Book - Text-Book of Embryology 17
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Bailey FR. and Miller AM. Text-Book of Embryology (1921) New York: William Wood and Co.
- Contents: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Nervous System
General Considerations
There are certain features of the nervous system in general and particularly of the vertebrate nervous system, the comprehension of which makes the processes of development of the nervous system in man more intelligible. First, the nervous systems of the lower Vertebrates are in many respectssimpler than those of higher forms and their variations throw light upon thecauses which determine neural structures. Second, as the nervous systems of all Vertebrates develop from the same germ plasm, there are resemblances between certain features of both the embryonic and adult systems of lower vertebrates and certain developmental stages in the higher. Certain structures met with in lower adult forms may be regarded as representing stages of arrested development although specialized and aberrant in many respects of structures found in higher forms. Vestigial structures in the developing nervous systems of higher forms may be regarded as recurring developmental necessities in the attainment of the adult form.
Stated in the most general terms, coordination of bodily activities in response to both external and internal conditions is the biological significance of the nervous system. This implies a transmission of some form of change from one part to another or, in other words, conduction. This functional necessity is shown structurally in the elongated form of the histological elements of the nervous system. That such changes habitually pass along each element or neurone in some one direction seems to find a natural structural expression in the receptive body and dendrites of the neurone, and in its long transmitting axone.
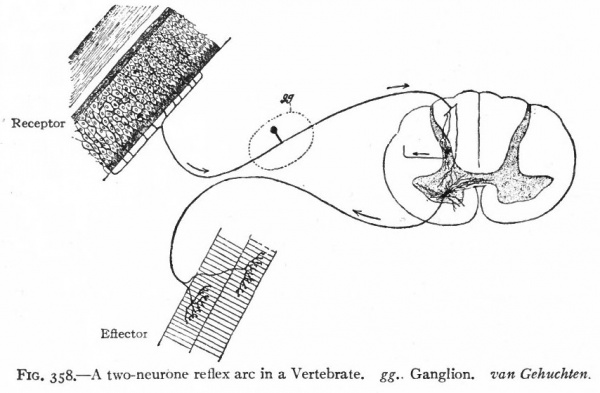
It is also evident that coordination can only be performed by a transmission of a change from some given structure either back to that structure or to some other structure to cause a responsive change. We thus have not only in the vertebrate, but at a very early stage in the invertebrate nervous system, a differentiation into afferent and efferent components, the two together usually being termed the peripheral nervous system. The histological elements of these components are the afferent and efferent peripheral neurones. All structures which are so affected as to transmit the change to the afferent peripheral neurones may be conveniently termed receptors, those structures affected by the efferent peripheral neurones may be termed effectors (Sherrington). Receptors include various "sensory" structures whose principal function appears to be to limit to some particular kind of stimulus the changes affecting the afferent nervous elements connected with. them. Effectors include various structures (muscles, glandular epithelia) whose activities are influenced by the nervous system (Fig. 358). A primitive nervous mechanism, thus composed of (i) afferent peripheral neurones which transmit the stimulus from a receptor to (2) efferent peripheral neurones which in turn transmit the stimulus to an effector, is a simple, two-neurone reflex arc (Fig. 358).
At the same time these neurones, as they increase in number, are obviously brought into relation with each other with more economy of space by having common meeting places. This, together with the factor noted below, leads to the concentration of an originally diffuse nervous system, spread out principally in connection with the outer (ectodermal) surface, into a more centralized (ganglionic) type of nervous system, which at the same time has in part retreated from the surface layer (ectoderm) from which it was originally derived
Fig. 358. A two-neurone reflex arc in a Vertebrate. gg.. Ganglion, van Gehuchten.
Furthermore, when we consider the great number of receptors and effectors in even simple forms, it is apparent that for effective coordination there must be a considerable degree of complexity of association between the afferent and efferent neurones. These associations may be to some extent accomplished by various branches of the afferent and efferent neurones coming directly into various relations with each other, but it is also evident that when a certain degree of complexity is reached, such an arrangement would necessitate an extraordinary number of afferent and efferent neurones or an extraordinary development of branches of each where they connect. Accordingly we find a second category of neurones, the intermediate or central neurones which mediate between the afferent and efferent peripheral neurones. These central neurones, together with portions of peripheral neurones in immediate relation with them, form, in all fairly well differentiated nervous systems, including those of all Vertebrates, the central as distinguished from the peripheral nervous system.
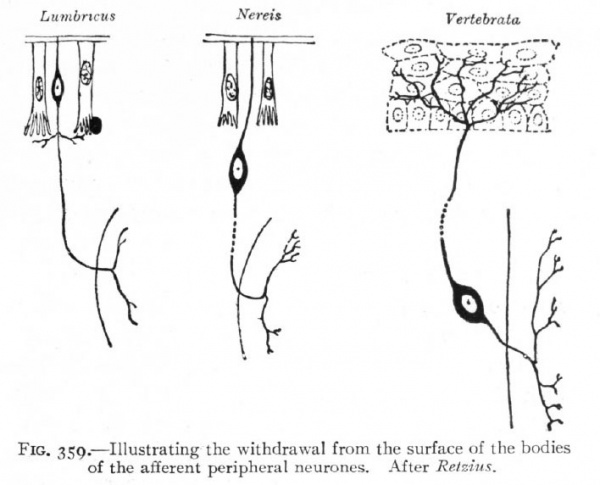
Fig. 359. Illustrating the withdrawal from the surface of the bodies of the afferent peripheral neurones. After Retzius.
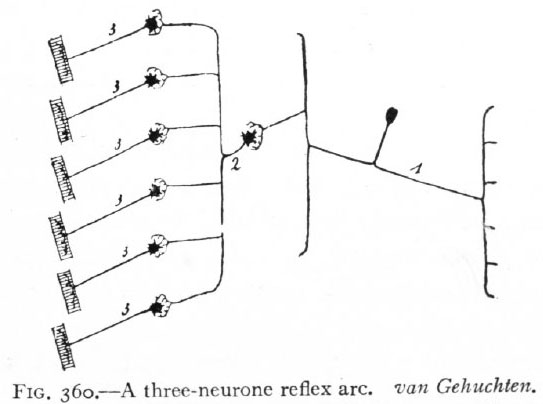
Fig. 360. A three-neurone reflex arc. van Gehuchten. Afferent peripheral neurone; 2, intermediate or central neurone; 3, efferent peripheral neurones.
The change or stimulus would now pass from receptor through (i) afferent peripheral neurones, (2) intermediate neurones, (3) efferent peripheral neurones to effector. This arrangement constitutes a three-neurone reflex arc (Fig. 360), and is evidently capable of complicated combinations which may be further increased in complexity by the intercalation in the arc of other intermediate neurones. Finally, in the central nervous system certain structures consisting of intermediate neurones are developed which represent the mechanisms for certain coordinations of the highest order. Such are the higher coordinating centers (suprasegmental structures of Adolf Meyer).
As a result of the preceding, it follows that in seeking the explanation for various nervous structures there must always be kept in mind, first, their correlation with peripheral structures and, second, the degree of development of the central coordinating mechanism represented by the intermediate or central neurones. The most important features common to the nervous systems of all Vertebrates owe their uniformity either to a corresponding uniformity in the peripheral receptors and effectors, or to a uniformity in the coordinations of the stimuli received and given put by the central nervous system. Variations in structure are due to variations of either the peripheral or central factor above mentioned. In the lower Vertebrates the former factor plays a relatively more important part than in the higher Vertebrates, the central apparatus being simpler; while in the development of the higher vertebrate nervous systems the dominating factor is the increasing complexity of the central mechanism. The superiority of the nervous system of man does not consist, in the main, of superiority in sense organs or motor apparatus, but in the enormous development of the intermediate neurone system.
General Plan of the Vertebrate Nervous System
The Vertebrate is an elongated bilaterally symmetrical animal progressing in a definite direction, primitively perhaps by alternating lateral contractions performed by a segmented lateral musculature. Associated with these characteristics are the bilateral character of the nervous system and its transverse segmentation, shown by its series of nerves, a pair to each muscle segment. The definite direction of progression involves a differentiation of the forward extremity of the animal, such as the location there of. the mouth and respiratory apparatus and the development there of specialized sense organs, the nose, eye, ear, lateral line organs, and taste buds, which increase the range of stimuli received by the animal and thereby render possible a greater range of responsive activities in obtaining food and in reproduction. As a natural outgrowth of these specializations, the highest development of the central coordinating mechanism also takes place at the forward end or head. This concentration and development of various mechanisms in the anterior end is usually termed cephalizatian, and is a tendency exhibited also by various groups of Invertebrates in which the same general conditions are present.
The typical vertebrate nervous system, then, consists of a bilateral central nervous system connected by means of a series of segmental nerves with peripheral structures (receptors and effectors) and exhibiting at its anterior extremity a higher development and specialization in both its peripheral and central parts.
The general features of the typical vertebrate nervous system are best revealed by a brief examination of certain stages in its development.
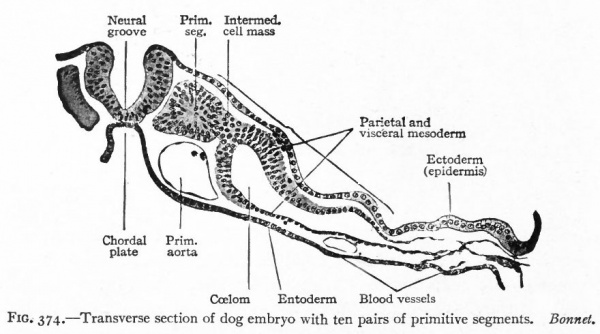
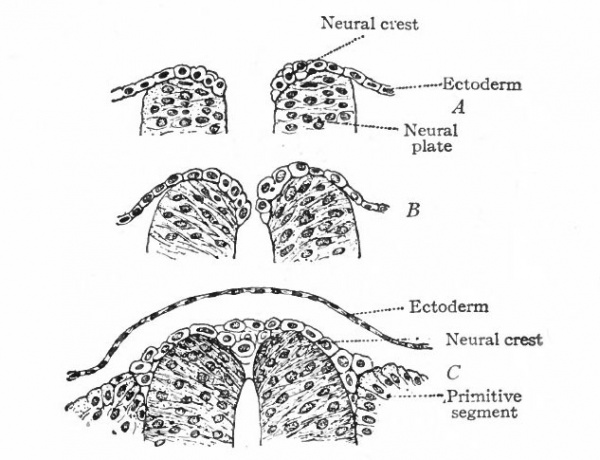
The entire nervous system, except the olfactory epithelium and parts of certain ganglia (see p. 422), is derived ontogenetically from an elongated plate of thickened ectoderm, the neural plate. This plate extends longitudinally in the axis of the developing embryo, its position being usually first indicated externally by a median groove, the neural groove (Fig. 372), the edges of the plate being elevated into the neural folds (Fig. 373). The neural folds are continuous around the cephalic end of the plate, but diverge at the caudal end, enclosing between them in this region the blastopore. Even at this stage, the neural plate is usually broader at its cephalic end, thereby indicating already the future differentiation into brain and spinal cord (Fig. 375). The neural folds now become more and more elevated (Fig. 374), presumably due in part to the growth of the whole neural plate, and finally meet dorsally and fuse, thus forming the neural tube (Figs. 52 and 391). The fusion of the lips of the neural plate to form the neural tube usually begins somewhere in the middle region of the plate and thence proceeds both forward and backward (Fig. 83). The last point to close anteriorly is usually considered as marking the cephalic extremity of the neural tube, and is called the anterior neuropore.
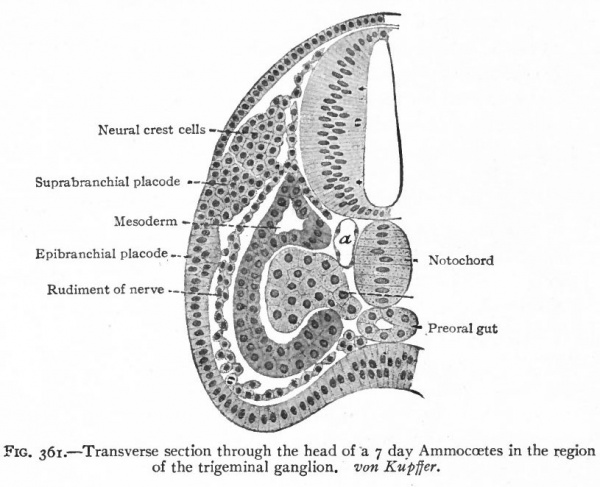
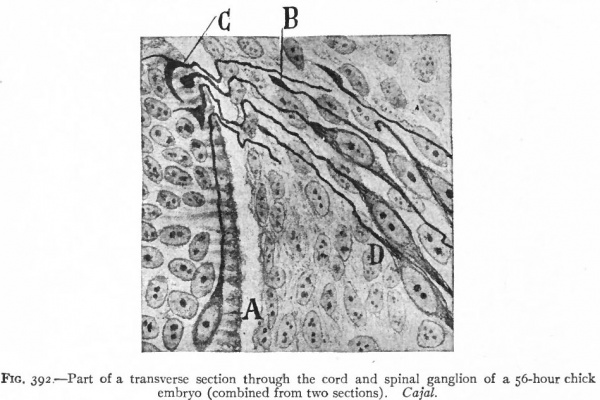
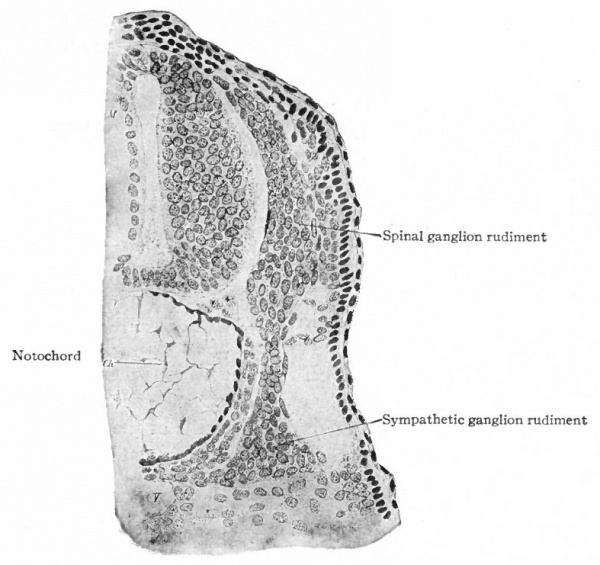
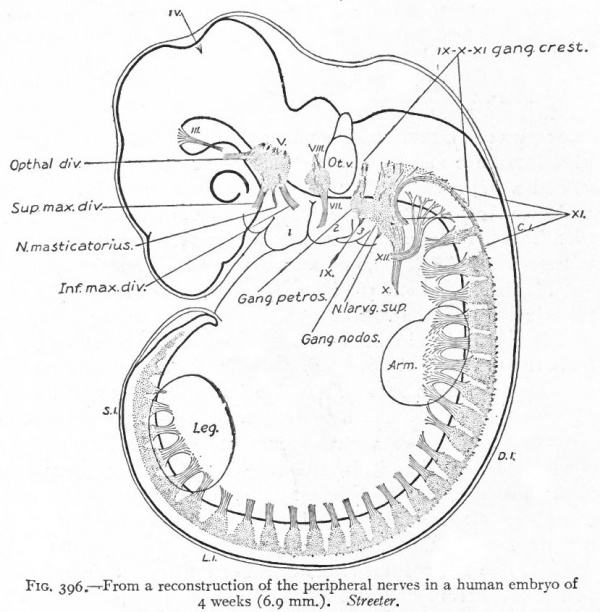
Even before the neural plate closes to form the tube, there is often a differentiation of cells along each edge, forming an intermediate zone between the neural plate and the non-neural ectoderm (Fig. 391). As the neural plate becomes folded dorsally into the neural tube these two zones are naturally brought together at the point of fusion of the dorsal lips of the neural plate. The two zones thus brought together are not included in the wall of the neural tube, but form a paired or unpaired ridge of cells lying along its dorsal surface. This ridge of cells is called the neural crest (Fig. 391). Later, each half of the neural crest separates from the other half and from the neural tube and passes ventrally down along the sides of the tube, at the same time becoming transversely divided into blocks of cells (Fig. 396). These masses of cells are the rudiments of the cerebrospinal ganglia and differentiate into the afferent peripheral neurones, and into some at least of the efferent peripheral visceral neurones (sympathetic) as well as some other accessory structures (see pp 459 to 464). The peripheral processes of these ganglion cells (afferent peripheral nerve fibers) pass to the receptors, the central processes (afferent root fibers) enter the dorsal part of the nerve tube (Fig. 392). In the case of the special sense organs there is an interesting tendency on the part of portions of the neural tube, either evaginations (optic vesicles, olfactory bulbs), or ganglia, to fuse with ectodermal thickenings (placodes) at the site of the future sense organs. There appear to be often two series of ganglionic placodes in the head, a dorsal (suprabranchial) series and a ventral (epibranchial) series, the latter being often known as gill cleft organs. The former appear to be especially connected with the development of the acustico-lateral system, the latter probably with the gustatory (see p. 432 )- (Fig. 361). The bodies of the efferent neurones (except the sympathetic) remain in the neural tube, lying in its ventral half, and send their axones out as the efferent peripheral nerve fibers to the effectors.
Fig. 361. Transverse section through the head of a 7 day Ammocoetes in the region of the trigeminal ganglion. von Kupffer.
The formation of the neural plate and its closure into a tube are the embryological expression of the above noted tendency of highly specialized neural structures to concentrate and withdraw from the surface (p. 418). The same is true of the less highly specialized placodes, in which this process is not carried so far. The neural plate may thus be regarded as the oldest placode. The afferent peripheral neurones would naturally originate from the borders of this plate, such portions being the last to separate from the non-neural ectoderm or outer surface. They may be regarded as the youngest portions, phylc genetically, of the plate, and there seems to be some variation among Chordates as to the degree of inclusion of the afferent peripheral neurones in the plate.
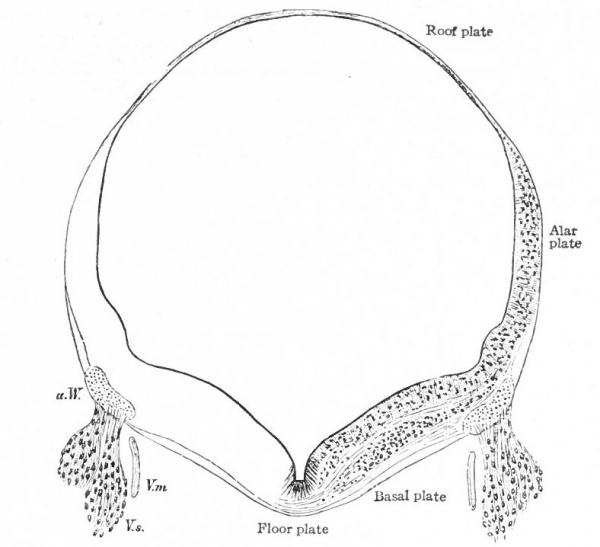
In the neural tube thus formed, there can be distinguished four longitudinal plates or zones : A ventral median plate (floor plate}, a dorsal median plate (roof plate), where the fusion occurred, and two lateral plates (e.g., Fig. 404).
Two points are to be noted : First, that the neural plate is a bilateral structure and the future development of the tube will naturally take place principally in the side walls or lateral plates of the formed tube; second, that the primary connection between the two side walls is the ventral median plate, the dorsal median plate having been produced by a secondary fusion. This being the case, the ventral connection between the two lateral plates will naturally be more extensive and possibly more primitive than the dorsal. The ventral and dorsal median plates do not usually develop nervous tissue, but bands of vertical elongated ependyma cells. In places the roof plate expands into thin membranes which are covered with vascular mesodermal tissue forming chorioid plexuses, such as the chorioid plexuses of the lateral, third and fourth ventricles (Fig. 370).
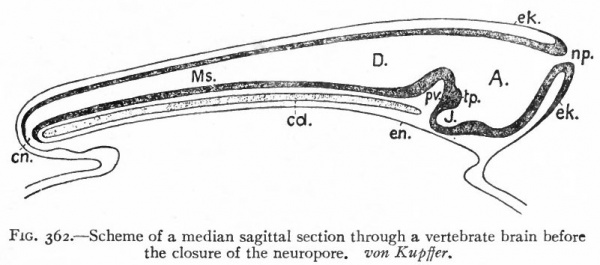
Fig. 362. Scheme of a median sagittal section through a vertebrate brain before the closure of the neuropore. von Kupffer.
- A., Archencephalon; D., deuterencephalon; Ms., medulla spinalis (spinal cord); cd., notochord; en., neuronteric canal; ek., ectoderm; en., entoderm; J., infundibulum; np., neuropore; pv., ventral cephalic fold; tp., tuberculum posterius
It has already been seen that even at its first appearance the neural plate exhibits a differentiation into an anterior expanded part, the brain, and a posterior narrower part, the spinal cord. After closure, in many Vertebrates at least, a three-fold division can be made out: (1) A caudal part of the neural tube, the spinal cord, which gradually expands cranially into (2) the caudal part of the brain (deuterencephalon, v. Kupffer) (Fig. 362). These two parts lie above the notochord and all the typical cerebrospinal nerves are connected with them. (3) Cranially, at the anterior end of the notochord, the brain wall expands ventrally forming the third portion (archencephalon) . At the forward extremity is seen the anterior neuropore. The deuterencephalon is thus an epichordal part of the brain, while the archencephalon is prechordal. At the boundary between the two is a ventral infolding of the brain wall the ventral cephalic fold (plica encephali ventralis). At this stage the brain resembles that of Amphioxus in many respects. From each side wall of the archencephalon an evagination appears, the optic vesicle (Fig. 376) which develops into the retina and optic nerve.
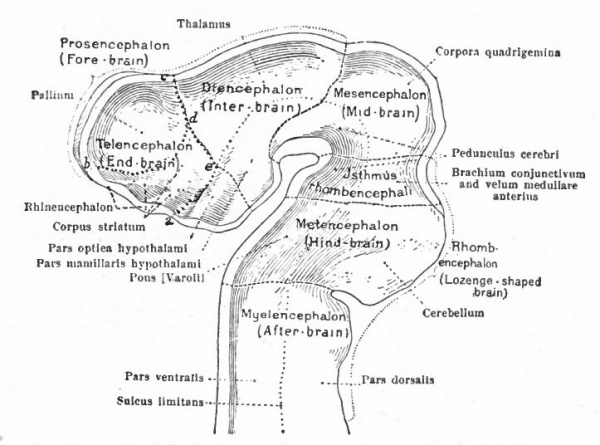
In the next stage (Fig. 363), there is a tendency for the neural tube to bend ventrally around the anterior end of the notochord. This bending is the cephalic flexure. At the same time the dorsal wall above the cephalic fold becomes expanded and is marked off from that part of the dorsal wall lying caudally by a transverse constriction, the rhombo-mesencephalic fold, and from the part of the dorsal wall lying cranially by another transverse fold at the site of the future posterior commissure. The middle part of the brain, the roof of which is thus marked off, is the mid-brain or mesencephalon. Its floor is the middle projecting part of the ventral cephalic fold. The cephalic expansion of the brain, practically the former archencephalon, is now the of the three primary brain expansions, fore-brain or prosencephalon and the caudal expansion, is the rhombic brain or rhombencephalon.
