and umbilical veins during the development of the human liver. J. Anat. , 231, 718-735. PMID: 28786203 DOI.
- ↑ 5.0 5.1 Uemura M, Igarashi H, Ozawa A, Tsunekawa N, Kurohmaru M, Kanai-Azuma M & Kanai Y. (2015). Fate mapping of gallbladder progenitors in posteroventral foregut endoderm of mouse early somite-stage embryos. J. Vet. Med. Sci. , 77, 587-91. PMID: 25648459 DOI.
- ↑ Raparia K, Zhai QJ, Schwartz MR, Shen SS, Ayala AG & Ro JY. (2010). Muscularis mucosae versus muscularis propria in gallbladder, cystic duct, and common bile duct: smoothelin and desmin immunohistochemical study. Ann Diagn Pathol , 14, 408-12. PMID: 21074688 DOI.
Reviews
Kruepunga N, Hakvoort TBM, Hikspoors JPJM, Köhler SE & Lamers WH. (2018). Anatomy of rodent and human livers: What are the differences?. Biochim Biophys Acta Mol Basis Dis , , . PMID: 29842921 DOI.
Lemaigre FP. (2010). Molecular mechanisms of biliary development. Prog Mol Biol Transl Sci , 97, 103-26. PMID: 21074731 DOI.
Causey MW, Miller S, Fernelius CA, Burgess JR, Brown TA & Newton C. (2010). Gallbladder duplication: evaluation, treatment, and classification. J. Pediatr. Surg. , 45, 443-6. PMID: 20152372 DOI.
Roskams T & Desmet V. (2008). Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) , 291, 628-35. PMID: 18484608 DOI.
Bani-Hani KE. (2005). Agenesis of the gallbladder: difficulties in management. J. Gastroenterol. Hepatol. , 20, 671-5. PMID: 15853977 DOI.
Delalande JM, Milla PJ & Burns AJ. (2004). Hepatic nervous system development. Anat Rec A Discov Mol Cell Evol Biol , 280, 848-53. PMID: 15382016 DOI.
Articles
Salazar MC, Brownson KE, Nadzam GS, Duffy A & Roberts KE. (2018). Gallbladder Agenesis: A Case Report. Yale J Biol Med , 91, 237-241. PMID: 30258310
Botsford A, McKay K, Hartery A & Hapgood C. (2015). MRCP imaging of duplicate gallbladder: a case report and review of the literature. Surg Radiol Anat , 37, 425-9. PMID: 25757833 DOI. Ahmed M & Aurangzeb. (2010). Triplication of gallbladder. J Coll Physicians Surg Pak , 20, 766-7. PMID: 21078254 DOI.
Blidaru D, Blidaru M, Pop C, Crivii C & Seceleanu A. (2010). The common bile duct: size, course, relations. Rom J Morphol Embryol , 51, 141-4. PMID: 20191134
Peloponissios N, Gillet M, Cavin R & Halkic N. (2005). Agenesis of the gallbladder: a dangerously misdiagnosed malformation. World J. Gastroenterol. , 11, 6228-31. PMID: 16273658
Search Pubmed
July 2010
Search Bookshelf Gall Bladder Development
Search Pubmed Now: Gall Bladder Development | Cholangiocyte Development |
Additional Images
See also Gall Bladder Histology
Chapter XVIII. The Organs of Digestion Keith, A. (1902) Human Embryology and Morphology. London: Edward Arnold.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 1) Embryology Gastrointestinal Tract - Gallbladder Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Gastrointestinal_Tract_-_Gallbladder_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G