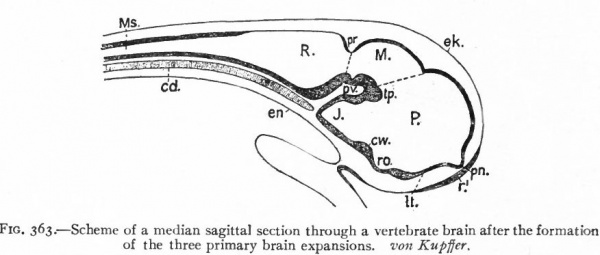
Fig. 363. Scheme of a median sagittal section through a vertebrate brain after the formation of the three primary brain expansions. von Kupffer. P.. prosencephalon; M., mesencephalon; R., rhombencephalon ; Ms., spinal cord; cw., chiasma eminence; J., infundibulum; It., lamina terminalis; pv., ventral cephalic fold; pn., processus neuroporicus; pr., rhombo-mesencephalic fold; r. 1 , unpairecTolfactory placode; ro., recessus (prae-?) opticus; tp., tuberculum posterius.
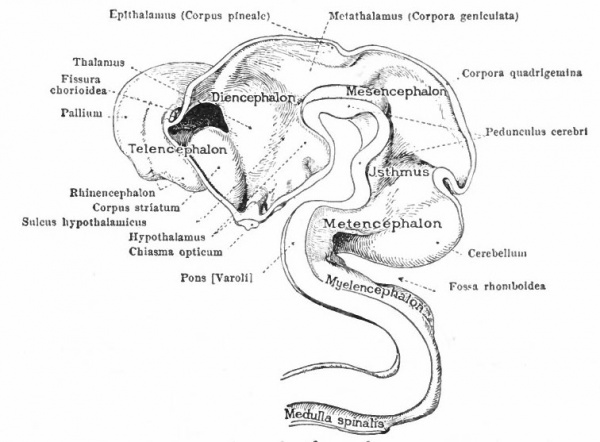
These three primary brain expansions ("vesicles"), the fore-brain, midbrain and rhombic brain, are constant throughout the Vertebrates. Beginning at the location of the former neuropore (processus neuroporicus) and passing caudally along the floor of the fore-brain we have the lamina terminalis or endwall of the brain, containing a thickening which indicates the site of the future anterior (cerebral) commissure, next the recessus praopticus, then another thickening, the chiasma eminence, and finally a diverticulum, the recessus postopticus and infundibulum (Fig. 363).
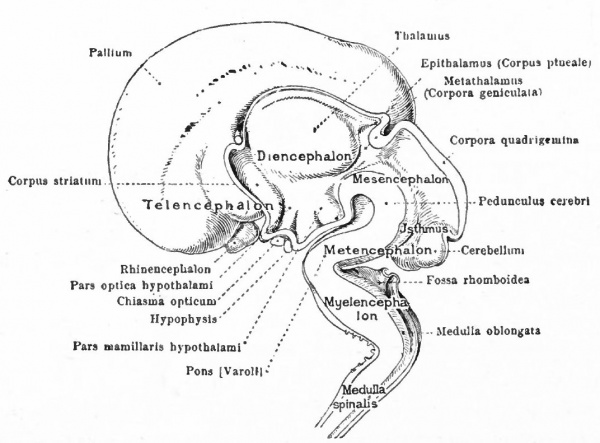
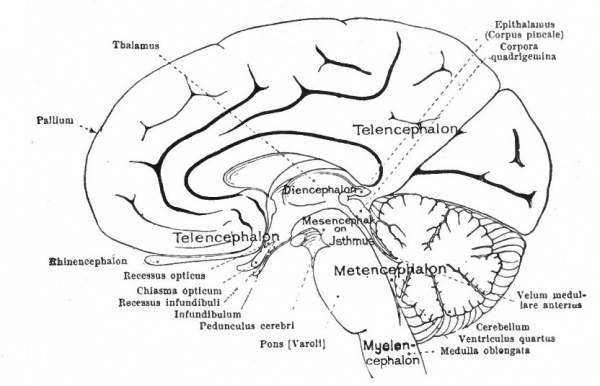
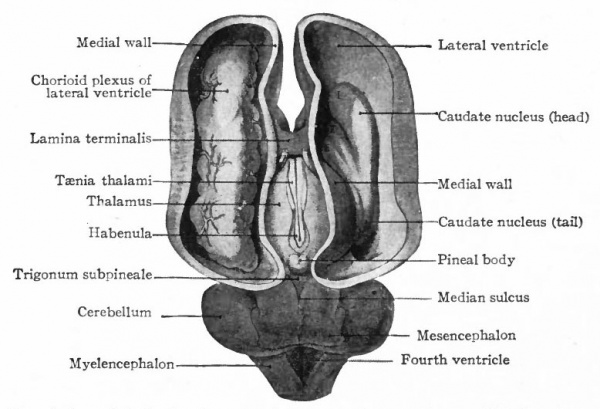
At a later stage (Fig. 364), there appear two evaginations in the roof of the fore-brain, the anterior epiphysis or paraphysis and the posterior epiphysis or epiphysis proper (pineal body). Immediately caudal to the paraphysis is a transverse infolding of the brain roof, the velum transversum. The line aa (Fig. 364) extending from this fold to the optic recess indicates the location of a fold in the side walls in some forms and is taken by some as the boundary between two subdivisions of the fore-brain, the end-brain or telenccphalon and the inter-brain or diencephalon. Cranial to the epiphysis proper, is a commissure in 'the dorsal wall (commissura habenularis) connecting two structures which develop in the crests of the side walls, the ganglia habenula.
From the dorsal part of the telencephalon is developed the pallium. The ventral anterior part evaginates toward the olfactory pit, its end receiving the olfactory fibers. This region is often termed the rhinencephalon. Thickenings of the basal lateral walls of the telencephalon form the corpora striata.
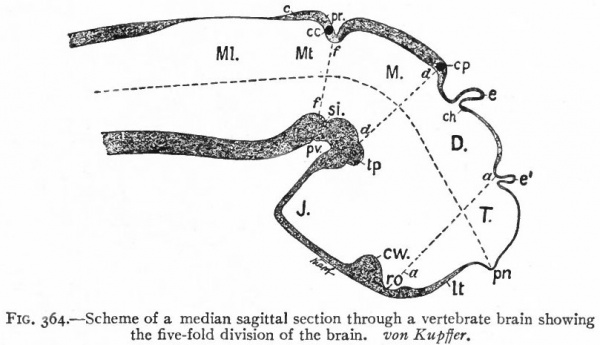
Fig. 364. Scheme of a median sagittal section through a vertebrate brain showing the five-fold division of the brain. von Kupffer.
- T., Telencephalon; D., diencephalon; M., mesencephalon; Mt., metencephalon; Ml., myelencephalon; c., cerebellum; cc., cerebellar commissure; ch., habenular commissure; cp., posterior commissure; cw., chiasma eminence; e., epiphysis; e*., paraphysis; J., infundibulum; lt. t lamina terminalis; pn., processus neuroporicus; pr., rhombo-mesencephalic fold; pv., ventral cephalic fold; ro., recessus (prae-) opticus; si., sulcus intraencephalicus posterior; tp., tuberculum posterius. The lines aa., dd and ff indicate the boundaries between four divisions.
The roof of the mesencephalon finally develops the "optic lobes." The dckened part of the roof lying immediately caudal to the rhombo-mesencephalic fold develops into the cerebellum. The part of the tube of which this forms the roof is often called the hind-brain or metencephalon, while the rest of the lombencephalon is then termed the after-brain or myelencephalon. The roof of i is portion, which has become very thin in the course of its development, forms epithelial part of the tela chorioidea of the fourth ventricle. The conicted portion of the tube between the rhombic brairv and mid-brain is the \thmus.
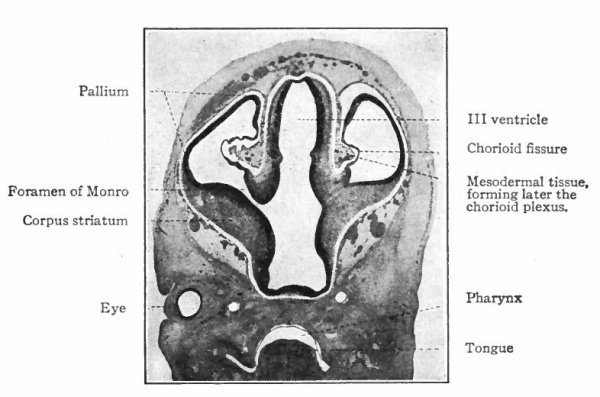
The above subdivisions of the three primary expansions into five parts (end-, inter-, mid-, hind- and after-brains), especially the subdivisions of the rhombic brain, do not have the morphological value of the three primary divisions but have a certain value for descriptive purposes. The cavities of the brain are the ventricles and their connecting passages, namely, the third ventricle of the diencephalon and the fourth ventricle of the rhombencephalon, the two being connected by the mid-brain cavity (aquceductus Sylvii). The telencephalon usually develops a more or less paired character, its cavities being then paired diverticula of the unpaired fore-brain cavity and known as the lateral ventricles.
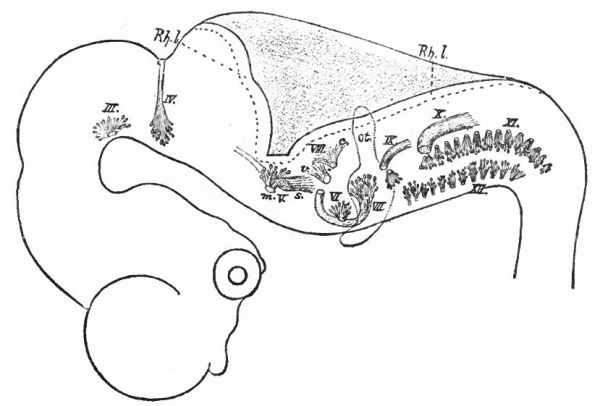
Before the closure of the brain part of the neural tube, transverse constrictions appear across the neural plate. The transverse rings into which the tube, when completed, is thus divided are known as neuromeres. They are held to represent a primitive segmentation of the head, similar, perhaps, to that exhibited by the spinal nerves and segmental somatic musculature (primitive segments) of the trunk. The neuromeres may appear before the head somites. To what extent they correspond to the somites or to the visceral segmentation (p. 430) and also to the cranial nerves is a matter of dispute. Concerning their number there have been various views, the evidence inclining to three in the fore-brain, two in the mid-brain and six in the rhombic brain (Fig. 365). Their presence and number are most in doubt in the cephalic end of the tube, the highly modified prosencephalon.
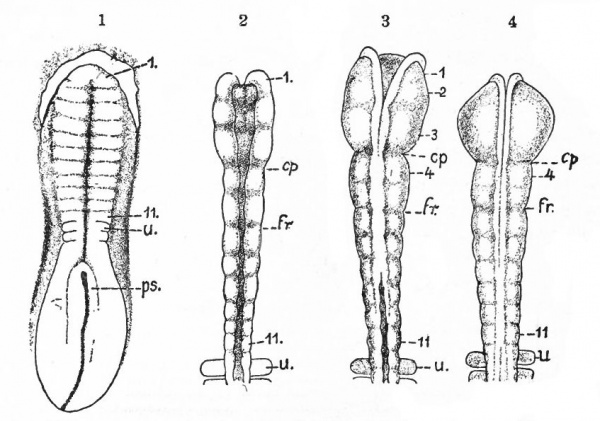
Fig. 365. Chick embryos; 1, of 22 hours' incubation; 2, of 24 hours; 3, of 25.5 hours; 4, of 26 hours. Showing respectively 2, 5, 6, and 7 primitive segments. Hill.
- cp., Caudal limit of fore-brain ; fr., caudal limit of mid -brain; u., first primitive segment; ps., primitive streak; I-II, neuromeres.
The general features of the vertebrate nervous system which especially illuminate conditions met with in the human nervous system are the following: (1) The correlation between the peripheral structures (receptors and effectors) and the nervous system. (2) The distinction between the epichordal and prechordal portions of the brain. The latter (fore-brain) is, in accordance with its anterior position (comp. p. 420), the most highly modified part of the neural tube. (3) The distinction between the segmented and suprasegmental parts of the brain (Adolf Meyer).* The segmental part of the brain is that portion in more immediate connection with peripheral segmental structures. Its epichordal part is spinal-like and most clearly segmental. Its prechordal part, both as to its peripheral and central portions, is so highly modified that its segmental character is more obscure. It and the rest of the prechordal brain are most conveniently treated together as fore-brain. The suprasegmentai parts of the brain, or higher coordinating centers, are the cerebellum, midbrain roof and the pallium (cerebral hemispheres). Their general functional significance has been mentioned (p. 420). Some of their general structural characteristics are : First, that they are each expansions of the dorso-lateral walls of the neural tube; second, that in them the neurone bodies are placed externally and in layers (cortex), the nerve fibers (white matter) lying within; third, that each appears to have originally had an especially close relation with some one of the three great sense organs of the head, the olfactory, visual or acustico-lateral system; fourth, that each is connected with the rest of the brain by bundles of centripetal and centrifugal fibers, and often there are specialized groups of neurone bodies in other parts of the brain for the origin or reception of such bundles. Each higher center has also its own system of association neurones.
It will accordingly be most convenient to consider : (1) the spinal cord, (2) the segmental part of the epichordal brain, (3) the cerebellum, (4) the midbrain roof, (5) the prosencephalon.
Spinal Cord and Nerves
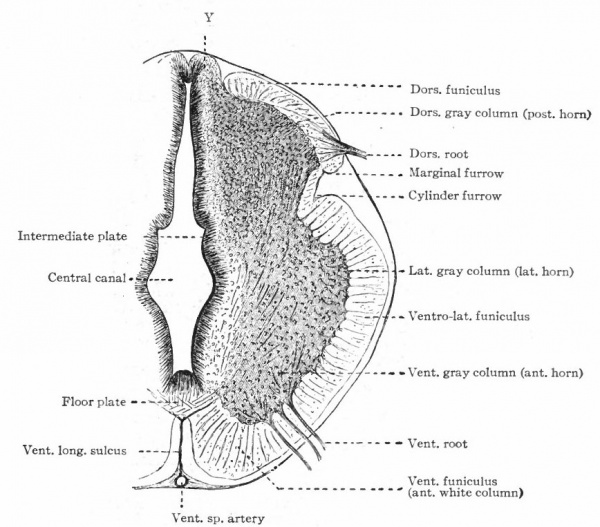
As already brought out, there are two principal morphological differences between the afferent and efferent peripheral neurones. First, the neurone bodies of the former are located outside the neural tube, while the neurone bodies of the latter lie within the walls of the neural tube. Second, the afferent nerves enter the dorsal part of the lateral walls of the tube, while the efferent nerves leave the ventral part of the lateral walls, their neurone bodies lying in this ventral part. The effect of this upon the structural arrangements within the tube is the production in the tube of two columns of neurone bodies, a dorsal gray column for the reception of the dorsal or afferent roots and a ventral, gray column containing the efferent neurone bodies.
* This distinction apparently ignores the fact that the primitive neuromeric segmentation of the neural tube involves its dorsal as well as its ventral walls and thus "suprasegmental" as well as "segmental " structures were originally segmental. This may be granted, but while the demonstration of the primitive segmentation of the neural tube may be valuable as showing the primitive mechanism which has undergone later modifications, the importance of such later modifications renders the above distinction necessary. The main significance of the nervous system is its associative character and its progressive development is not as a segmental, but as a more and more highly developed associating mechanism.
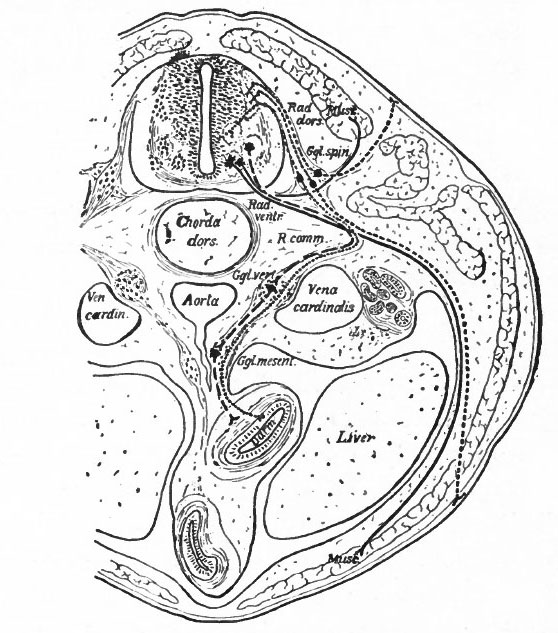
Fig. 366. Transverse section through the body of a typical Vertebrate. Showing the peripheral (segmental) nervous apparatus. Froriep.
- Small dots, afferent visceral neurones; coarse dots, afferent somatic neurones; dashes, efferent visceral (ventral root and sympathetic) neurones; lines, efferent somatic neurones. Darm, gut; Ggl. spin., spinal ganglion; Ggl. vert., vertebral sympathetic ganglion; Ggl. mesent., mesenteric sympathetic ganglion. The peripheral sympathetic ganglionic plexuses (Auerbach and Meissner) are not shown. Muse., muscle; Rad. dors., dorsal root; Rad. vent., ventral root; R. comm., white ramus communicans. Two sympathetic neurones are represented as intercalated in the visceral efferent pathway. It doubtful if there should be more than one.
Another important differentiation arises apparently from the important physiological difference in general character between the activities of what may be termed the internal (visceral or splanchnic) and the external (somatic) structures. Internal activities are to a certain extent independent of activities which have to do more with the reactions of the organism to the external world, and consequently their nervous mechanisms have a more or less independent character, forming what is often called the autonomic (sympathetic) system. This independence is exhibited structurally by the intercalation in the peripheral pathway of additional neurones, whose bodies form visceral ganglia connected in various ways among themselves and probably having their own reflex arcs or plexuses. These ganglia are nevertheless to some extent under the control of the efferent neurones of the central nervous system, some of which send their axones to such ganglia (Fig. 366). There are thus in the central nervous system two categories of efferent peripheral neurones, those innervating visceral structures "via sympathetic ganglia and those innervating somatic structures. The bodies of the somatic efferent neurones are located in the ventral gray matter of the nerve tube, while the bodies of the splanchnic efferent neurones are believed to occupy more central and lateral positions in the lower half of the gray matter of the neural tube (Fig. 366). It is uncertain whether there are similar afferent splanchnic neurones in the sympathetic ganglia, and thus distinct from those in the spinal ganglia, or whether these all lie in the spinal ganglia and are consequently not fully differentiated from the somatic afferent neurones.
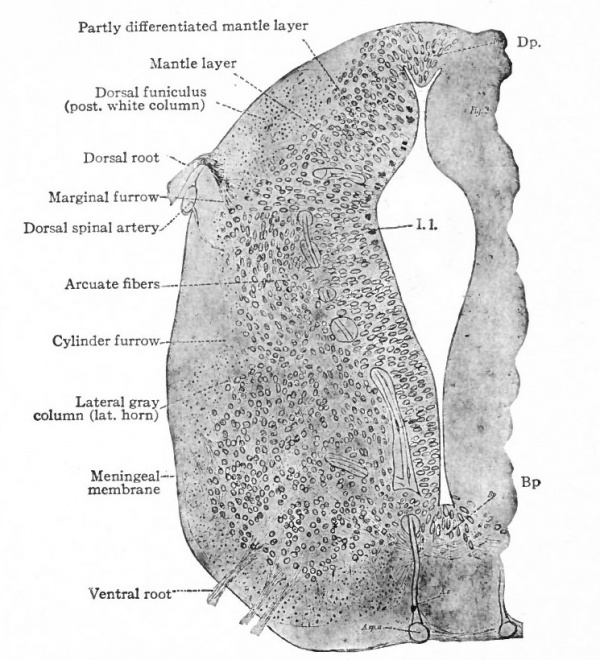
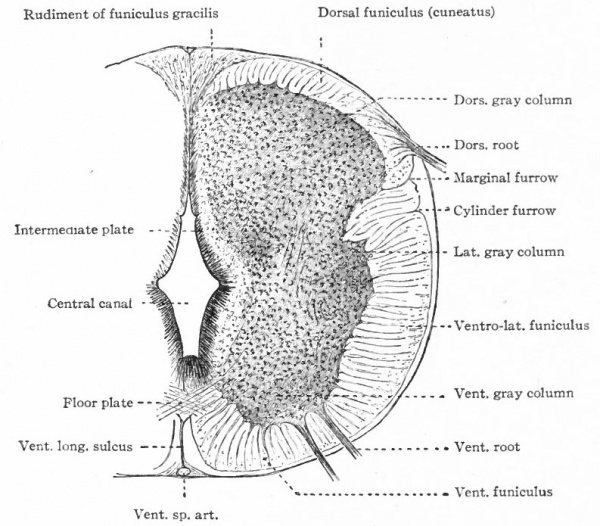
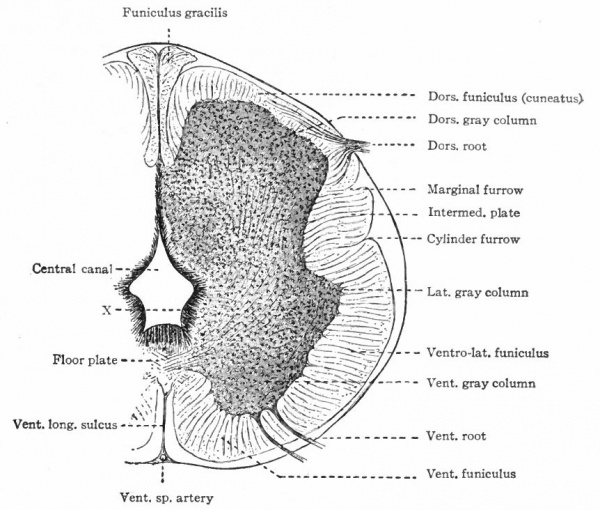
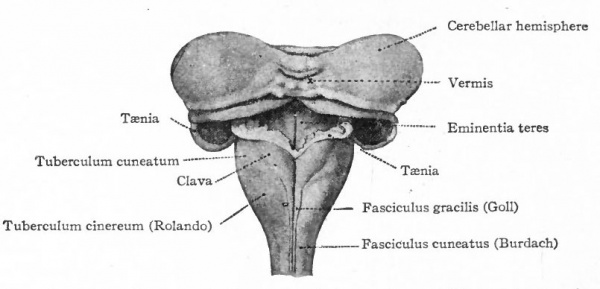
The muscular segmentation of the trunk has already been mentioned and also the corresponding segmental arrangement of the spinal nerves. Local extensions of this musculature and of its overlying cutaneous surface in the form of fins and limbs cause corresponding increase in the size of those segments of the cord innervating them. This is due to the increased number of afferent fibers and consequent increase in the dorsal white columns and in the receptive dorsal gray columns, also to the increase in the number of efferent peripheral neurones whose bodies occupy the ventral gray column (e.g., cervical and lumbar enlargements). (Compare also the differentiation in the cervical cord and lower medulla of the columns and nuclei of Goll for the lower extremities and those of Burdach for the upper extremities).
In general, the intermediate neurones of the cord fall into two categories; intersegmental (ground bundles), connecting cord segments, and those sending long ascending bundles to suprasegmental structures (see pp. 442 and 443.)
The Epichordal Segmental Brain and Nerves
The principal peripheral structures which exert a determining influence on the structure of the epichordal brain are: The mouth, the respiratory apparatus (gills and later lungs), and two specialized sensory somatic structures, the acustico-lateral system and the optic apparatus.
In the gills we have essentially a series of vertical clefts forming communications between the pharynx and the exterior, the intervals between the clefts being the gill arches. The musculature of the gill arches is morphologically splanchnic (pp. 272 and 280). The gill or branchial musculature is in closer relations with stimuli from the external world than is the visceral musculature of the body. As a result of this the former is not of the smooth involuntary type, like the visceral musculature of the body, but is of the striated voluntary type, like the somatic musculature. The branchial receptors are naturally visceral in character and there is also in this region a series of specialized visceral receptors, the end buds of the gustatory system. The development of this whole specialized visceral apparatus in this region of the head has apparently caused a corresponding reduction of the somatic musculature.
The musculature of the mouth is also splanchnic, the mouth itself beingregarded by many morphologists as a modified pair of gill clefts which has replaced an older mouth lying further forward in the region of the hypophysis. The existence of this series of gill clefts has naturally caused a branchiomeric pir splanchnic segmentation of the musculature of this region as opposed to the somatic muscular segmentation seen in the trunk. Whether these two kinds of segmentation correspond in this region is uncertain. (In this connection see Fig. 390 and p. 466.)
In the acustico-lateral system three parts may be distinguished : (1) a remarkable series of cutaneous sense organs, extending in lines over the head and body and known as the lateral line organs; (2) the vestibule, including the semicircular canals; (3) the cochlea (organ of hearing proper Cor ti's organ) . In the higher Vertebrates, the lateral line organs have disappeared, owing to a change from a water to a land habitat; the labyrinth has remained unchanged, and the cochlea has undergone a much higher development and specialization.
Regarding the optic apparatus, it is sufficient to point out here that its motor part, the eye muscles, is usually taken to represent the sole remaining somatic musculature belonging to the head proper.
The peripheral nerves of the epichordal part of the brain have fundamentally the same arrangements as the spinal nerves, namely, the peripheral afferent neurone bodies are separate from the nerve tube, forming ganglia, while the bodies of the efferent neurones are located centrally in the morphologically ventral portions of the lateral walls of the nerve tube. There are, however, important differences, clearly correlated with the peripheral differentiations and specializations outlined above, and affecting the afferent and efferent nerves.
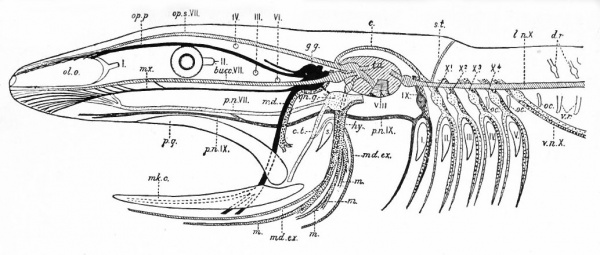
Fig. 367. Diagram showing the principal branches of the cranial nerves in a fish. (Modified from Wiedersheim.)
- mk. c., Meckel's cartilage; ol. o., olfactory organ; p.q., palato-quadrate; s., spiracle; I-V, brachial clefts; I, II, III, IV, VI, the first, second, third, fourth and sixth cranial nerves. The remaining nerves are differently shaded.
- Black - The afferent general somatic system (trigeminal, Vth nerve); g.g., Gasserian ganglion; md., mandibularis; mx., maxillaris; op. p., opthalmicus profundus.
- Oblique Shading - The lateral line system from its center (t.a.), the tuber acusticum. Bucc. VII, buccalis branch of VII; md. ex., external mandibular branch of VII; l.n. X, lateralis nerve, with its supra-temporal branch (s.t.) and its commissural connection (c) with op. s.
- Light dots - The afferent brachio-visceral (splanchnic) system (dark gray for the IX) and heavier dots the efferent brachio-visceral system.
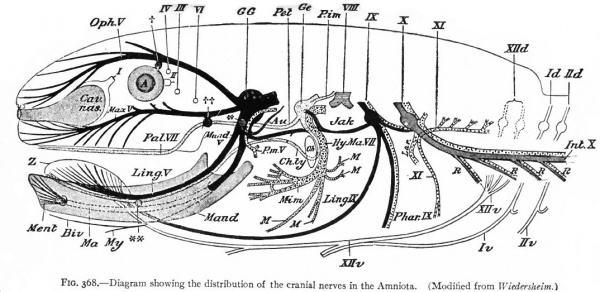
Fig. 368. Diagram showing the distribution of the cranial nerves in the Amniota. (Modified from Wiedersheim.)
First to be considered is the afferent- part of the trigeminus (Figs. 367 and 368). The peripheral branches of the ganglion (semilunar or Gasserian ganglion) of this nerve innervate that part of the external (somatic) surfaces of the head (skin and stomodaeal epithelium) which have not been encroached upon by the spinal afferent nerves. This nerve is accordingly more strictly comparable with the afferent spinal nerves. The central processes of the semilunar ganglion cells, after entering the brain, form a separate descending bundle, the spinal V. It is interesting to note that the terminal nucleus of this bundle of fibers is the morphological continuation in the brain of the dorsal gray column of the cord. The extensiveness of the area innervated by the trigeminus may be partly due to disappearance or specialization of anterior somatic nerves and also to the growth of the head.
The organs of the lateral line are innervated by a quite distinct system of ganglionated afferent nerves whose central connections are nearly identical with those of the acoustic (Fig. 367). With the disappearance of the lateral line organs and the specialization of the cochlear part of the ear vesicle, there is a disappearance of the lateral line nerves (comp. Figs. 367 and 368) and a wellmarked division of the acoustic nerve into vestibular and cochlear portions, the former innervating the older vestibule-semicircular canal portion, the latter, the more recent cochlea. Centrally, the vestibular nerve forms also a descending bundle of fibers and has its own more or less specialized terminal nuclei. The latter is also true of the cochlear nerve.
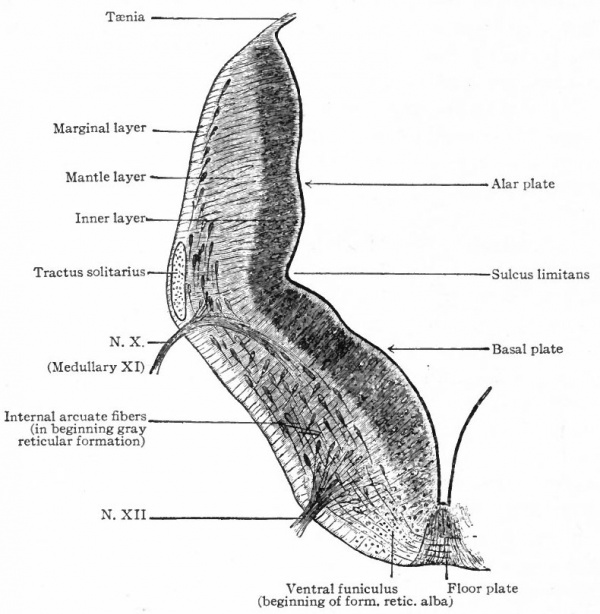
The afferent portions of the facial, glossopharyhgeal and vagus nerves innervate the splanchnic receptors of the pharyngeal and branchial surfaces as well as of a large part of the viscera. The facial, glossopharyngeal and vagus also innervate the specialized splanchnic receptors, the gustatory system mentioned above. This system of taste buds has a very extensive development in certain lower Vertebrates, especially the Bony Fishes. In the latter the system of nerves innervating these structures is naturally much more extensive and its central terminations and nuclei cause important modifications of the medulla. In Mammals the remnants of this system are represented by the taste buds in the mouth, the nerves innervating them being the chorda tympani branch of the facial and the lingual branch of the glossopharyngeal (Fig. 368). The central branches of the ganglia of these three nerves, after entering the brain, form a descending bundle of fibers, the tractus solitarius (or communis).
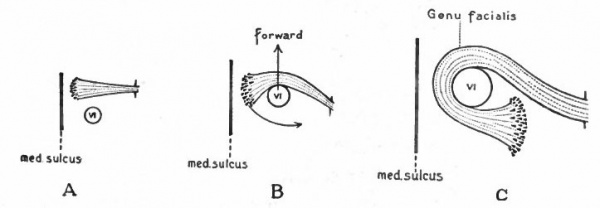
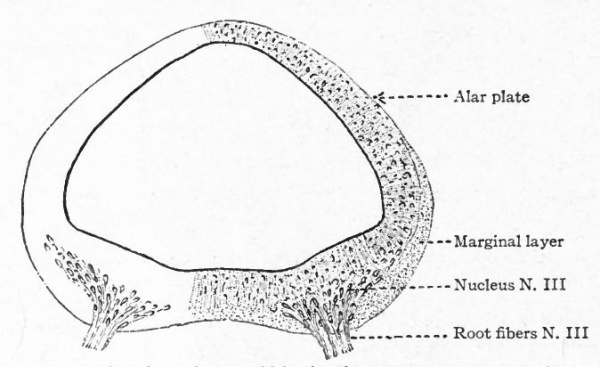
The somatic musculature of the head, as above mentioned, is usually taken to be represented by the eye muscles and, later, the tongue muscles. The tongue is one of the newer structures, rising in importance with the change to a land habitat, and its muscles are probably an invasion from the neck region caudal to the branchial arches (p. 290). The eye muscles are innervated by the III, IV and VI cranial nerves, the tongue muscles by the XII which is a more recent addition to the cranial nerves. All of these nerves are characterized by having their neurone bodies located in the most medial (morphologically most ventral) portions of the lateral brain walls, and they all, except the IV, emerge near the mid-ventral line. In these respects they resemble the major or somatic part of the ventral spinal roots. (For illustration see Figs. 389, 367 and 368).
The splanchnic musculature of the jaws and the branchial arches is innervated by the efferent portions of the V, VII, IX, X (and XI). The neurone bodies or nuclei of origin of these nerves lie more laterally than those of the III, IV, VI and XII, and their axones also leave the nerve tube more laterally along with the incoming afferent fibres. These nerves all exhibit a characteristic segmental arrangement corresponding to that of the gill clefts. The VII, IX, and the various nerves making up the X, divide dorsal to the corresponding gill clefts into prebranchial and postbranchial branches, also giving off suprabranchial branches. The efferent element, or component, forms a part of each postbranchial branch. These relations are shown clearly in the accompanying diagrams (Figs. 367 and 368). Part of the vagus also innervates the viscera and this nerve is thus divisible into branchial and visceral portions.
Two peculiarities may be noted in regard to these splanchnic nerves : First, that the afferent portions have ganglia resembling those of the spinal nerves; second, that the branchial efferent portions consist simply of one neurone proceeding all the way from the nerve tube to the muscle innervated, thus resembling the somatic rather than the visceral nerves of the trunk. As already noted (p. 429), these nerves regulate activities somatic in character but involving splanchnic structures. It is thus seen that the dominating factor is functional rather than morphological present functional necessities modify those of the past.
With the change from a water to a land habitat and the accompanying disappearance of gills and appearance of lungs, we have various suppressions and modifications of the branchial musculature (Fig. 368). There are two striking specializations of the branchial musculature. One is the origin of the facial (mimetic) musculature in the highest Vertebrates. This is derived from the muscles of the hyoid arch, innervated naturally by extensions of the facial nerve. The other is a specialization of muscles, probably of the caudal branchial arches, into cervico-cranial muscles (head-movement), innervated by what may be considered a caudal extension of the vagus nerve, namely, the spinal accessory (p. 466). The splanchnic laryngeal musculature and its nerves show a certain degree of specialization (sound-production) in higher forms. The efferent V is naturally a large constant nerve, in correlation with the uniformly developed jaw musculature in all jaw-bearing (gnathostome) Vertebrates (Figs. 367 and 368). These various changes in peripheral structures are thus due either to environmental influences or to developments within the central nervous system (p. 420). One of the most important environmental influences is the change from a water to a land habitat. The influence of the central nervous system is shown in the further development and specialization of a number of peripheral structures as motor "instruments" of suprasegmental mechanisms.
The effects, then, of the peripheral arrangements upon the arrangements within the neural tube are:
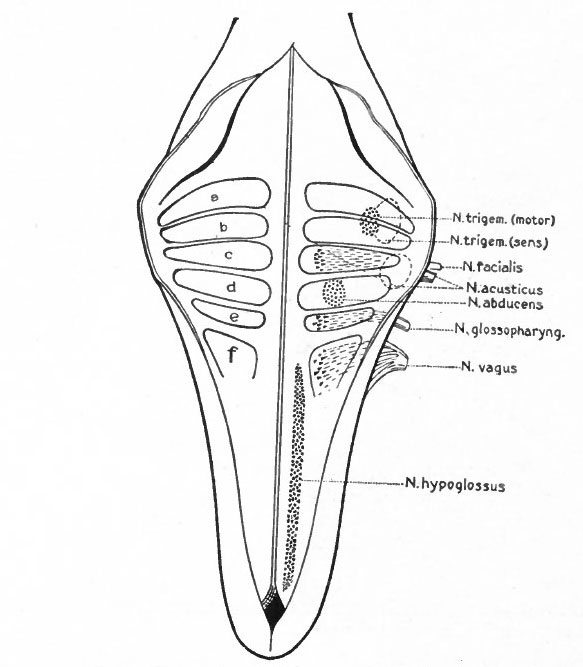
- The formation of separate tracts and terminal nuclei for (a) the unspecialized somatic afferent V nerve (spinal V and posterior horn) ; (b) the specialized somatic vestibular nerve (descending or spinal VIII and various terminal nuclei) and also the cochlear nerve and its various terminal nuclei; (c) the splanchnic afferent nerves (tractus solitarius and its terminal nuclei).
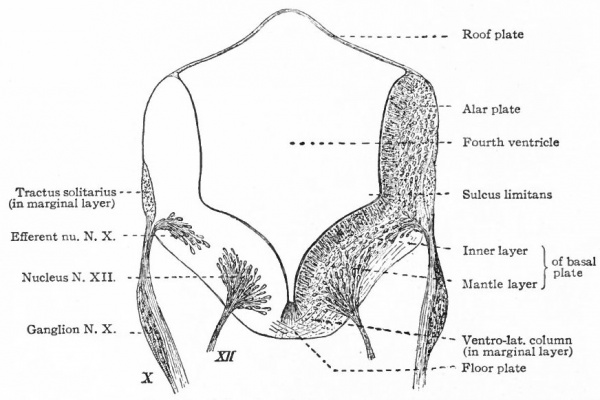
- The separation of the efferent neurone bodies lying in the neural tube into two main longitudinal series of nuclei (a) the somatic efferent nuclei, occupying a more medial position, their axones emerging from the neural tube as medial ventral nerve roots; (b) the splanchnic efferent nuclei occupying a more lateral position, their axones emerging laterally and forming mixed roots with the incoming afferent fibers (Fig 369).
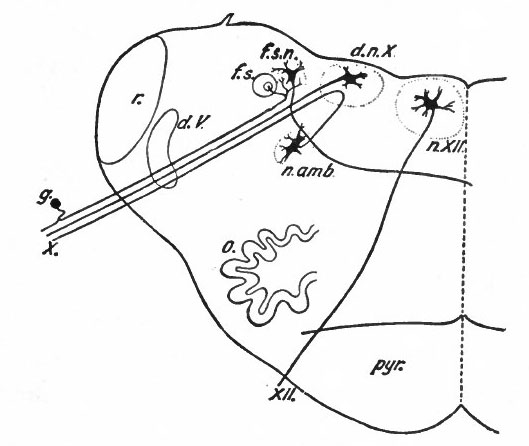
Fig. 369 Diagram of a transverse section through the lower human medulla showing the origin of the X and XII cranial nerves. Schafer.
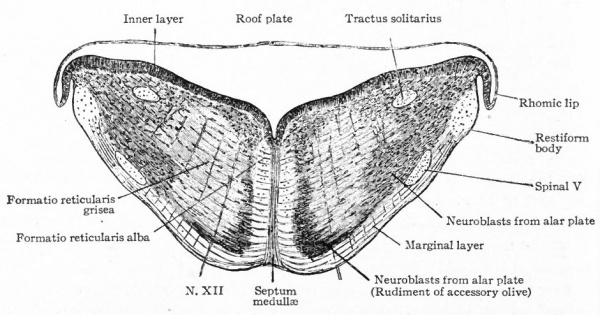
- Ganglion cell of afferent vagus sending central arm (root fiber) to solitary tract (f. s.) and collateral to the nucleus of the solitary tract (f. s. n.). It is not certain that the axones of the cells of this terminal nucleus take the course indicated in the figure, n. amb., nucleus ambiguus and d. n, X, dorsal efferent nucleus of the vagus, both of which send out axones as the efferent root fibers of the vagus. These two represent the lateral or splanchnic efferent nuclei of this region, n. XII, nucleus of the hypoglossus the axones of which pass out medially as efferent root fibers of the XII. This nucleus represents the medial or somatic efferent nuclei of this region. f. s.. tractus solitarius or descending roots of vagus, glossopharyngeus and facial; d. V., descending spinal root of the trigeminus; r., restiform body; o., inferior olivary nucleus (olive"); pyr.. pyramid.
The intermediate neurones of the epichordal segmental brain, as well as of the cord, fall into two general systems. One of these is the system of inter segmental neurones, connecting various segments of the segmental brain and cord. This system may be collectively termed the ground bundles (of the cord) and reticular formation (of the brain). These neurones may be regarded as not only furnishing the various reflex communications between the afferent and efferent cerebrospinal peripheral neurones, but as also forming a system upon which the descending neurones from the higher coordinating centers (suprasegmental structures) act, before the efferent peripheral neurones are reached. This system may thus be regarded in general as more closely associated with the efferent than with the afferent peripheral neurones. Certain tracts in this system and their nuclei of origin have reached a considerable degree of differentiation, due principally to association with higher centers. Among these differentiated reticulo-spinal tracts may be mentioned the medial longitudinal fasciculus, the rubro-spinal tract, and the various tracts from Deiters' nucleus. The other system consists of nuclei which are associated with the afferent axones as their terminal nuclei, the axones of which form long afferent tracts to suprasegmental structures. Especially well-marked differentiations of nuclei and tracts of this system are usually due both to its connections with peripheral structures and with the higher centers. The principal afferent suprasegmental tracts to the cerebellum are mentioned below (p. 436). Those to mid-brain roof and (via added neurones) to pallium are the medial fillet or lemniscus from the nuclei of the columns of Goll and Burdach, the lateral lemniscus from the cochlear terminal nuclei and other ascending tracts from terminal nuclei of peripheral afferent neurones.
The Cerebellum
The other great factor (see p. 420) affecting the structure of the epichordal brain is the development in it of two higher coordinating centers or suprasegmental structures, the cerebellum and optic lobes. The cerebellum is a development of the dorsal part of the lateral walls of the tube just caudal to the isthmus and was probably primarily developed in correlation with the acustico-lateral system, especially with the lateral line and vestibulo-semicircular canal portions (p. 430). Due probably to the fact that it is thus an important "equilibrating" mechanism, the cerebellum has acquired other important connections besides its original ones with the acustico-lateral system. In the vertebrate series it is especially developed in all active balancing forms (Fig. 370). In Mammals it has acquired important connections with the greatly enlarged pallium (cerebral hemispheres), in accordance with its general regulative influence (static and tonic) upon motor reactions. The great development of the cerebellum has profoundly modified the anatomical arrangements of the rest of the brain and cord, owing to its numerous and massive connections. The following important masses of gray matter and fiber bundles may be mentioned as cerebellar afferent connections: Clarke's column cells, and other cells in the cord, and the spino-cerebellar tracts; the lateral nuclei, inferior olives and the restiform body in the medulla; part of the pes pedunculi, the pontile nuclei and middle peduncle of the cerebellum. The superior cerebellar peduncle to the red nucleus, together with tracts to Deiter's nucleus, belong to the cerebellar efferent connections. The cortico-pontile portion of the pes, the pontile nuclei and the middle peduncle represent the most recently developed cerebral connections (comp. pp. 440-442 and Fig. 371).
The Mid-brain Roof
This expansion of the dorsal part of the neural tube constitutes a higher coordinating center for impulses received by various somatic nerves spinal, cochlear and optic. Owing to its being, in all forms below mammals, the principal visual center, the optic part (optic lobes) varies in proportion to the development of the eye, animals with poorly developed eyes having small optic lobes. In mammals, the optic part (anterior corpora quadrigemina or colliculi) is relatively less important, owing to a taking over of a portion of its coordinating functions by the neopallium (pp. 440, 442) , but the cochlear part (posterior corpora quadrigemina or colliculi) has increased in importance, owing to the rise of the cochlear organ (organ of Corti). The centripetal and centrifugal connections of the mid-brain roof are not so massive or extensive and consequently do not modify the other parts of the brain and cord as profoundly as do those of the cerebellum. It sends descending tracts to afterbrain and cord segments.
The Prosencephalon
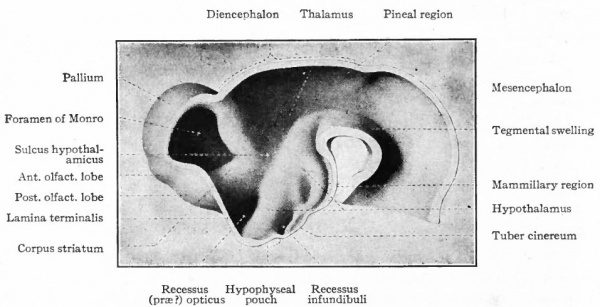
The division of this part of the brain into the telencephalon and diencephalon has already been indicated (p. 425). In the diencephalon may be noted (i) the absence of the notochord ventral to the brain, thereby permitting a ventral expansion of the brain walls, the hypothalamus, associated with an organ not well understood, the hypophysis; (2) certain more or less vestigial structures, such as the pineal eyes (epiphyses), and other primitive structures, such as the ganglia habenulae, in the dorsal part, this dorsal portion being collectively termed the epithalamus; (3) nuclei in (i) and (2) connected with olfactory and gustatory tracts; (4) receptive nuclei for the optic tract and the cochlear path from the posterior colliculus; (5) receptive nuclei for secondary tracts from the end stations of more caudal somatic ganglia (nuclei of Go 11 and Burdach and medial lemniscus). The last two (4 and 5) constitute the thalamus and increase in importance in the higher vertebrates (see p. 440, Fig. 371).
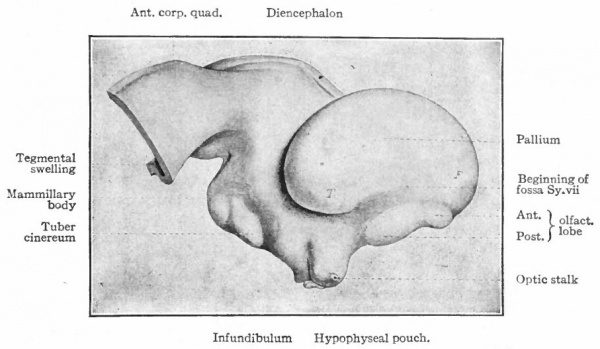
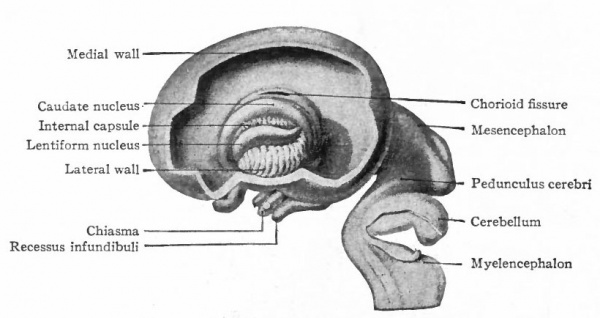
In the telencephalon there may be roughly distinguished an anterior and basal part, the rhinencephalon, in especially intimate relations with the olfactory nerve; a thickening of the basal wall, the corpus striatum ; and a thinner- walled dorsal part, the pallium. The latter may be regarded in a sense as a dorsal development of the corpus striatum and first appears as a distinct structure in the Amphibia.
The peripheral or segmental apparatus which are connected with the prosencephalon are the highly modified optic and olfactory organs. While the optic apparatus primarily originates from the prechordal brain, in the lower Vertebrates its highest coordinating center, as mentioned above, lies partly in the epichordal portion (optic lobes). It is possible that this connection is secondary and contingent upon two functional necessities, the importance of correlation with stimuli coming via more caudal nerves (cochlear and spinal nerves) , and the innervation of its motor apparatus by epichordal nerves, the III, IV and VI. With the development of the neopallium in Mammals (see p. 447) and the consequent projection of visual stimuli upon it, the lower prechordal (thalamic) centers form part of the newer pathway to the neopallium and thus increase in importance, while the optic lobes recede, assuming the position of a reflex center, especially for the visual motor apparatus.
The olfactory nerves enter the anterior extremity of the brain and are connected by secondary and tertiary tracts with regions lying more caudally, where in some cases the olfactory stimuli are associated with gustatory and probably with visual stimuli. One of these regions is the hypothalamus which receives both olfactory and gustatory tracts (Herrick) . More dorsal olfactory pathways pass to the epithalamus. Both epithalamus and hypothalamus give rise to descending systems which doubtless ultimately reach efferent nuclei. In fact, this part of the brain presents, apparently, a complicated primitive mechanism for the correlation especially of olfactory and gustatory stimuli, also to some extent of visual stimuli and stimuli via the trigeminal nerve, the whole forming a sort of oral sense, probably controlling the feeding activities (Edinger).
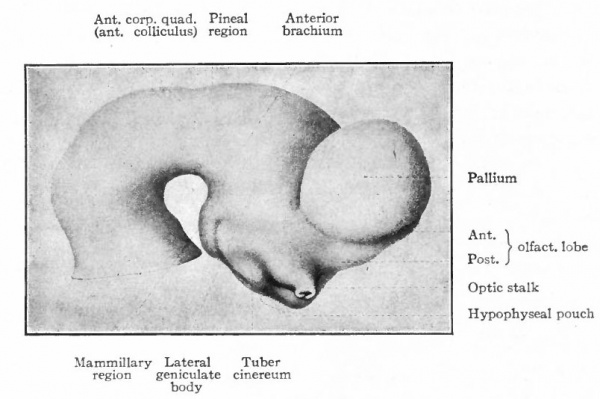
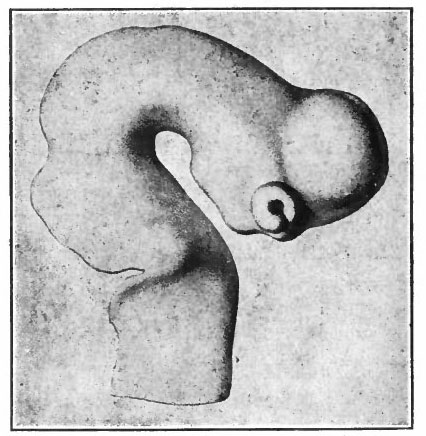
The next factor in the further development of this part of the brain is the rise in importance of the pallium upon which at first are projected mainly olfactory stimuli (Fig. 370).
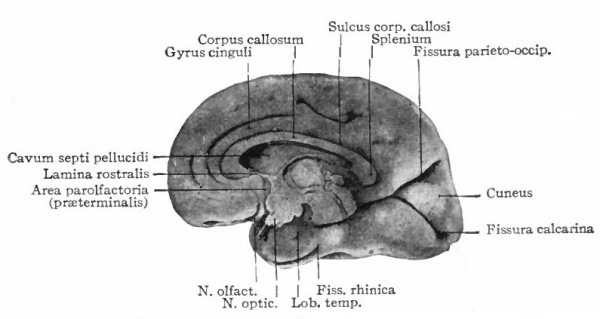
A further and still more extensive development of the pallium arises when other kinds of stimuli are projected to a considerable extent upon it, thus giving rise to a distinction between the older olfactory pallium (archipallium) and the newer non-olfactory pallium (neopallium} . The latter appears first in the lateral dorsal portion of the pallial wall and by its subsequent development the archipallial wall is rolled inward upon the mesial surface of the hemispheres. Further changes consist in the extension caudally of this portion pari passu with the extension caudally of the neopallium and then the practical obliteration of its middle portion by the great neopallial commissure, the corpus callosum (Fig. 370, G and H).
In addition to the increasing projection of stimuli from all parts of the body upon the neopallium and the consequent increase in centripetal fiber terminations and in centrifugal neurone bodies lying in its walls, a second factor in the development of the neopallium is the enormous increase of its association neurones. It is the latter feature which especially distinguishes the human from other mammalian brains.
The biological significance of these changes lies in the fact that there is thus produced a mechanism not only for the association of all kinds of stimuli, but also for very complex coordinations between these stimuli. In this way an extensive symbolization and formulation of individual experience (memory, language, etc.) can take place. The formulated experience of one generation can be immediately transmitted (by education in the broad sense of the term) to the plastic late-developing neopallia of the next generation. In this way a racial experience may be rapidly built up without the direct intervention of the slow processes of heredity and natural selection and each generation profit by the accumulated experience of past generations to a much greater extent. The nervous mechanism, the pallium, is provided by inheritance; experience is not inherited but " learned." The pallial associative mechanisms are continuously modified by their activities, thus affecting the character of subsequent pallial reactions (associative memory). Such reactions are usually termed psychical or conscious, as distinguished from the reflex reactions of other parts of the nervous system.
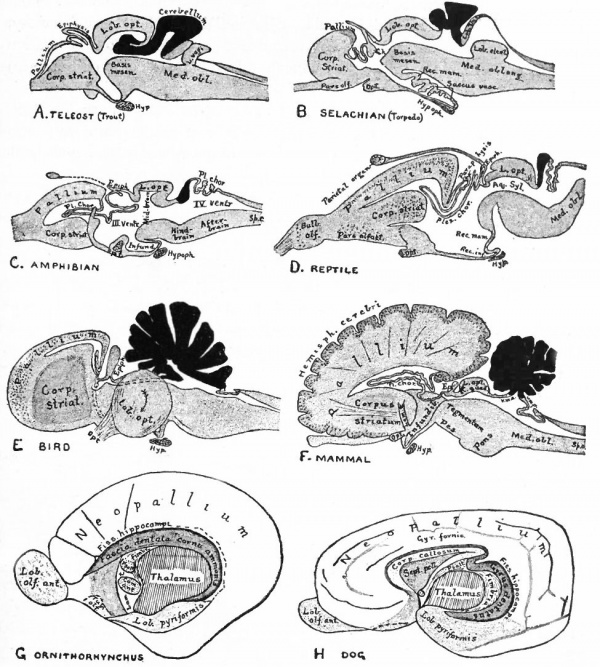
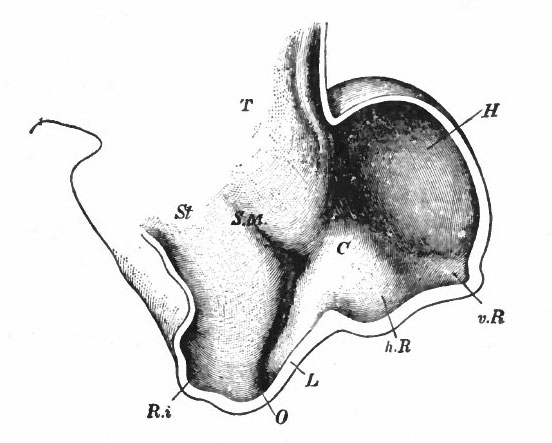
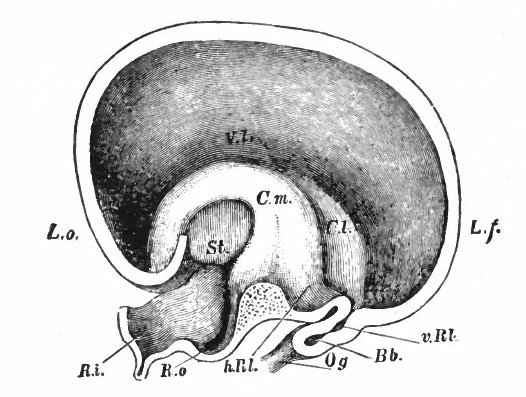
Fig. 370. A-F are sagittal sections showing structures lying in the median line and also paired structures (e.g., pallium) lying to one side of the median line. (Edinger) The cerebellum is black. It is doubtful whether the membranous roof in A indicated as pallium is strictly homologous with that structure in other forms, In B, Pallium indicates prepallial structures.
- Aq. SyL, Aquseductus Sylvii; Basis mesen., basis mesencephali; Bulb, olf., bulbus olfactorius; Corp. striat., corpus striatum; Epiph., epiphysis; G. h., ganglion habenulae; Hyp., hypophysis; Infund., infundibulum; Lam. t., lamina terminalis; Lob. elect., lobus electricus; L. vagi, lobus vagi; L. opt., mid-brain roof; Med. obi., medulla oblongata; Opt., optic nerve; Pl.chor., plexus chorioideus; Rec. inf., recessus infundibuli; Rec. mam., recessus mammillaris; Saccus vase., saccus vasculosus; Sp. c., spinal cord; ventr., ventricle; v. m. a., velum medullare anterius; v.m. p., velum medullare posterius.
- G and H show the mesial surface of the cerebral hemispheres in a low (G) and high (H) Mammal. G. Elliot Smith, Edinger, slightly modified.
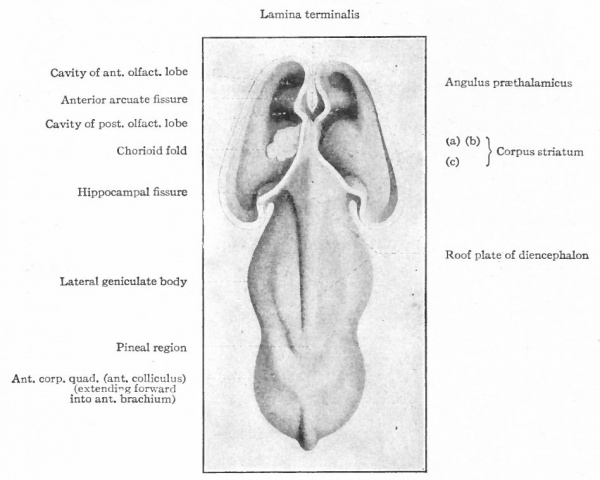
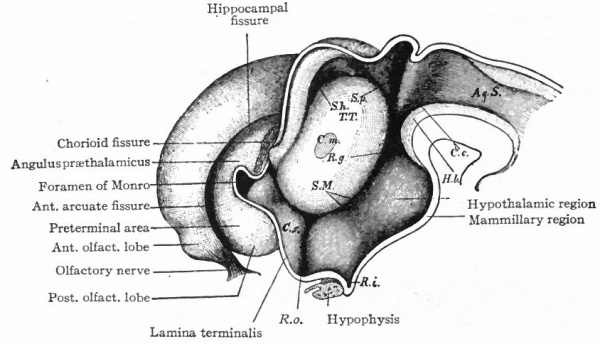
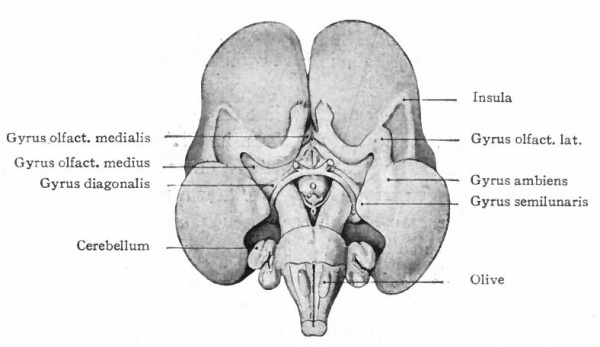
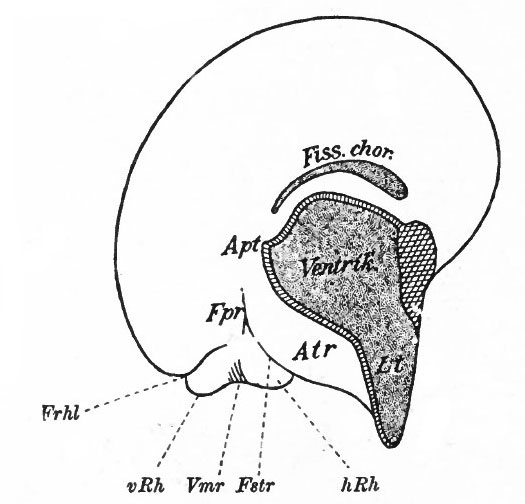
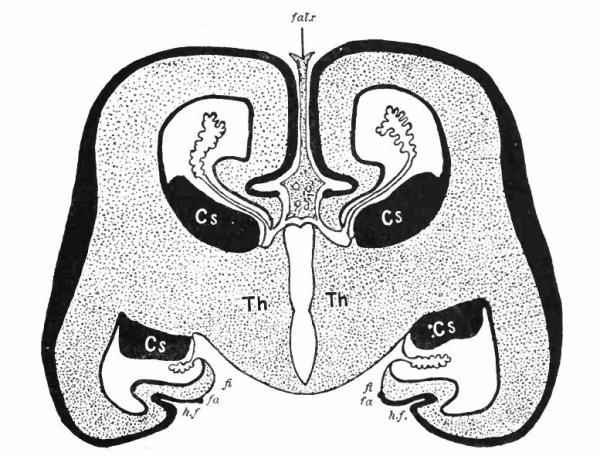
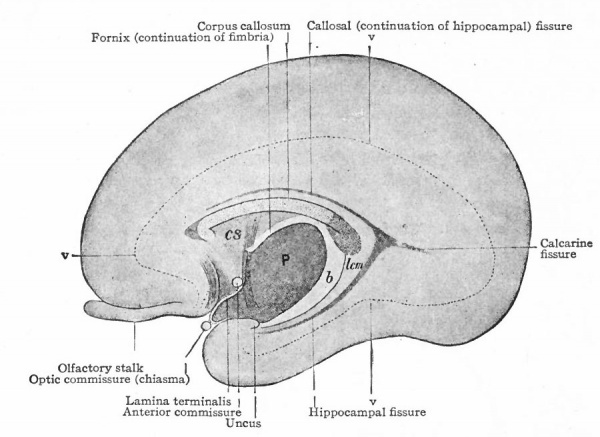
The exposed gray matter of the olfactory regions is shaded, the darker shade indicating the archipallium (preterminal area and hippocampal formation), the lighter shade indicating the rhinencephalon, which consists of the anterior and the posterior (principally pyriform) olfactory lobes. In Amphibia and Reptiles the hippocampal formation includes all or nearly all of the mesial surface. As the early neopallium appears in the lateral hemisphere walls, the neopallial commissural fibers first pass across the median line in the ventral or anterior commissure. With the increase of the neopallium and its extension on the mesial hemisphere walls, its commissural fibers pass across more dorsally via the archipallial or fornix commissure (psalterium) forming the neopallial commissure or corpus callosum, the great development of which nearly obliterates the anterior hippocampal formation.
Com. ant., Anterior commissure; corp. callosum, corpus callosum; Fimbr., fimbria; Fiss. hippocampi, hippocampal fissure; Lam. t., lamina terminalis; Lob. olf. ant., anterior olfactory lobe; Lob. pyrfformis pyriform lobe; Psalt., psalterium (fornix commissure); Sept. pell., septum pellucidum; Tuo. olf., tuberculum olfactorium. Only a part of the gray (cortex) of the hippocampal formation appears, as the gyrus dentatus, on the mesial surface; the remainder forms an eminence, the cornu Ammonis, on the ventricular surface. This invagination is indicated extenu'lyby the hippocampal fissure. The exposed fiber bundle forming the edge of this formation (fimbria) passes forward (fornix and its commissure) and thence descends, as the anterior pillar of the fornix, behind the anterior commissure. The anterior pillar is partly indicated by a few lines in this region in the figure.
In the course of these developments the pallium or cerebral hemispheres have enormously increased in size until in man they overlap all the other parts of the brain. Naturally the extensive connections of the neopallium with the rest of the brain have profoundly modified the latter. Among the new structures which have on this account been added to the older structures of the rest of the brain, the following may be mentioned: (i) The centripetal connections of the neopallium, consisting mainly of what are usually termed the thalamic radiations. These consist essentially of a system of neurones passing from the above mentioned termini in the thalamus of general somatic, acoustic and optic ascending systems to certain areas in the cerebral hemispheres. In this system we can distinguish (a) the continuation of the fillet (general somatic) to the central region (somaesthetic area) of each hemisphere; (b) the optic radiation from the lower thalamic optic center (lateral geniculate body) to the calcarine (visual) area of the hemisphere; (c) the acoustic radiation from the medial geniculate body of the thalamus to the upper temporal region (auditory area) of the hemisphere. Associated with these last two connections are the increase of the geniculate bodies and the diminution of the mid-brain in importance already alluded to (p. 437). (2) The centrifugal connections consisting of (a) the pyramids passing from the precentral area of each hemisphere to various lower efferent neurones, or neurones affecting the latter, and forming part of the internal capsule and pes pedunculi ; (b) fibers from various parts of the hemisphere, forming the greater part of the rest of the internal capsule and pes, and terminating principally in the pontile nuclei whence a continuation of this system (the fibers of the middle peduncle), passes to the cerebellar hemisphere. The great increase in size of the cerebellar hemispheres, of the contained nuclei dentati, and probably of the superior cerebellar peduncles are further effects of this new connection, which has already been alluded to (see Cerebellum, p. 436 ), ( Fig. 371)
Another important effect of the development of the pallium is the assumption by man of the upright position, due both to the specialization of the hand to execute pallial coordinations and its consequent release from locomotion, and also to the overhanging of the eyes by the enlarged cranium. The great increase of cerebellar connections may be partly due to the new problems of equilibrium connected with the upright position.
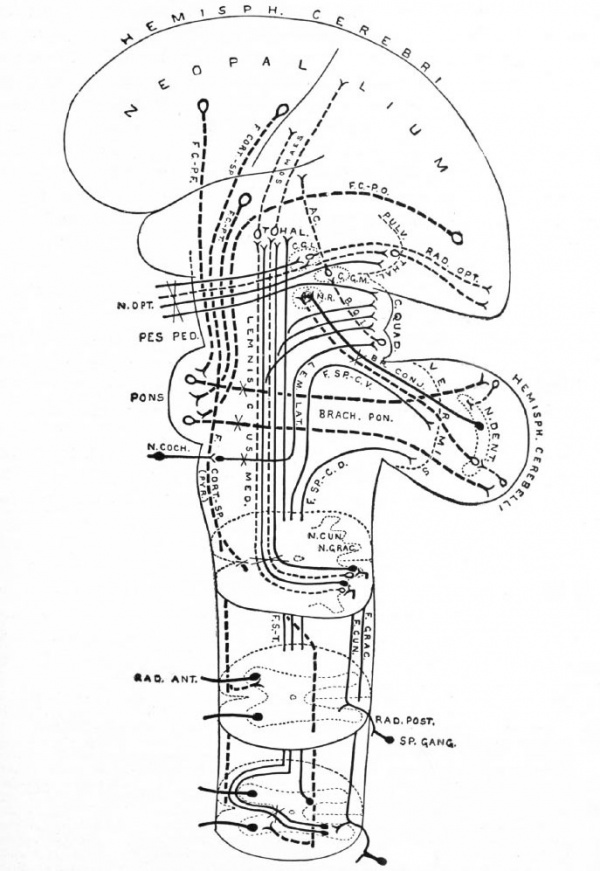
Fig. 371. Principal afferent and efferent suprasegmental pathways (excepting the archipallial connections, the efferent connections of the mid- brain roof and the olivo-cerebellar connections) Neopallial connections are indicated by broken lines. Intersegmental connections are omitted Some peripheral elements are indicated. Each neurone group (nucleus and fasciculus) is in dicated by one or several individual neurones. Decussations of tracts are indicated by an X
- ac., Acoustic radiation, from medial gemculate body to temporal lobe; br. conj., brachium conjunctivum (superior cerebellar peduncle); brack, pon., brachium ponds (middle cerebellar peduncle); b.q. i., brachium quadrigeminum inferias (a link in the cochlear pathway) ; c. g. I., lateral or external geniculate body; c. g. m., medial or internal geniculate body; c. quad., corpora quadrigemina; f.cort.-sp., cortico-spinal fasciculus (pyramidal tract); f.c. p.-f. frontal cortico-pontile fasciculus (from frontal lobe); f.c.-p.t., temporal cortico-pontile fasciculus (from temporal lobe); f.c.-p.o., occipital cortico-pontile fasciculus (from occipital lobe); f.ctm.f fasciculus cuneatus (column of Burdach); f.grac., fasciculus gracilis (column of Goll) ; f. s.-t., tract from cord to mid-brain roof and thalamus (sometimes included in Gowers' tract); f.sp.-c.d., dorsal spino-cerebellar fasciculus (tract of Flechsig); f.sp.-c.v., ventral spino-cerebellar fasciculus (tract of Gowers, location of cells in cord uncertain) ; lem. lot., lateral lemniscus or lateral fillet; lemniscus -med., medial lemniscus or fillet (the part to the thalamus is mainly a neopallial acquisition); n.coch., cochlear nerve; n. cun., (terminal) nucleus of the column of Burdach; n.grac., nucleus of the column of Goll; n.dent., nucleus dentatus; n. opt., optic nerve; n.r., nucleus ruber (red nucleus); pes ped., pes pedunculi (crusta); pulv. thai., pulvinar thalami; pyr., pyramid; rod. ant., ventral spinal root; rod. post,. dorsal spinal root; rod. opt., optic radiation (from lateral geniculate body, and pulvinar (?), to calcarine region); somaes., bundles from thalamus to postcentral region of neopallium; s p. gang., spinal ganglion; ihal., thalamus.
General Development of the Human Nervous System during the First Month
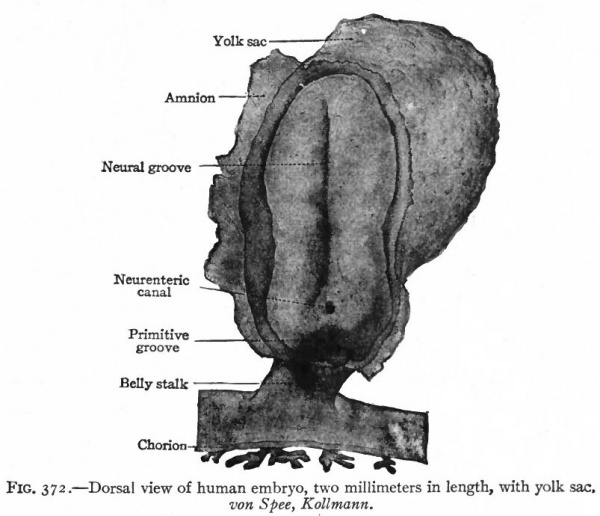
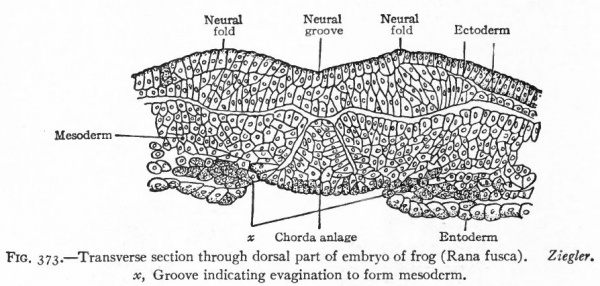
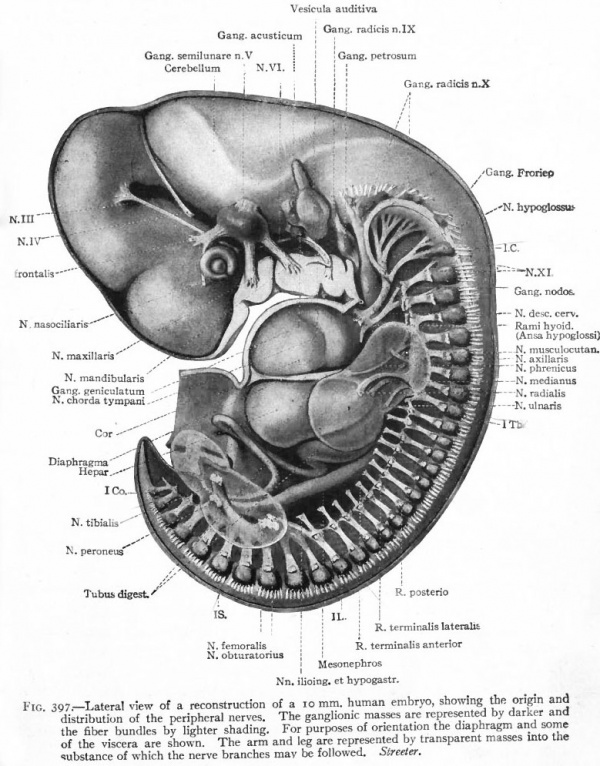
One of the earliest stages in the development of the human nervous system is shown in the 2 mm. embryo of about two weeks (Fig. 372). This shows the stage of the open neural groove. The appearance of a transverse section of the neural plate, groove and folds, in other forms, is shown in Figs. 373 and 374.
The neural folds now become more and more elevated and finally meet, thus forming the neural tube as previously described (p. 421). The fusion of the neural folds begins in the middle region and thence extends cranially and caudally. The stage of partial closure of the neural tube is shown in Eternod's figure of a human embryo of 2.1 mm. (Fig. 375, b). This order of closure indicates, to some extent, the order of subsequent histological development; the extreme caudal and cephalic extremities are more backward than the parts which close first. The last point to close anteriorly marks, as stated previously (p. 42 1), the cephalic extremity of the neural tube and is the anterior neuropore. As indicated in Eternod's embryo, the anterior end of the neural plate is broader even before its closure; thus when the tube is completed its anterior end is more expanded. This expansion is the future brain, the narrower caudal portion being the future spinal cord. Before the closure of the brain part of the tube the beginnings of the three primary brain vesicles are also indicated (Fig. 84). At this stage the neural plate shows no differentiation into nervous and supporting elements. The neural tube is composed of the two lateral walls and the median roof and floor plates (comp. p. 423) (Figs. 307 and 404).
Fig. 372. Dorsal view of human embryo, two millimeters in length, with yolk. von Spee, Kollmann. The amnion is opened dorsally.
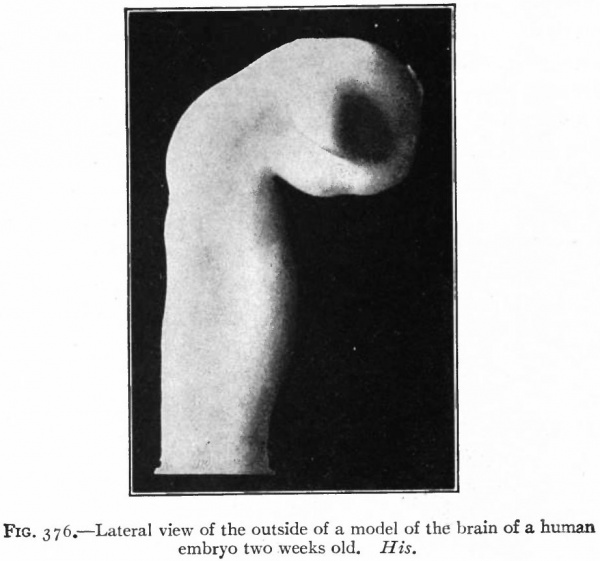
The appearance of the anterior end of the neural tube with the closure completed, except the anterior and posterior neuropores, is shown in the model of one half of the tube. The external appearance and also the inner surfaces are shown in Figs. 376 and 377. At this stage the cephalic flexure (see p. 424) is already quite pronounced, the cephalic end of the brain tube being bent ventrally at about a right angle to the longitudinal axis of the remaining portion of the tube. This bending begins before the closure of the cephalic part of the neural tube (Fig. 84). From each side of the brain near the cephalic extremity is an evagination of the brain wall, the beginning of the optic vesicles.
Fig. 373. Transverse section through dorsal part of embryo of frog (Rana fusca). x, Groove indicating evagination to form mesoderm. Ziegler.
The process of evagination and consequently the location of the vesicle begins before the closure of the tube. Dorsal and anterior to the optic vesicles can be seen a slight unpaired protrusion of the dorsal wall, the beginning of the pallium. The area basal to it and extending a short distance into the anterior wall of the optic vesicle is the site of the future corpus striatum (Figs. 376 and 377).
Fig. 374. Transverse section of dog embryo with ten pairs of primitive segments. Bonnet.
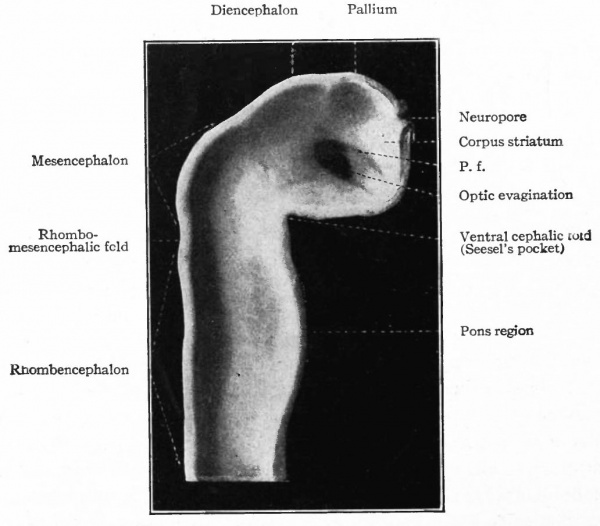
Caudal to the pallium and separated from it by a slight constriction (indicated best by the ridge on the inner wall) is another protrusion of the dorsal wall, the roof of the diencephalon. Still further caudally and separated from the roof of the diencephalon by another slight constriction is another expansion of the dorsal wall, the roof of the mid-brain or of the mesencephalon which arches over the cephalic flexure. It is separated by another constriction (plica rhombo-mesencephalicd) from the rhombic brain or rhombencephalon, which latter tapers into the cord. A ventral bulging of the rhombencephalon indicates the future pans region (Figs. 376 and 377).
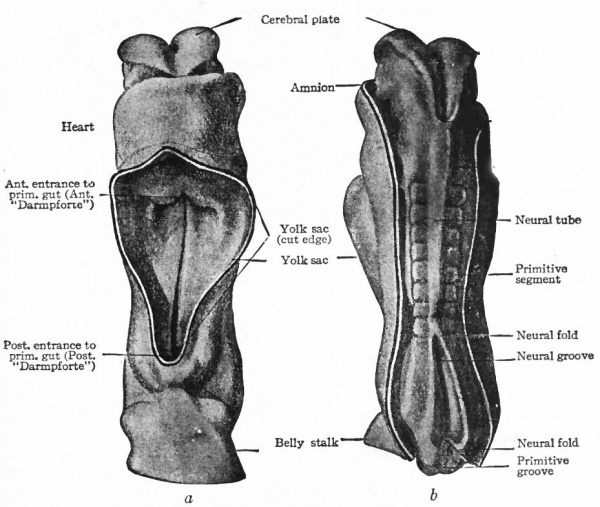
Fig. 375. (a) Ventral view; (&) dorsal view of human embryo with 8 pairs of primitive segments (2.11 mm). Eternod. From models by Ziegler. In b the amnion has been removed, merely the cut edge showing; in a the yolk sac has been removed.
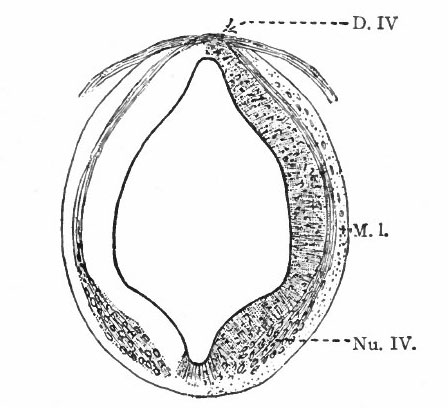
Even at this early stage the cavity of the caudal part of the rhombencephalon is expanded dorsally due to an expansion of the roof plate, which forms only the narrow dorsal median part of the rest of the tube. This expansion reaches its maximum about opposite the auditory vesicle.
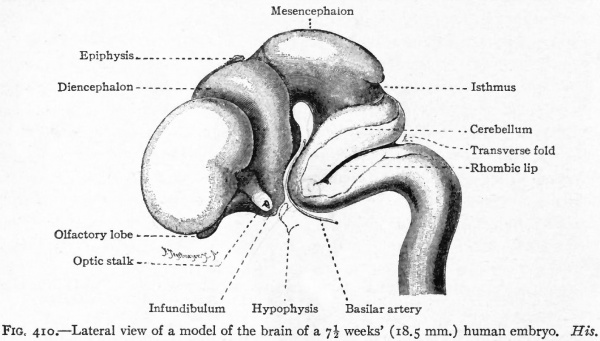
Fig. 376. Lateral view of the outside of a model of the brain of a human embryo two weeks old. His.
Fig. 377. Lateral view of inner side of the same model shown in Fig. 414. His. P.f. is the ridge corresponding to the peduncular furrow on the outer side.
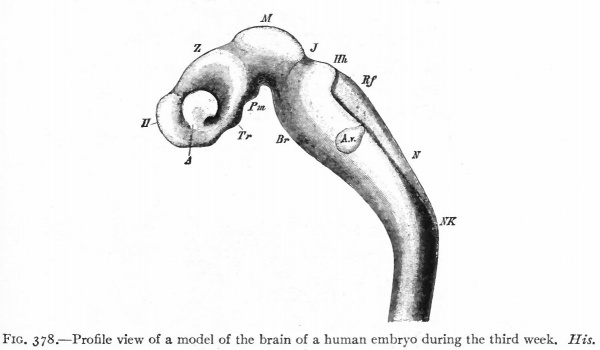
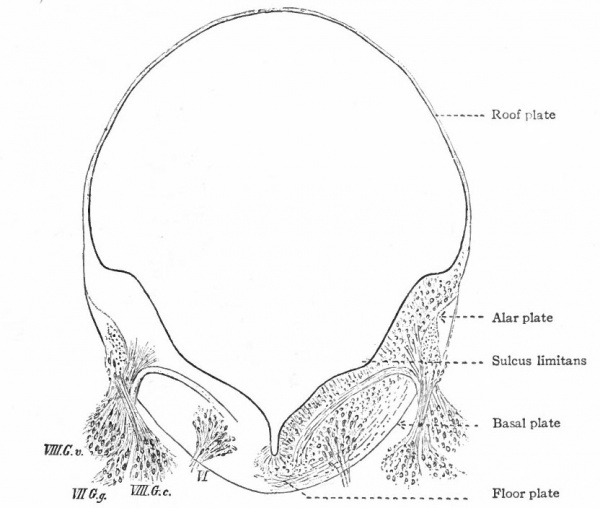
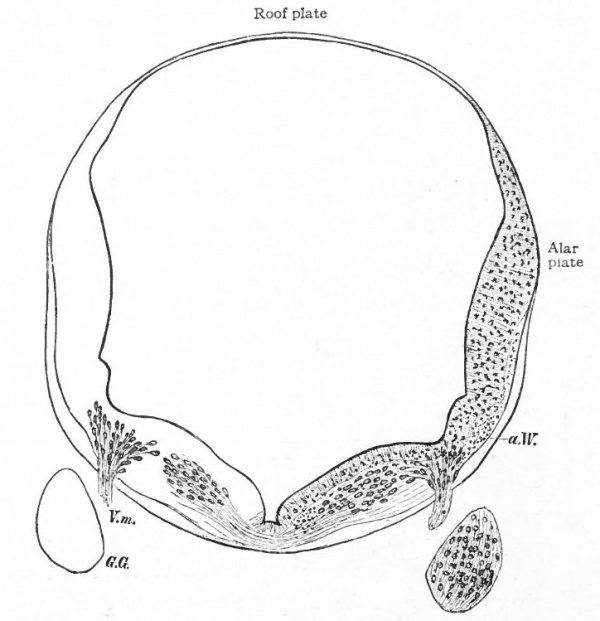
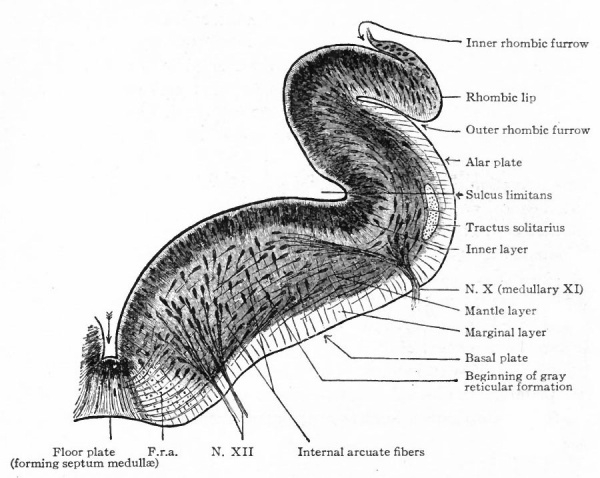
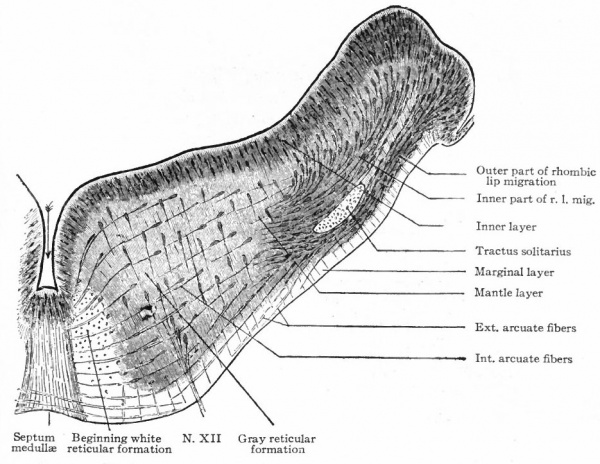
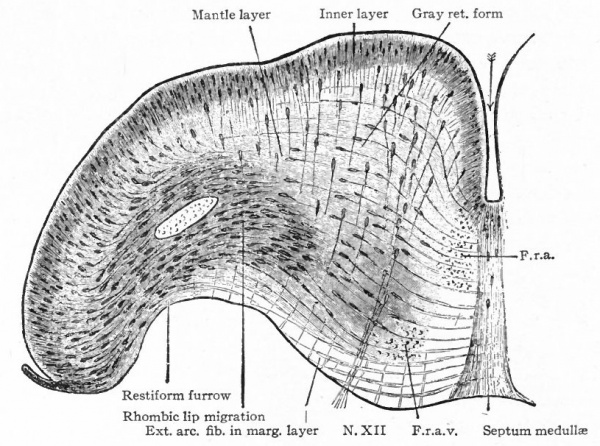
The principal changes in form during the next two weeks are the following (Figs. 378 and 434): The cephalic flexure becomes still more pronounced so that the anterior end of the neural tube is folded back upon the ventral side of the rest 01 the brain, an effect probably enhanced by the expansion of the ventral wall of the anterior portion (Figi 378 and 434). In the space thus enclosed the dorsum sellae is subsequently formed. Associated with this increase of the cephalic flexure is an increased prominence of the mid-brain roof. The pontine flexure has begun, there being now a bending of the whole tube in the pons region, the concavity of the bend being dorsal. At the same time there is a corresponding tendency for the roof of the rhombencephalon to become shorter and wider. There is also a further thinning of the above mentioned expanded portion of the roof plate in this region, and associated with this a thrusting of the thick lateral walls outward at the top so that they come to lie almost flat instead of vertically as in the cord. From the cord to the place of greatest width above mentioned, this dorsal thrusting apart of the lateral rhombic walls obviously becomes more and more pronounced. In front of this region of greatest width, the roof plate becomes narrower and the dorsal parts of the walls (alar plates) form the rudiment of the cerebellum, the rest of the rhombic brain forming the medulla oblongata. Each lateral wall of the rhombic brain is now divided into a dorsal longitudinal zone or plate (alar plate) and a ventral zone or plate (basal plate) by a longitudinal furrow along its inner surface, the sulcus limitans. A study of the external appearances and transverse sections of this part of the brain tube will make these relations clear (Figs. 418, 398 to 401 and 489). Neuromeres are also present at this stage (see p. 459). In the meantime the neural tube has also become bent ventrally at the junction of the brain and cord, forming the cervical flexure. The pallium has increased in size and now forms a considerable prominence on the brain tube. Its boundaries are also much more clearly marked off (see Fig. 433). On the inner side of the tube, the area below the bulging of the pallium is the corpus striatum. Externally, just below the bulging, we have the region where the olfactory lobes are differentiated. The proximal part of the optic evagination has become longer and narrower. The ventral expansion of the diencephalon is the hypothalamus, the portion of the diencephalon dorsal to the latter being the thalamus. Two slight protrusions of the ventral wall of the hypothalamus have appeared; the caudal one is the mammillary region, the anterior one the infundibulum. The cavity of the diencephalon (third ventricle) is connected by the mid-brain cavity (iter or aqu&ductus Svlvii) with the rhombic brain cavity or iourth ventricle.
Fig. 378. Profile view of a model of the brain of a human embryo during the third week. His. A, Optic vesicle; A.v., auditory vesicle; Br, pons region; H, pallium; Hh. cerebellum; J, isthmus; M, mid-brain; AT and Rf, medulla; NK, cervical flexure; Pm, mammillary region; Tr, infundibulum; Z, inter-brain or diencephalon.
Histogenesis of the Nervous System
The neural plate is at first a simple columnar epithelium. The various processes by which this is converted into the fully formed nervous system are : (i) cell proliferation; (2) cell migration; (3) cell differentiation. These processes are not entirely successive in point of time, but overlap each other. Cell division is present from the first, increases to a certain period in development and then practically ceases; cell migration is partly a necessary concomitant and resultant of cell division, and cell differentiation is in part due to the growth of the cytoplasm and is in part a result of environmental differences produced by these processes. In development the following stages may be distinguished :
(1) Stage of indifferent epithelium; (2) appearance of nerve elements (neurones) and resulting differentiation into supporting and nerve elements; (3) growth of neurones and resulting differentiation and development of (a) peripheral neurones, (b) lower intermediate or intersegmental neurones, (c) neurones of higher centers and neurone groups in connection with them (suprasegmental neurones). These stages do not occur simultaneously throughout the whole neural tube, some parts being more backward in development than others (p. 443) . In general the spinal cord and epichordal segmental brain are most advanced in development. Furthermore, the ventral part of the brain tube precedes the dorsal. The most backward part of the whole neural tube is the pallium.
The various phases of differentiation of the neurone are (1) the development of the axone and, later, of its branches; (2) the growth of the dendrites; (3) the formation of accessory coverings or sheaths, the neurilemma and the myelin (medullary) sheath. The principal internal differentiations are (i) the appearance of the neurofibrils; (2) the chromophilic bodies of Nissl; (3) pigment. These latter may all be regarded as products of the nucleus and undifferentiated cytoplasm of the nerve-cell.
Epithelial Stage
Development of Neuroglia.
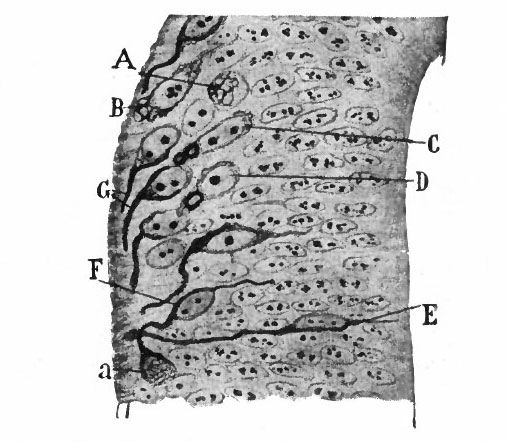
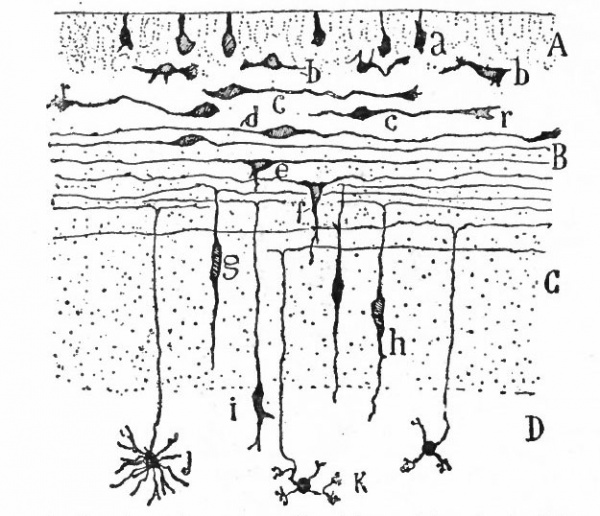
From the very first, the neural plate exhibits dividing cells similar to those seen in the non-neural ectoderm. The cell divisions are indirect and the mitoses are confined to the outer part of the ectoderm, occurring between the outer ends of the resting epithelial cells (Fig. 370). These dividing cells have been termed by His germinal cells. When the neural tube is formed, the mitoses are still confined to the outer, now the luminal, surface, this being a general phenomenon in developing epithelial tubular structures. As a result the daughter nuclei migrate away from the lumen.
In the most advanced parts of the neural tube (see p. 438), the mitoses increase in number up to about the fourth to sixth week of development, and then diminish anc 1 finally nearly disappear about at the end of two months. At about the time the blood vessels penetrate the tube, the mitoses are no longer entirely confined to the proximity of the lumen.
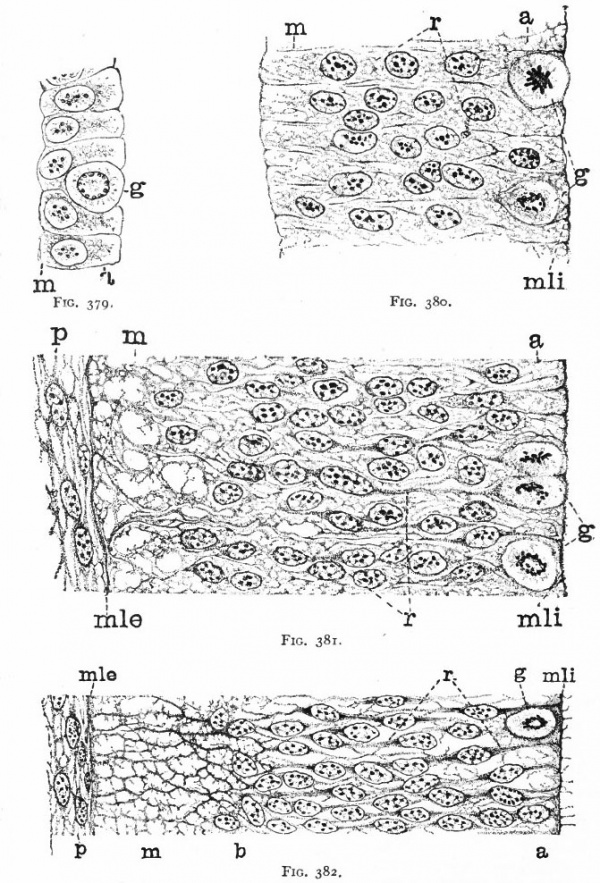
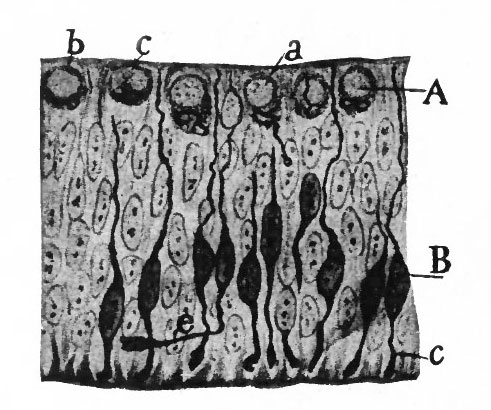
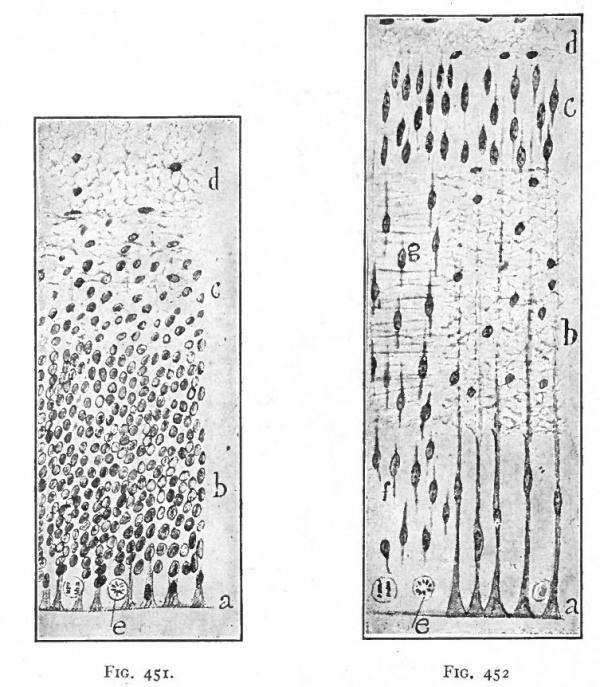
As a result of proliferation, the epithelial wall very early assumes the ap. pearance of a stratified epithelium at least there are several strata of nuclei. There are at this stage in many forms two layers, an outer or marginal layer, free of nuclei, and an inner or nuclear layer (Figs. 380 and 381). In a human embryo, however, of about two weeks this division into layers is yet hardly evident, though there are several strata of nuclei. Apparently these layers are not well-marked until the radial arrangement of the myelospongium, as described below, has become more pronounced.
Accompanying the above changes, changes also manifest themselves in the character of the cells. At about the time of the closure of the neural tube, the cell boundaries become indistinct and finally practically obliterated, thus forming a syncytium, the myelospongium. At the same time, the syncytium becomes very alveolar in structure and a general spongioplasmic reticulum is formed (Figs. 380 and 381) by the anastomosing denser strands (trabeculae) of protoplasm. At a very early stage (two weeks), these trabeculae unite along the inner and outer walls of the neural tube forming internal and external limiting membranes. The nuclei of the neural tube have at first an irregular arrangement in the reticulum, at least in the human embryo. This is followed by a more radial arrangement of both nuclei and protoplasmic filaments (Fig 382), forming nucleated radial masses of protoplasm the sponglioblasts (Figs. 381 to 384). There is some dispute as to the loss, complete or incomplete, of identity of the epithelial cells in the formation of the spongioblasts. According to Hardesty, they are formed by a collapse of the epithelial cells and a rearrangement of their denser parts into axial filaments. The radial arrangement does not extend into the outer part of the neural tube which, retaining its irregular reticular character, is now non-nucleated in the human embryo and forms the marginal layer. The increase in the thickness and circumference of the walls of the tube and the resulting tensions may be a factor in this arrangement of the protoplasmic filaments. At the boundary between the marginal and nuclear layers the reticulum appears to be especially dense.
Fig. 379. From the neural tube of an embryo rabbit shortly before the closure of the tube, g, Germinal or dividing cell; w, peripheral zone, position of the later marginal layer. His.
Fig. 380. Pig of 5 mm, unflexed. Just after closure of the neural tube. Segment of a vertical section of the lateral wall of the tube, g, Germinal cells; m, beginning of marginal layer; mli, internal limiting membrane; r, radial columns of protoplasm. The resting nuclei lie in the inner or nuclear layer. Hardesty.
Fig. 381. Pig of 7 mm, unflexed. Segment from the ventro-lateral wall of the neural tube; g y Germinal cells; mli, internal limiting membrane; mle, external limiting membrane r radial, axial filaments of the syncytial protoplasm; p, beginning of pia mater. Hardesty.
Fig. 382. Pig of 10 mm "crown-rump" measurement. Segment from lateral wall of neural tube. b, boundary between nuclear layer and marginal layer (m). Other references same as in 381. Hardesty. a indicates the zone in which the dividing cells are located. Later, it is composed of the inner ends of the ependyma cells (column layer of His}.
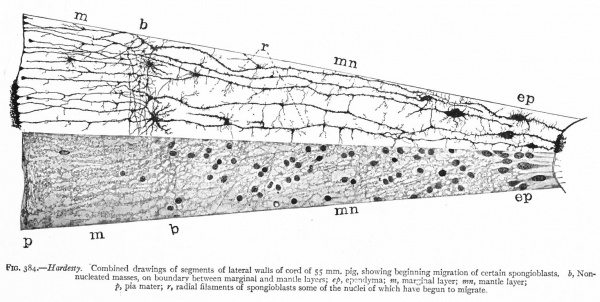
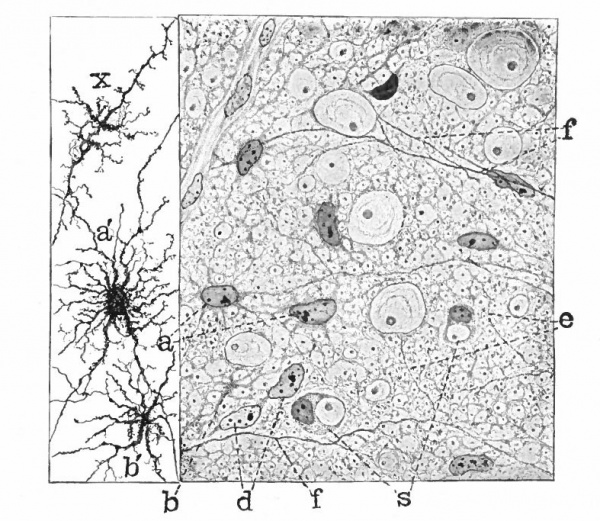
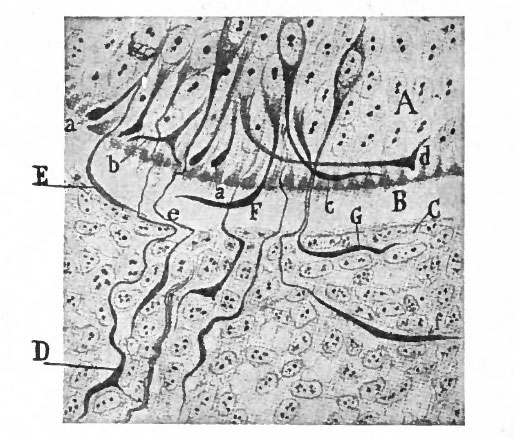
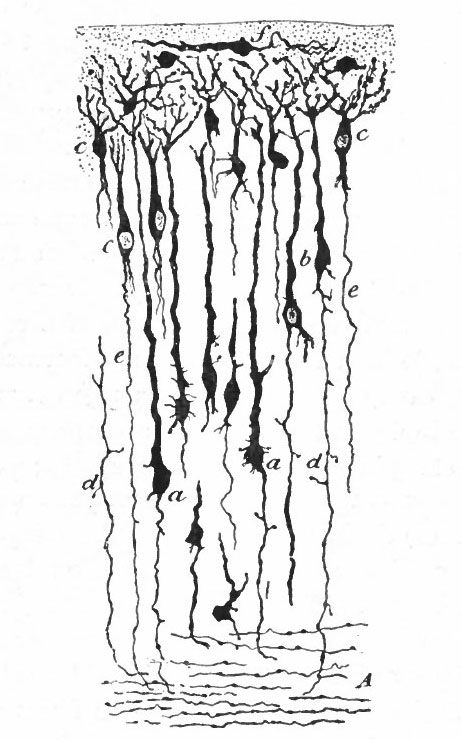
With the further increase and development of the nervous elements (see p. 455) the radial arrangement of the spongioblasts noted above becomes more and more obliterated. As shown by Golgi preparations, in their migration from the lumen (Fig. 384) the spongioblasts lose their connection with the lumen, their peripheral processes become abbreviated and disappear, and they finally differentiate into the irregular branching neuroglia cells (Fig. 385). According to Hardesty, there is simply a general nucleated mass which changes form pari passu with changes in the enclosed . differentiating nervous elements, finally assuming shapes dependent upon the character of the spaces between the formed nervous elements. An exception to this is a layer of nucleated elements which remain next the lumen and form the ependyma cells which still send radial extensions into the wall of the neural tube (Figs. 383 and 384). These cells develop cilia projecting into the lumen.
A still later differentiation in the supporting elements of the tube is the appearance of neuroglia fibers a product of the spongioblastic protoplasm, but differing from it chemically (Fig. 385). The exact relation of these neuroglia fibers to the nucleated neuroglia cells in the adult is a matter of dispute.
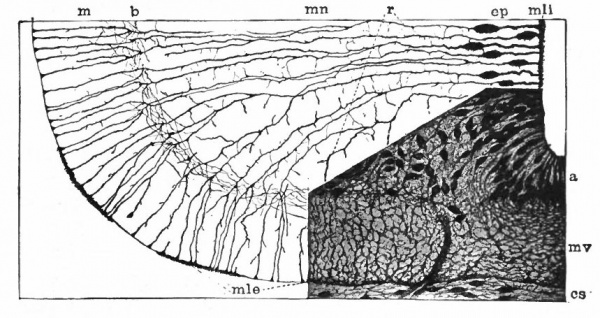
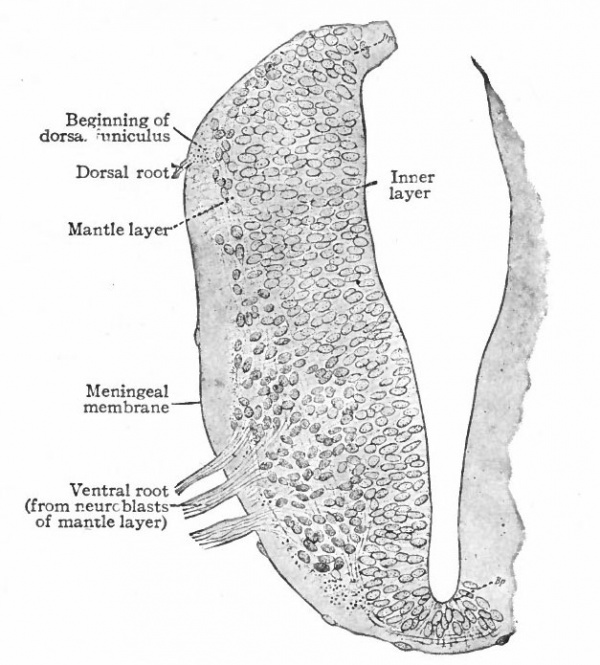
Fig. 383, Hardesty. Combination drawing from sections of pig of 15 mm. The upper part is from a section of the same stage as the lower but stained by the Golgi method. By migration and differentiation the mantle layer has been formed. The cells remaining near the lumen form the ependyma layer (ep.). b, Boundary between mantle and marginal layers; ep, ependyma; mli and mle, internal and external limiting membranes; mv, differently arranged mid-ventral portion of the marginal layer; r, radial filaments; cs, connective tissue syncytium.
Fig. 385. Hardesty Combination drawing from transverse sections of the spinal cord of 20 cm. pig. Showing the first appearance of neuroglia fibers, a, Neuroglia cell as shown by the Benda method of staining; a', similar cell by the Golgi method; b and b r , non-nucleated masses; d, free nuclei; e and f, differentiating neuroglia fibers; s, "seal-ring" cells, enveloping myelinating nerve-fibers.
With the penetration of blood vessels into the neural tube a certain amount of mesodermal tissue is brought in. How much of the supporting tissue of the nervous system is derived from the mesoderm is uncertain, but it is most probable that it is relatively small in amount and is confined principally to the connective tissue of the walls of the blood vessels.
Early Differentiation of the Nerve Elements
It has been seen that some of the actively dividing cells (germinal cells) at first simply increase the ordinary epithelial elements of the tube which in turn form the myelospongium, the spongioblasts and finally the ependyma and the neuroglia. Other daughter cells produced by the division of the germinal cells differentiate into nerve cells as described below. Still others probably migrate outward as indifferent cells, which later proliferate and form cells which differentiate into neuroglia and nerve cells.
According to recent researches (Cajal), by means of the silver stain of Cajal the first indication of the differentiation of cells into nerve cells is the appearance of neurofibrils in the cytoplasm of cells near the lumen. The part of the cell in which the neurofibrils first appear is called the fibrillogenous zone (Held) and is usually in the side furthest from the lumen. The cells in which these appear are apparently without processes, and are accordingly termed apolar cells (Cajal). (Fig. 386.)
Fig. 386. Section through the wall of the fore-brain vesicle of a chick embryo of 3.5 days. Cajal.
- A, b arid c, Differentiating nerve cells in apolar stage, the neurofibrils are black; a, cell in a stage transitional to the bipolar stage; B, bipolar cells; c (at lower right corner), cone of "growth" of developing axone; e, tangential axone. The cells in the bipolar stage have migrated out ward, but the neuroblast or mantle layer has not yet been differentiated.
The next step in the development of many, but probably not all, of these cells is their transformation into bipolar cells by the outgrowth of two neurofibrillar processes, one directed toward the lumen, the other, usually thicker, toward the periphery, the cell body at the same time beginning to migrate outward (Fig. 386). This bipolar stage may be regarded as conditioned to some extent by the radial arrangement of the other elements, due in turn partly to the original epithelial structure and partly, possibly, to tensions produced by the growth of the tube. It is also interesting as recalling conditions in sensory epithelia and in the cerebrospinal ganglia. The bipolar stage is most common probably in those parts where the elements show a radial arrangement in the adult. Such are the layered cortices of the mid-brain and pallium. Nerve cells maintaining a connection, by central processes, with the luminal wall have been described in lower Vertebrates. This connection may be explained as due to a persistence of the central processes of cells in the bipolar stage.
The next stage is a monopolar stage produced by the atrophy of the luminal process. Cells in this stage are the neuroblasts of His, the peripheral processes being the developing axones (Fig. 387). As seen in ordinary stains, the above differentiation of the neuroblasts is marked by a corresponding differentiation of the nuclear layer into an inner layer retaining its previous characteristic radial arrangement, and an outer layer characterized by fewer nuclei more irregularly arranged. The latter layer is the mantle, or neurone layer (Fig. 404) . There are now three layers: (i) inner (nuclear), (2) mantle (neurone) and (3) marginal. The mantle layer is thus produced by the migration and differentiation of cells into neuroblasts. While this process may begin near the lumen (apolar nerve cell of Cajal) and progress as the cell has moved somewhat further away (bipolar stage), the monopolar stage is probably reached only when such cells form a part of the mantle layer. In other words, the mantle layer is created by the migration to a certain location and differentiation to a certain stage of the primitive nerve cells. The mantle layer, as previously stated, probably also contains indifferent cells which may by further proliferation and subsequent differentiation become either glia or nerve cells. * The looser arrangement of the cells of the mantle layer is probably in some measure due to the growth of the dendrites which appear soon after the axones. It may be also due to the beginning vascularization of the tissues with resulting transudates (His) which usually, however, begins somewhat later. The association in time of vascularization and further growth of neurocytoplasm (dendrites) is significant. When the cell-proliferation near the lumen has ceased, the supply of new cells ceases, and as the cells of the inner layer continue to differentiate into cells of the mantle layer, the inner layer, being no longer replenished from within, is reduced to the single layer of cells which remain behind as ependyma cells (p. 451).
Fig. 387. Dorsal portion of the lumbar cord of a chick embryo of three days. Cafal. A, B, Cells in the apolar stage with fibrillogenous zones; B shows transition to the bipolar stage; E, further advanced bipolar cell; G, cells in monopolar stage or neuroblasts of His; a, giant cone of growth. These cells have migrated to the outer part of the nuclear layer, thereby forming the beginning of the mantle layer.
- It is an open question as to how late in development these " extraventricular " cell-divisions, involving " indifferent " cells, may occur. The neuroglia cells, however, like other supporting elements, preserve this capacity of division indefinitely, as shown by the increase in neuroglia cells in pathological conditions.
Differentiation of the Peripheral Neurones of Cord and Epichordal Segmental Brain
Efferent Peripheral Neurones. The differentiation of a mantle or neurone layer from the outer part of the original nuclear layer is practically universal throughout the whole neural tube. It appears first and is consequently most advanced, however, in the ventral part of the lateral walls of the cord and epichordal brain. The axones of neuroblasts occupying the basal plate of this region of the neural tube grow out through the external limiting membrane and emerge as the efferent ventral root fibers. The appearance of these early root fibers in the duck is shown in Fig. 388. The process is similar in the human embryo and begins about the third week. The neurones thus differentiated are the efferent peripheral neurones.
Fig. 388. Ventral part of wall of lumbar cord of 70-hour duck embryo, showing efferent root fibers first emerging from cord (combined from two sections). Cajal. A, Spinal cord; B, perimedullary space; C, meningeal membrane; a, b, cones of radially directed axones; c, d, cones of transversely directed axones; Z>, bifurcated cone; E,F, cones crossing perimedullary space; G, aberrant cones.
In some forms, at least, cells appear to migrate out from the tube along with the efferent root fibers. Their fate is not certain, but they probably either metamorphose into the neurilemma cells or possibly form part of the sympathetic ganglia (see p. 492). In general the questions affecting the differentiation of the efferent fibers are the same as for the afferent and are further dealt with later (pp. 462-465).
The majority of the efferent root fibers pass to the differentiating somatic muscles which they innervate, forming specialized terminal arborizations (the motor end plates). The fibers to the dorsal musculature form, together with the afferent fibers (p. 460), the dorsal branch of the peripheral spinal nerve; others form part of the ventral branch which sends a branch mesially toward the aorta. Some of the fibers of the mesial branch take a longitudinal course. This mesial branch is the white ramus communicans and terminates in the various sympathetic ganglia which are later formed along its course (p. 461).
Fig. 389. Diagram (lateral view) of the brain of a 10.2 mm human embryo (during the fifth week). Showing the roots of the cranial nerves. His. Ill, Oculomotor; IV, Trochlear; V, Trigeminus (m, efferent root, s, afferent root) ; VI, Abducens; VII, Facial; VIII, Acoustic (c, cochlear part, v t vestibular part); IX, Glossopharyiigeus; X, Vagus; XI, Spinal accessory; XII, Hypoglossus. ot., Auditory vesicle; Rh.l., rhombic lip. The two series of efferent roots (medial and lateral) are clearly shown.
(Comp. Figs. 225, 227, 394 and 36^.) The fibers to the sympathetic ganglia ire the visceral (splanchnic) fibers of the ventral root. There are a few other fibers which grow dorsally from neuroblasts in the ventro-lateral walls of the cord and thence out via the dorsal root (Fig. 392). They also are probably visceral.
In the cord the splanchnic fibers, with the exception above noted, issue with the somatic fibers in a common ventral root. In the epichordal segmental brain, however, there is a differentiation of the efferent neuroblasts of the basal plate into two series of nuclei, a medial and a lateral. The medial series consists of the nuclei of the XII, VI, IV and III cranial nerves, and their axones grow out as medial ventral root fibers (except the IV) (Fig. 389) to the differentiating muscles of the tongue and eyeball which they respectively innervate. These muscles are probably somatic and their nerves are the somatic efferent cranial nerves corresponding with the greater part of the fibers of the ventral roots of the cord (compare p. 432). The lateral series consists of the nuclei of the efferent portions of the roots of the XI, X, IX, VII and V cranial nerves and their axones grow out as lateral roots (Fig. 389) to the differentiating striated branchial (splanchnic) muscles (sternocleidomastoideus, trapezius, pharynx, larynx, face and jaw) and also to muscles of the viscera (via sympathetic?). The lateral nuclei and their roots are thus splanchnic. (Cf. pp. 302-3, 462, 464.) Their root fibers, with the incoming afferent fibers, form the mixed roots of these nerves. The positions of these various nuclei and their roots are clearly indicated in Figs. 389, 398-401, 409 and 413 and require no further description. Additional details are mentioned in connection with the afferent cranial nerves. In the region of the vagus nerve, there are differentiated two series of lateral nuclei, a ventro-lateral (nucleus ambiguus X) and a dorso-lateral (dorsal efferent nucleus X) (comp. Fig. 369). Fig. 414 apparently indicates the beginning of this differentiation. The significance of the dorso-lateral nucleus is uncertain. It possibly sends fibers to the sympathetic system.
Fig. 390. Diagram of the floor of the 4th ventricle of a 10 mm human embryo. Illustrating the rhombic grooves and their relations to the cranial nerves. The point of attachment of the acoustic and the sensory root of the trigeminal nerve is shown by dotted circles; the motor nuclei are represented by heavy dots. Streeter.
At about this period six transverse rhombic grooves are plainly marked in the floor of the fourth ventricle, standing in relation with the nerves of this region (Fig. 390). They are ordinarily regarded as neuromeric, but the above relation would indicate that they have primarily a branchiomeric character (Streeter). It will be noticed that each of the three main ganglionic masses of this region (p. 465) corresponds to two of the grooves. (Comp. p. 435).
The further development of the efferent neurones exhibits phases common to many other nerve-cells with a large amount of cytoplasm (somatochrome cells). The further development of the neurofibrils of cell body and dendrites is, according to some observations, at first confined to the peripheral portions, leaving a clear zone in the vicinity of the nucleus. The chromophilic substance first appears as distinct granules about the end of the second month, there being apparently a diffuse chromophilic substance present before this period. The chromophilic granules also are first differentiated in the peripheral portions of the cell. A still later differentiation is the pigment, which probably does not appear till after birth. This increases greatly in amount in later years and is then an indication of senility of the nerve-cell.
Fig. 391. Three stages in the closure of the neural tube and formatiqn of the neural crest (spinal ganglion rudiment). From transverse sections of a human embryo of 2.5 mm. (13 pairs of primitive segments, 14-16 days), -von Lenhossek.
Afferent Peripheral and Sympathetic Neurones. It has already been mentioned (p. 421) that in the closure of the neural tube certain cells forming an intermediate band between the borders of the neural plate and the nonneural ectoderm are brought together by the fusion of the lips of the plate and form a ridge on the dorsal surface of the neural tube, this ridge being known as the neural crest (Fig. 391).
In the SPINAL CORD, at three weeks, the neural crest has separated from the cord and split into two longitudinal bands. The ventral border of each band shows a transverse segmentation into rounded clumps of cells, forming the rudiments of the spinal ganglia which later become completely separated. The efferent roots have begun to develop but the afferent roots appear later (fourth week, Fig. 396). The cells composing these rudiments are polyhedral or oval rather than columnar and proliferation still proceeds among them A differentiation of these cells soon begins. Some, usually larger cells begin to assume a bipolar shape. Their central processes grow toward the dorsal part of the lateral walls (alar plate) of the neural tube which they enter (Fig. 392), becoming afferent (dorsal) root fibers. These fibers enter the marginal layer and there divide (Figs. 392 and 403) into ascending and descending longitudinal arms which constitute the beginning of the dorsal (posterior) juniculus of the cord. The peripheral processes of the developing ganglion cells grow toward the periphery, uniting with the ventral root and forming with it the various branches of the peripheral spinal nerve (compare Figs. 22 5> 227,394 and 366). Other peripheral branches pass as a part of the white ramus communicans to the sympathetic ganglia through which they proceed to the visceral receptors. These latter fibers are thus visceral afferent fibers.
Fig. 392. Part of a transverse section through the cord and spinal ganglion of a 56-hour chick embryo (combined from two sections). Cajal. A, Efferent cell of dorsal root; B, cone of growth of central process (afferent dorsal root fiber) of spinal ganglion cell; C, bifurcation of afferent root fibers in cord, forming beginning of dorsal funiculus or dorsal white column of cord.
It is now known that the spinal ganglion is a much more complicated structure and has more forms of nerve cells than was formerly realized. The differentiation into these various types has not yet been fully observed. The bipolar cells, however, become unipolar in the manner shown in Fig. 393. The cell body first becomes eccentrically placed with reference to the two processes and then, as it were, retracts from them, remaining connected with them by a single process. This change may economize space.
According to most authorities, many of the cells of the neural crest do not cease their migration by forming spinal ganglia, but undifferentiated cells wander still further ventralward and form, probably also undergoing still further proliferation, the rudiments of the various sympathetic ganglia, becoming subsequently differentiated into the sympathetic cells. By this migration there is first formed a longitudinal column of cells ventral to the spinal ganglia (Fig. 395) and, later, in relation with the white communicating rami (Fig. 394). This column becomes segmented (seventh week), forming ultimately the ganglia of the vertebral sympathetic chain. In the meanwhile, the cells of the column proliferate in places, forming rudiments which, by migration and further differentiation, form the ganglia of the various prevertebral sympathetic plexuses (cardiac, cceliac, pelvic, etc.). Further migrations lead to the formation of the ganglia of the peripheral plexuses (Auerbach, Meissner, etc.). All these ganglia, probably, are innervated by fibers from the white ramus, along whose course they apparently migrated. The axones of their cells pass to visceral structures either in the same segment or, via the longitudinal chain, to those of other segments. Some also join the branches of the peripheral spinal nerves (gray ramus}. Fibers of the white ramus also pass longitudinally in the chain to vertebral ganglia of other segments. The possibility previously mentioned (p. 456) of a contribution to the sympathetic ganglia by cells migrating out along with the ventral roots must be kept in mind. It would seem a priori more probable that these latter would furnish the efferent sympathetic cells, but the efferent cells predominate in the sympathetic and must thus be regarded as derived partly or wholly from the neural crest which furnishes at least the major part of all the sympathetic cells.
Fig. 393. Section of spinal ganglion of 12-day chick embryo. Cajal. Showing various stages of the change from the bipolar to the unipolar condition. A,B, Unipolar cells; C, D, F, G, cells in transitional stage; E, bipolar cell; H, immature cell. The neurofibrils are well shown.
Fig. 394. From a transverse section of a chick embryo of 4.5 days. Neumayer.
It seems probable that not all the cells of the neural crest form nerve cells, but some, usually smaller cells, become closely applied to the spinal ganglion cells, forming amphicytes, while others (lemmocytes) wander out along the nerve fibers and become the neurilemma cells, forming the neurilemma. These cells in this case would be quite strictly comparable to the glia cells of the neural tube. According to another view, the neurilemma cells are of mesodermal origin. While this point cannot be considered entirely determined, it seems fairly certain that in some types at least the former view is correct, removal of the neural crest having resulted in the formation of efferent nerves without neurilemma cells (Harrison). The modification into neurilemma cells seems to be accomplished by their enveloping the axones and becoming closely applied to them.
The peripheral nerve grows toward the periphery as a bundle of fibers which forms, as seen in many stains, a common fibrillated mass, dividing at its extremity into the developing branches of the nerve. The lemmocytes closely envelop each of these growing tips, but proximally only envelop the main nerve trunk (Bardeen). The final clear separation of the fibrillated mass into the individual nerve fibers is accomplished, according to Gurwitsch, by these accompanying cells forming septa within the mass and finally enveloping each axone as its neurilejnma sheath. Growth in bundles appears to be characteristic also of the axones (tracts and fasciculi) of many neurone groups in the central nervous system.
Fig. 395. From a transverse section through a shark (Scyllium) embryo of 15 mm. Showing the origin of the sympathetic ganglion. Onodi. In mammals the cells are more scattered and their origin from the spinal ganglion rudiment not so clear.
Owing to the presence of these migrating cells as well as of mesodermal cells, the peripheral nerves in their earlier stages appear cellular in character; later the fibrous elements predominate, the nuclei becoming more scattered and changing into the flatter nuclei characteristic of the neurilemma (Fig. 394). According to one view (Balfour;, the nerve fibers themselves are differentiated from the cytoplasm of these cell-strings and are thus multicellular structures. Still another view is that of Hensen, according to which the fibers are a differentiation in situ from preexisting syncytial bridges uniting the parts connected subsequently by the formed nerve fibers. This differentiation may not be primarily connected with the neuroblasts (Apathy, Paton) . An intermediate view between this and the outgrowth view of His is that of Held, according to which the neurofibrillar substance is an outgrowth from the neuroblast body, or at least a differentiation proceeding from that body, but always within the preexisting cellular bridges of Hensen. The differentiating fiber is thus always intracellular instead of intercellular as according to the His-Cajal view. The experiments of Harrison above alluded to, in which the accompanying migrating cells were eliminated and naked axones (axis-cylinders) nevertheless developed, apparently disposes of the cell-string theory of Balfour. The growth of the fibers in the marginal layer of the central nervous system is also unfavorable to this theory. The apparently proven capacity of growing axones to find their way through foreign tissues (aberrant regenerating nerve fibers, Cajal), through ventricular fluid (Cajal), and even through serum (Harrison) seems to throw the weight of evidence in favor of the view of His. The latter is the view adopted in this description, though many of the most important facts of development are not perhaps entirely irreconcilable with any of these views. The general conception of the neurone is affected by these questions and the related question of anastomoses between the nervous elements, whether present at all, and if present, whether primary or secondarily acquired.
From the above it would seem that the cells of the neural crest have the capacity of differentiating into afferent neurones, efferent (sympathetic) neurones and supporting cells. Other cells of the neural crest differentiate into the chromafnne cells of the suprarenal glands and similar structures (p. 396).
There are several views as to the development of the myelin sheath. According to one view (Vignal), it is a product of the neurilemma cells, being formed in a manner analogous to the formation of fat by fat cells. According to Wlassak, the various substances composing the myelin (fat, lecithin and protagon) are first found in the central nervous system in the protoplasm of the spongioblasts, their probable original source being the blood of the meningeal blood vessels. Later, the myelin is laid down around the axones, appearing first as drops or granules. The same process takes place in the peripheral nervous system. The supporting elements of the nervous system thus would have a chemical as well as a mechanical function. Another view (Gurwitsch) is that the myelin is a product of the axone and is, at its first appearance, quite distinct from the neurilemma cells.
As the appearance of the myelin sheath is a final stage in the development of the neurone, the various neurone systems would naturally becorr Trwelinated in about the same sequence in which their axones develop. This is probably true in a general way, but the development of both axones and sheaths requires further study before any law can be exactly formulated. Coarse fibers apparently become medullated early, the sheaths of such fibers being usually thicker.
Although the myelin sheath is apparently an accessory structure, its formation is of great importance, not only from the above reason, but also because its appearance possibly indicates the assumption by the neurone of its capacity for the precise performance of its final functions. The functional significance of the myelin sheath is not, however, entirely clear. Its importance is enhanced by the fact that its integrity depends upon the integrity of its neurone and that we possess precise stains for demonstrating both its normal and abnormal conditions.
In the region of the RHOMBENCEPHALON, the neural crest very early exhibits a division into three masses: a glossopharyngeo-vago-accessorius, an acusticofacialis, and a trigeminus. These masses soon become separated from each other and from the neural tube, the glossopharyngeus also showing a partial separation from the vago-accessorius mass (Fig. 396).
The vago-accessorius group, at about three weeks, is a mass of cells much larger at the cranial end and continuous by a narrow band of irregular cells with the spinal neural crest. The cranial end of the mass shows a partial division into a dorsal and ventral part. The former becomes the ganglion of the vagus root, the latter the ganglion of the trunk (nodosum). The glossopharyngeus mass likewise shows a division into a dorsal group of cells, the future ganglion of the root and a ventral group, the future ganglion of the trunk (petrosum). The two ventral groups are associated with epidermal thickenings (placodes), but it is doubtful whether any ganglion cells are derived from the thickenings. These thickenings probably represent the thickenings associated in water-inhabiting Vertebrates with the development of certain sense organs, either lateral line or epibranchial (see p. 422). At this stage there are no afferent fibers, the cells not yet being differentiated into neurones. Some fibers found among the cells are efferent (see p. 458). The glossopharyngeus cells lie in the region of the third branchial arch, the vagus in the region of the fourth.
During the fourth and fifth weeks the processes of the cells begin to develop (Fig. 396), and the cell masses finally become definite ganglia with afferent root fibers passing into the neural tube and peripheral processes passing outward, forming, with the associated efferent fibers, the peripheral branches of the nerves in question (Fig. 397). The root and trunk ganglia of the vagus and glossopharyngeus, respectively, are also now connected by fiber bundles instead of cellular strands. At the same time there is a diminution of cells in the caudal part of the vago-accessorius group, this part finally being composed almost exclusively of efferent fibers emerging from the lateral surface of the medulla and cord. A few groups of cells (accessory root ganglia) persist, however, and develop into ganglion cells, some being found there at birth (Streeter) . This would indicate the presence of a small and hitherto undetected afferent element in the spinal accessory nerve, which is usually regarded as purely efferent. The spinal accessory nerves are thus identical with the vagus in their early development and consist at first of a homologous series of efferent roots and ganglia. This indicates that the spinal accessory might be regarded as a specialized part of the vagus extending caudally into the cord (Streeter) (see p. 434) *
Fig. 396. From a reconstruction of the peripheral nerves in a human embryo of 4 weeks (6.9 mm). Streeter.
- UI-XII, III to XII cranial nerves; C.I, D. /,, L.I., 5. /., ist cervical, ist dorsal, ist lumbar, and ist sacral nerves, respectively; i, 2, 3, branchial arches; Ot. v., auditory vesicle; IX-X-XI gang, crest, ganglionic or neural crest of IX, X and XI cranial nerves. Fiber masses are represented by fine lines, ganglion cell masses by dots.
From this point on, the further development of the efferent fibers of the X and XI nerves and of the peripheral processes of their ganglia is the further growth of the various branches of these nerves and their connection with the differentiating structures innervated by them. At the same time there is an increasing concentration of the cells, thereby forming more definite ganglionic masses. The changes taking place are similar to those exhibited in the differentiation of the spinal nerves (p. 460), The central relations of the nerves of this region of the medulla are shown in Fig. 398. (Comp. Fig 369). The glossopharyngeus at the same time develops its branches, most of the peripheral fibers running in the third arch (lingual branch). Somewhat later (i 2 to 14 mm. embryo) another bundle (tympanic branch) (Fig. 397) passes forward to the second arch. This forms the typical branchiomeric arrangement in which there is a forking of the nerve into prebranchial and postbranchial branches, the latter being larger and containing the efferent element (see p. 434 and Fig. 367).
- According to another view (Bremer) , the spinal accessory nuclei and roots are to be regarded as representing a specialization of lateral nuclei of the ventral gray column of the cord whose root fibers pass in the dorsal branches of the spinal nerves to the dorsal trunk musculature (p. 45 7 > comp. Fig. 366). According to this view, the muscles innervated by the XI would be somatic. The possible pomology of the lateral efferent nuclei and roots of the medulla with those dorsal root fibers of the cord which arise from cells in the ventral gray column (p. 457 and Fig. 392) may be mentioned in this connection.
Fig. 397. Lateral view of a reconstruction of a 10 mm. human embryo. Showing the origin and distribution of the peripheral nerves. The ganglionic masses are represented by darker and the fiber bundles by lighter shading. For purposes of orientation the diaphragm and some of the viscera are shown. The arm and leg are represented by transparent masses into the substance of which the nerve branches mav be followed. Streeter.
Fig. 398. Transverse section through the rhombic brain of a 10.2 mm human embryo (during the fifth week). X, Vagus; XII, Hypoglossus. His.
While the ganglia of the facialis and acusticus are derived from the same mass of cells (p. 465, Fig. 396) and are later still in very close apposition, it must be remembered that they are totally different in character. At four weeks they are differentiated from each other (Fig. 399). The relations of the two ganglia are shown in Figs. 397 and 399. It is probable that the ganglion of the facial (geniculate ganglion) shows an early differentiation into dorsal and ventral parts similar to the ganglia of the IX, and X, and also has associated placodes. The peripheral branches of the cells of the geniculate ganglion develop into the great superficial petrosal and chorda tympani. Both of these nerves enter into secondary relations with the V. There is some doubt as to whether the chorda is a prebranchial or postbranchial nerve (Fig. 397; also compare p. 432 and Figs. 367 and 368^
The VII, XX and X are, as already mentioned, branchial (splanchnic) nerves and the central processes of their ganglia ail have a common destination; they grow into the lateral surface of the medulla oblongata, enter the marginal layer of the alar plate, and there bend caudally, forming a comrion descending bundle of fibers in the marginal layer, the tractm solita.ius (Figs. 398 and 432; see also pp. 432, 435).
Fig. 399. Transverse section through the acoustic region of the rhombic brain of a 10.2 mm human embryo. VI, Abducens and its nucleus; VII G.g., geniculate ganglion; VIII G. c., cochlear ganglion of acoustic nerve; VIIIG.v., vestibular ganglion of VIII nerve. His.
The acoustic ganglionic mass is elongated at an early stage, and is in < onr.ection with an ectodermal thickening (placode) which gives rise to the acoustic receptors (p. 558). From the upper part of the mass a bundle of peripheral processes forms a branch which subsequently innervates the ampullae of the superior and lateral semicircular canals and the utricle, while from the lower part a branch develops to the ampulla of the posterior canal and to the saccule. The nerve and ganglion (ganglion of Scarpa] is thus at first vestibular and at this stage the cochlear part of the ear vesicle is not indicated as a separate outgrowth. As the lower border of the vesicle grows out into the cochlea, the lower border of the ganglion becomes thickened and develops into the cochlear ganglion (the ganglion spirale). It will be recalled that the vestibular part of the ear is the older part phylogenetically, the cochlea being a more recent specialized diverticulum of the older structure. (See p. 552 and Figs 464 and 465.)
The central processes of the acoustic ganglionic mass first develop from the upper part, forming the vestibular nerve root which enters the marginal layer of the medulla. A portion at least of its fibers bends caudally, forming a descending tract. The central processes of the cells of the cochlear ganglion, forming the cochlear nerve root, pass dorsally, cross the vestibular ganglion and enter the medulla dorsal and lateral to the vestibular root fibers (Fig. 399).
Fig. 400. Transverse section through the rhombic brain in the region of the trigeminus (V) nerve of a 10.2 mm human embryo. a.W., Spinal V; G.G., Gasserian ganglion; V.m., efferent root of V nerve. His.
The trigeminus is the most anterior of the ganglionic masses (Fig. 396). Embryological evidence has been brought to show that it consists of two or more nerves which subsequently fuse. Placodes have also been described. It is possible that such placodes represent those belonging to the most anterior division of the lateral line system in lower forms, and probably in this case would not properly belong to the V (comp. Fig. 367). From the ganglionic mass (Gasserian or semilunar ganglion) the three principal branches ophthalmic, maxillary and mandibular are formed, the two latter passing into the maxillary process and mandibular arch, respectively (Fig 397). The central processes, forming the afferent root (portio major} of the V, enter the marginal layer of the alar plate of the rhombencephalon and form a descending bundle, the spinal V (Figs. 400, 401, 402 and 432).
Fig. 401. Transverse section through the trigeminal region of the rhombic brain of a 10.2 mm human embryo. a. W., Spinal V; V. s., Gasserian ganglion; V. m., part of efferent root of V nerve. His.
Fig. 402. Part of a transverse section through the rhombic brain of a chick embryo toward the fourth day, showing the trigeminal roots. Cajal. Aj part of the efferent (masticator) nucleus of the V; B, efferent root of the V; C, bipolar cells of the Gasserian ganglion; D, beginning of descending tract (spinal V) formed by the central processes of C.
The trigeminus exhibits its spinal-like character in the behavior of its visceral portion (comp. p. 461). Cells of the ganglionic mass migrate further peripherally and form sympathetic ganglia (ciliary, otic, sphenopalatine (?) submaxillary(?) ). As in the cord, the question has arisen whether efferent roots may not also contribute a portion. Cells have been described as migrating with the oculomotor root fibers and forming part of the ciliary ganglion (Carpenter).
Besides those already described (cerebrospinal, sympathetic), the only other peripheral neurones of the nervous system are connected with the PROSENCEPHALON and are a part of the eye and nose. The visual receptors (rods and cones) and peripheral afferent neurones (bipolar cells) appear to be represented by portions of the retina and are described elsewhere (Chap. XVIII).
In the nose there is first a placode (p. 422) from which neuroblasts develop. Some of these migrate toward the neural tube and probably differentiate into lemmocytes, a few becoming ganglion cells.* The majority of the neuroblasts remain in the olfactory epithelium, sending their axones (fila olfactoria) into the olfactory bulb, the peripheral afferent olfactory neurones thus apparently displaying the primitive ectodermal location of afferent peripheral neurones (p. 418 and Fig. 359). (Comp. p. 551.)
Development of the Lower (Intersegmental) Intermediate Neurones
It has already been seen how, by migration and by differentiation of the cells during migration, the nucleated layer comprising the greater part of the thickness of the wall of the neural tube is differentiated into two layers an inner nucleated layer retaining its earlier characteristics, and an outer nucleated (mantle) layer, composed largely of the differentiating neuroblasts and characterized in ordinary staining by more widely separated nuclei. It has also been seen that this differentiation takes place earlier and more rapidly at first in the ventral part of the lateral walls (basal plate) , and that the first cells to migrate and differentiate are those whose axones grow out through the neural wall and pass out as the ventral root fibers.
Not much later than the above differentiation of the efferent peripheral neurones, axones of other neuroblasts also grow toward the periphery of the tube but do not pass beyond its wall. Such neuroblasts become intermediate neurones (p. 419). The migrating bodies of these neuroblasts are checked at the inner boundary of the marginal layer, but their growing axones enter the marginal layer and there, apparently on account of their inability to penetrate the external limiting membrane, turn cranially or caudally, or bifurcate, and form longitudinal ascending and descending fibers. These longitudinal fibers constitute a part of the future white columns (see p. 477), and their cells are therefore often called column cells. Many axones from such cells in all parts of the lateral walls (heteromeric or commissural column cells) pursue a ventral course through the mantle layer, arising around near the periphery and crossing the floor plate, ventral to the lumen, to become longitudinal ascending and descending fibers in the marginal zone of the opposite side. These early decussating axones form, in the cord, the beginning of the anterior commissure (Fig. 403). Other neuroblasts, the axones of which do not cross the median line, become tautomeric column cells.
- The latter are probably transient, but possibly in some forms persist as the ganglion cells of the nervus terminalis of Pinkus.
Fig. 403. Part of a section through the lumbar spinal cord of a 76-hour chick embryo. Cajal. A, Ventral root; B, spinal ganglion; C, bifurcation of dorsal root fibers forming beginning of dorsal funiculus; a, b, c, neuroblasts showing various stages of differentiation into intermediate neurones, some, at least, (c) becoming heteromeric column cells; d, efferent neurone.
It is about this time that the afferent root fibers enter the marginal layer of the dorsal part (alar plate) of the lateral wall and form in the marginal layer various bundles of longitudinal fibers above described (dorsal funiculus, actus solitarius, descending vestibular, and spinal V) (Figs 403, 404, 398, 399, 401, 402 and 432). In the cord the ascending arms grow to a greater length than the descending. In the rhombic brain the reverse is usually the case.
The longitudinal fibers of the afferent roots and of the intermediate neurones thus form an external layer occupying the marginal layer of the neural tube. This is the beginning of the differentiation into white and gray matter, i.e., into that part of the neural tube containing only the axones of the neurones and into that part containing the cell bodies and the beginnings and terminations of the axones. The terminations of axones are formed by a turning of the longitudinal fibers into the mantle layer or gray matter to form there terminal arborizations. Later, the longitudinal fibers develop branches (collaterals) which also pass into the gray matter. The differentiation of the white matter is completed several months later by the myelination of the nerve fibers.
The longitudinal axones of intermediate neurones which are formed at this period in the cord and epichordal brain are located ventrally near the median line. These medial tracts occupy the position of the future medial longitudinal fasciculi, the reticulo-spinal and ventral ground bundles, and may be regarded on both comparative anatomical and embryological grounds as a primitive system of long and short ascending and descending tracts mediating between cerebrospinal afferent and efferent peripheral neurones, and not having at this period connections with the higher centers. Other more lateral tracts of this character are formed somewhat later, the whole forming the beginning of the reticular formation + ventro-lateral ground bundle system (compare Figs. 404, 411, 414 and 416).
While merging more or less imperceptibly into the following stages, it may in a general way be said that at this stage of development there is differentiated what might be termed the primary and probably the oldest coordinating mechanism of the nervous system, most clearly segmental in character and having general features common not only to all Vertebrates, but to many Invertebrates. It is characterized by afferent and efferent peripheral neurones arranged segmentally and connected longitudinally in the central nervous system by crossed and uncrossed intersegmental intermediate neurones. (Compare pp. 435 and 436) . At the anterior end of this part of the nervous system (epichordal segmental brain) there are also exhibited differentiations due to fundamental vertebrate differentiations in the peripheral receptive and effective apparatus. Some of these are: (i) The differentiation of the splanchnic (visceral) receptive and motor apparatus, giving rise in the nervous system to (a) a separate system of afferent root fibers (tractus solitarius) including the more specialized gustatory apparatus; (b) a distinct series of lateral efferent nuclei. (2) The concentration of the non-specialized somat'c afferent innervation into one nerve (trigeminus and its central continuation, the spinal V). (3) The specialized somatic sense organ, the ear, with its older vestibular and newer cochlear divisions with central continuations of its nerves, including a vestibular descending tract.
These differentiations of the peripheral afferent apparatus lead to the later formation of special terminal nuclei for their central continuations and secondary tracts from these nuclei to suprasegmental structures (p. 436, Fig. 371).
The peripheral and intermediate neurones of the more highly modified cranial end of the tube, or FORE-BRAIN, appear to lag behind in development, but in its basal part the neuroblasts are beginning to be differentiated (fifth week) . In the development of the eye, the brain wall is evaginated, carrying with it the future retina comprising, apparently, the sensory epithelial cells or receptors (rods and cones), the afferent peripheral neurones (bipolar cells of retina) and the receptive or primary intermediate neurones (ganglion cells of retina and optic nerve). The histogenesis of these elements is dealt with elsewhere, but it may be pointed out here that the axones of the ganglion cells of the retina grow toward the inner side of the optic cup (away from the original luminal surface), pass thence in the marginal layer of the optic stalk, undergo a partial ventral decussation (optic chiasma) in the floor plate, and terminate in certain thalamic nuclei (lateral geniculate bodies) and in the roof of the mid-brain. The so-called optic nerve is thus obviously a central, secondary tract. The development of this tract does not apparently take place until a later period than the differentiation of the earlier secondary tracts of the cord and rhombic brain (after the sixth week) .
In the case of the olfactory organ, it has already been seen that the peripheral neurones develop at first apart from the neural tube and send their axones into the olfactory bulb. The latter is an evagination of the neural tube which receives the olfactory fibers, thereby constituting a complicated terminal nucleus for the latter. The axones of bulb cells (the mitral cells) which pass along the stalk of the bulb are thus the secondary tract of this system. Many of them decussate in the anterior commissure. Secondary (and tertiary) olfactory tracts find their way to caudal parts of the rhinencephalon and to hypothalamus, thalamus and epithalamus, forming, with other tracts, a highly modified prechordal intersegmental mechanism (p. 537). Other olfactory tracts proceed to the suprasegmental archipallium which develops efferent bundles to the segmental brain.
The embryological development of the peripheral apparatus, especially of its receptive portions, as shown by the various separate ganglionic rudiments (Fig. 396) and placodes, exhibits a segmental character which, though not in all respects primitive, is of practical value. These segments are (Adolf Meyer) : (1) The olfactory apparatus, nose, without efferent elements. (2) The visual apparatus, eye, with the eye-moving III and IV mid-brain nerves as its efferent portion. (3) The general sensory apparatus of the surfaces of the head and mouth, the afferent trigeminus, with the jaw-moving efferent trigeminus. (4) The auditory (and vestibular) apparatus, the ear (VIII nerve), with the VI (turning the eye to the source of sound) and VII (ear and face muscles) efferent nerves. In the latter, the original ear-moving apparatus has been replaced largely, in man, by the muscles of expression. (5) The visceral segment (IX, X, and XII nerves), not indicated externally in forms without gills. The afferent portion is concerned with taste and visceral stimuli, the efferent with tasting, swallowing, sound-production and other visceral functions. Overlapping with other segments is due to its visceral as opposed their somatic character. The apparent dislocation shown by the abducens is due to its common use by more than one segment.
Caudal to this is the mechanism for head movement (N. XI) , its afferent portion being the upper spinal nerves. Following this, there is the segmental series of spinal nerves which in places shows a tendency to fuse (plexuses) into larger segments (phrenic segment, limb segments) . All such modifications are expressions of more recent functional adjustments modifying preexisting ones.
These segments may be regarded as a series of reflex arcs, each one of which may have a certain amount of physiological independence but which are associated by intersegmental neurones. The latter class of intermediate neurones probably effects certain groupings of various efferent neurones, furnishing mechanisms which secure harmonious responses of groups of effectors involved in certain definite reactions (e.g., limb-movements, associated eye movements). These effector-associating mechanisms may be acted on directly (reflex) by afferent neurones or by the efferent arms of suprasegmental mechanisms.
Superadded to this segmental apparatus are the suprasegmental mechanisms which develop later, the pallium being the last to be completed. These receive bundles from the segmental nervous system and send descending bundles to the intersegmental neurones (pp.427, 435 and 436 and Fig. 371).
Further Differentiation of the Neural Tube
The Spinal Cord
From this time on, differences of structure between cord and epichordal segmental brain become more marked and make it more convenient to treat their later development separately. The ventral half of the cord for a considerable period maintains its lead in development. At four weeks (Fig. 404) this lead is not so pronounced as in the immediately following period. At this stage it will be noticed that the lumen is narrower in the ventral part, as if due to the greater thickening of the ventral walls (basal plates). The increase of the mantle layer (gray) of the basal plate marks the beginning of the ventral (anterior) gray column or horn. The increase in the basal plate may be partly due to neuroblasts migrating from the alar plate. These would be intermediate neurones. The development of the mantle layer at the expense of the inner layer, due to differentiation and migration of the cells of the latter, is well shown, but is more marked in the following stages.
As already mentioned, the axones of the heteromeric cells, many of which lie in the dorsal half of the lateral walls, after decussating (anterior commitsure), form longitudinal fibers in the marginal layer along the ventral surface of the opposite side, mostly mesial to the emerging ventral roots (Fig. 44) These longitudinal fibers are the beginning of the ventral (anterior) white columns or funiculi of the cord. The sides of the tube between the dorsal and ventral roots contain at first only a few longitudinal fibers the beginning of the ventrolateral juniculi. Their number soon rapidly increases, the fibers apparently coming from ventrally located tautomeric cells. The dorsal root fibers, as stated before (p. 460), form small round bundles in the marginal layer of the dorsal halves (Fig. 404). This is the beginning of the dorsal (posterior) white columns or funiculi,.
Fig. 404. Half of a transverse section of the spinal cord of a 4 weeks, (6.9 mm) human embryo. Dp, Roof plate; Bp, floor plate. His.
At four weeks there are blood vessels in the mesodermal tissue surrounding the neural tube. Branches of these soon penetrate the tube itself.
From its first appearance in the cord as an oval bundle, during the fourth week, the dorsal funiculus steadily increases in size, forming a "root zone" in the marginal layer of the dorsal half, but not reaching the roof plate (Fig. 405). This increase in size is probably produced in part by the addition on its inner side of overlapping ascending arms of dorsal root fibers from lower cord segments. The mantle layer of this part contains an increasing number of cells forming curved or arcuate fibers. (Fig. 405.) The increase in the mantle cells of the dorsal part marks the beginning of the dorsal (posterior) gray column or horn (terminal nucleus of the dorsal root fibers) . Later, other cells become differentiated from the inner layer which do not apparently form arcuate fibers (Fig 405) and which subsequently become part of the posterior horn. It is possible that the axones of some of these cells form the comparalively small ground bundles of the dorsal funiculus. During this period of development of the dorsal portions of the lateral walls the latter have approached each other, reducing the dorsal part of the lumen to a slit. The roof plate has undergone a slight infolding (Fig. 406). Ventral to the dorsal roots there is a groove running along each side of the cord (marginal furrow of His). At four and one-half weeks the number of fibers of the ventro-lateral funiculus has greatly increased and another groove has appeared parallel and ventral to the marginal furrow and forming the dorsal boundary of the ventrolateral funiculus (cylinder furrow of His) (Figs. 405 and 406). The portion of the lateral wall lying between these two grooves or furrows forms an intermediate plate which contains few fibers in its marginal layer at this period, and is thus backward in development. Grooves appear on the luminal wall, apparently corresponding approximately to the outer grooves.
Fig. 405. Half of a transverse section of the spinal cord of a 4.5 weeks (10.9 mm) human embryo. His. A.s., Artery in ventral longitudinal sulcus; A.sp.a., ventral (anterior) spinal artery; Bp, floor plate; Dp, roof plate; 7. 1., inner layer. The faint inner outline is the outline of the cord proper.
Fig. 406. Half of a transverse section of the spinal cord of a human embryo of 18.5 mm. (7.5 weeks). His.
The further growth of the dorsal funiculi and the concomitant growth of the associated gray matter, i.e., of the cells of the adjoining mantle layer, proceed until we have the conditions shown in Figs. 406 and 407. At the same time there is a further approximation of the dorsal portions of the lateral walls so that the widest part of the lumen is further ventral. At about eight weeks the portion of the wall near the median line, which has formed a ridge by the apposition of the two inner layers and the roof plate (Fig. 406 Y), and is uncovered as yet with fibers, differentiates a marginal layer (eight and one-half weeks, Fig. 407) into which fibers grow forming, on each side, in the upper part of the cord, the column of Goll or fasciculus gracilis (Fig. 408). Many of these fibers, at least, are the ascending arms of caudal dorsal root fibers, which are thus added mesially to the continuations of upper cord roots. It will be noted that there is now a massive dorsal gray column and that the original oval bundle has extended around on the mesial side of this gray column.
Fig. 407. Half of a transverse section of the spinal cord of a human embryo of 24 mm. (8.5 weeks). His.
While these changes are taking place, the dorsal portions of the lateral walls have fused, probably beginning at the most dorsal part, thus forming the dorsal septum. This may be accompanied by a certain amount of rolling in from the dorsal part indicated by the direction of the ependyma cells (Fig. 408). The growth of the ventral funiculi and gray columns results in the appearance and subsequent increasing depth of the. ventral longitudinal fissure. The cord now resembles the adult cord in many features, having well-marked white* and gray columns, but contains a disproportionately small amount of fibers. A further and later change consists in a rolling inward, as it were, of the dorsal gray column so that it becomes separated from the ventral gray column, and that portion of it formerly facing dorsally comes to face more mesially, the roots entering more dorsally. This change may be due partly to the development of the intermediate plate which has in the meantime taken place. In this plate axones of tautomeric cells have begun to form the limiting layer of the lateral funiculus. From the cells of the intermediate plate are formed the neck of the dorsal gray column, also the cells of Clarke's column and the their fibers become myelinated during the sixth month.
- The term "white" column is used for convenience, The funiculi do not become "white" until processus reticularis. In the course of these developments, the ventro-lateral ground bundles, formed primarily by heteromeric and tautomeric cord cells, receives various accessions. These are first the long descending intersegmental tracts from epichordal brain nuclei in the formatio reticularis which as they proceed down the cord naturally overlap externally the ground bundles already formed there. They include the medial longitudinal fasciculi; tracts from Deiters 1 nuclei and the rubro-spinal tracts which occupy the ventrolateral funiculi external to the ground bundles. In the lateral funiculi there are also added the ascending tracts from cord nuclei to suprasegmental structures.
Fig. 408. Half of a transverse section of the spinal cord of a human foetus of about 3 months. His.
These are the dorsal spino-cerebellar tracts from Clarke's columns, ventral spinocerebellar tracts, and tracts to mid-brain roof and thalamus (spino-tectal and thalamic). Finally (fifth month) the descending tracts from the pallium are added, the direct and crossed cor tico- spinal (pallio- spinal or pyramidal] tracts, the latter being thrust, as it were, into the lateral funiculus.
The development of the cord, then, is produced by (1) the proliferation of the epithelial cells and the formation of the nuclear and marginal layers; (2) the multiplication, differentiation and growth of the neuroblasts (mantle layer) ; (3) the development of the ventral roots; (4) formation of the funiculi (white columns when myelinated) by the growth into the marginal layer of (a) dorsal root fibers of the cord, the ascending arms of which overlap those root fibres entering higher cord segments, (b) cord neuroblasts forming intersegmental (ground bundle) tracts next to the gray matter, (c) descending intersegmental tracts from the segmental brain, representing continuations principally of cerebellar efferent tracts, (d) afferent suprasegmental tracts from cord nuclei, (e) descending pallio-spinal tracts. In addition to this, there are general factors of growth, such as increasing vascularization, increasing amount of neurone cytoplasm (especially dendrites) , increased size of axones and, finally, the acquisition by the latter of myelin sheaths.
The vertebral column grows faster in length than the inclosed spinal cord. The result of this is that the caudal spinal nerves making their exit through the intervertebral foramina are, so to speak, dragged caudalward and instead of proceeding outward at right angle to the cord, pass caudally to reach their foramina. The leash of nerve roots thus formed, lying within the caudal part of the vertebral column, constitutes the cauda equina. The coverings of the cord retain their original connections at the caudal end of the vertebral canal and form a prolongation of the cord membranes enclosing the thin, terminal part of the cord, the filum terminate.
The Epichordal Segmental Brain
In the fifth week, the walls of the rhombencephalon are comparatively thin. In the caudal region of the medulla oblongata (p. 447) , the dorsal part of each lateral wall is upright and is bent at a considerable angle with the ventral part (basal plate), the groove on the inner surface between the two being the sulcus limitans. The roof of this region is formed by the thin expanded roof plate (Figs. 398-401).
Anterior to this, the roof plate is not expanded, the alar plates almost meeting in the mid-dorsal line. This thicker part of the roof is the rudiment of the cerebellum. Its caudal edges are attached to the expanded roof plate.
In front of the cerebellum the tube is narrower and is compressed laterally. This part is the isthmus (Fig. 409) . Anterior to this, the roof plate and alar plates expand into the mid-brain roof, the basal and floor plates forming the basal part of the mid-brain.
Certain gross changes which from now on take place in the medulla may conveniently be noted here. At about this time (fifth week) the outer borders of the alar plate become folded outward and then downward, being thus turned back on the plate itself (Figs. 414 and 378). This fold is called the primary rhombic lip, and is most marked along the caudal border of ' the cerebellum. The folds of the lip then fuse, forming a rounded eminence composing the border of the alar plate to which the roof plate is attached laterally. Subsequently, the attachment to the roof plate is shifted dorsally in the medulla, caudally in the cerebellum. The portion of this lip which thins off into the roof plate is the tania of the medulla and the posterior velum and taenia of the cerebellum. The thin roof plate itself becomes tbe epithelial part of the tela chorioidea of the fourth ventricle. At the caudal apex of the fourth ventricle a fusion of the lips of the opposite sides forms the obex.
Fig. 409. Transverse section through the isthmus of a 10.2 mm human embryo. D.IV, Decussation of trochlear nerve; M. L, marginal layer; Nu. IV, nucleus trochlear nerve.
A further complication is due to the increasing pontine flexure by which the dorsal walls of the tube are brought close together (Fig. 410). The transverse fold of the tela thus produced is the chorioid fold. At about the same time lateral pocketings outward of the dorsal walls occur just caudal to the cerebellum which contain portions of the chorioid fold. These are the lateral recesses. By further growth and vascularization, the mesodermal part of the chorioid fold forms the chorioid plexus of the fourth ventricle (metaplexus). Finally, in the human brain an aperture appears in the caudal portion of the roof of the ventricle the foramen of Magendie (metapore) ; and, according to many authorities, one also occurs in the roof of each of the lateral recesses the foramina of Luschka. The roof of the fourth ventricle, where present, is thus composed of an inner ependymal epithelium the expanded roof plate of the neural tube and an outer mesodermal covering containing blood vessels. Other gross changes chiefly involve the basal plate. At the beginning of the fifth week this does not much exceed the alar plate in thickness and is separated from the opposite basal plate by an inner median sulcus (Fig. 414). The basal plate now increases in thickness and thereby both deepens the sulcus and contributes to a flattening out of the lateral walls, so that all portions by the sixth week lie approximately in the same horizontal plane (Fig. 416). Later, the floor plate increases in thickness more rapidly and the sulcus becomes shallower (eight weeks) (Fig. 417). The band of vertical ependyma fibers passing through it is the septum medulla. It is bounded on each side by a vertical extension of the marginal layer which for convenience will be referred to as the septal marginal layer (Figs. 415, 416 and 417).
Fig. 410. Lateral view of a model of the brain of a 7.5 weeks (18.5 mm) human embryo. His.
The histological condition of this part of the tube at the beginning of five weeks has already been described. The lateral walls consist of an inner layer of closely packed cells, of a mantle layer consisting of efferent neurones and a simple system of intermediate neurones, and an outer marginal layer containing the longitudinal bundles of incoming afferent roots and longitudinal axones of intermediate neurones (see p. 474). It has been seen that this condition has been brought about by the proliferation of cells near the tube cavity, which migrate outward, at the same time many of them differentiating into neuroblasts and nerve cells and thereby forming the mantle layer. As in the cord, the basal plate takes the lead and thus at first outstrips the alar plate, as shown in its greater thickness above mentioned. This process likewise terminates sooner in the basal plate, few cell divisions being present there at seven weeks. At about the end of the fifth week (see p. 489) the alar plate begins to develop very rapidly. Its period of proliferation is about terminated at the end of the second month. When the cell proliferation near the ventricle has ceased, the inner layer is reduced by outward migration to a single layer of epend] ma cells (compare pp. 455 and 456).
While the efferent nuclei continue to develop and the central continuations of the afferent neurones continue to grow in length, the principal differential ipns now taking place in the rhombic brain are those affecting the intermediate neurone systems.
The first of these to be considered is the further differentiation of the system of intersegmental neurones (p. 435). The earlier development of this system has been seen to involve especially the basal plate and the further development of the latter leads to the complete differentiation of the formatio reticularis which especially represents this system in the epichordal brain. It has already been seen (p. 474) that many of the intermediate neurones representing the beginning of this system seem to be at first heteromeric and form an internal arcuate system of fibers similar to those seen in the cord (pp. 473,477). They increase in number toward the median line and are especially numerous in the basal plate, where they, together with the medial efferent neurones (XII and VI cranial nerves), form an eminence of the mantle layer corresponding to the ventral gray column of the cord (Fig. 411). Many of the axones of these cells of the arcuate system cross the septum medullae, thus marking the beginning of the raphe, and form on each side a longitudinal bundle in the septal marginal layer (Fig. 411). These longitudinal bundles correspond to the first formation of the ventral funiculi of the cord. They must not, of course, be confused with the pyramids which appear much later. Whether these longitudinal bundles are also partly formed of axones of tautomeric cells is uncertain. Later, as the anterior horn swellings grow and the depth of the septum medullae and of the septal marginal layers increases (compare p. 484), more longitudinal fibers appear in the latter, the new ones apparently being added ventrally. Others also appear more laterally in the marginal layer (Figs. 415, 416 and 417). (Compare cord, p. 477-) At this time, also, fibers enter the marginal layer bordering the surface (as distinguished from the septal), pass along parallel with the surface, cross the septum, and proceed to various parts of the marginal layer of the opposite side. These fibers are the first external arcuate fibers as opposed to the prec eding internal arcuate fibers which traverse the mantle layer (gray) in the arcuate part of their course (Fig. 415).
The majority of the longitudinal fibers entering the septal marginal layers during the second month occupy approximately the position of the future mesial formatio reticularis alba (white reticular formation) and correspond in position to the fibers of the medial longitudinal fasciculi and reticulo-spinal tracts in the adult medulla, representing probably the same system as the medial part of the ventro-lateral funiculi of the cord (medial longitudinal fasciculi, reticulo-spinal and ventro-medial ground bundles of the cord). The medial longitudinal fasciculi are in part descending fibers from higher levels described later.
Fig. 411. Half of a transverse section of the medulla of a 10.2 mm human embryo. His.
In the basal plate, between the medial and lateral efferent nuclei, there are, even at the beginning of the fifth week, not only the efferent neurones and the heteromeric (commissural) neurones already mentioned, but other neuroblasts whose axones have a radial direction, i.e., toward the periphery. (Figs. 411 and 414.) The interlacing of these with the arcuate fibers forms the first indication of the formatio reticularis grisea (gray reticular formation). Later, longitudinal fibers are present here, giving rise to a condition more fully corresponding to that in the adult, analogous also to the condition in the lateral funiculi of the cord, especially in the processus reticularis.
In the region of the auditory segment an important neurone group appears which is possibly a differentiation of the extreme dorso-lateral portion of the basal plate. This is Deiters' nucleus, which apparently receives vestibular and cerebellar fibers and sends uncrossed descending bundles along the outer lateral part of the reticular formation and also ascending and descending crossed and uncrossed fibers along its outer mesial portion (part of the medial longitudinal fasciculus) . This nucleus thus represents, apparently, like the nucleus ruber and nucleus of Darkschewitsch (below), a differentiated portion of the intersegmental neurones in especial connection with suprasegmental efferent fibers which thereby act on many brain and cord segments.
The great development of the reticular formation here and caudally possibly causes a ventro-lateral displacement of the contained nucleus ambiguus and efferent facial nucleus and consequently the arched or hook-shaped course of their root fibers as seen in transverse section (Streeter) . At the same time, the nucleus of the VI, which originally was caudal to the VII, migrates cranially, carrying the facial efferent roots with it. This gives rise to the genu facialis (Streeter, Fig. 412).
Fig. 412. Diagram illustrating the development of the genu of the facial nerve in the human embryo. The drawings show the right facial nerve and its nucleus of origin, in three stages: the youngest, A, being a 10 mm. embryo, and the oldest, C, a new-born child. The relative position of the abducens (VI) nerve is represented in outline; its nerve trunk is not shown, as the structures represented are seen from above. Streeter.
In the mid-brain (Fig. 413), what appears to represent the basal plate forms an eminence, the tegmental swelling. Later there is differentiated from this the reticular formation of this region, containing various nuclei and traversed by radial, longitudinal and arcuate fibers, many of the latter arising from the later differentiating dorsal portions (corpora quadrigemina) of the lateral mid-brain walls. An important neurone group of the reticular formation system which appears in this region is the nucleus of Darkschewitsch. Its descending axones form a part of the medial longitudinal fasciculus and probably appear at the end of the first month. The nucleus ruber is probably differentiated from the forward extremity of the tegmental swelling which overlaps into a prechordal region (Fig. 425). Its axones (crossing as ForeVs decussation and forming the rubro-spinal tract) probably develop early. This neurone group apparently owes its great development principally to its close association with the cerebellum. These two long descending intersegmental tracts as they grow downward envelop the differentiating reticular formation of more caudal regions of brain (and cord) and thereby come to occupy an external position in the fully differentiated reticular formation.
The reticular formation is thus composed of a gray portion containing the neurone bodies and shorter tracts and a white portion composed of the longer tracts. Axones from certain nuclei (especially N. ruber, N. of Darkschewitsch and N. of Deiters) form long, principally descending, tracts which envelop the gray reticular formation mesially (medial longitudinal fasciculus including fibers from nuclei of Darkschewitsch and Deiters as well as other reticulospinal fibers) and laterally (rubro-spinal, lateral uncrossed tract from Deiters nucleus and other reticulo-spinal fibers) and constitute the white reticular formation. These long tracts descend to the cord and there similarly envelop its ventro- lateral ground bundles.
Fig. 413. Transverse section through the mid-brain of a 10.2 mm human embryo. His.
While the above differentiation of the reticular formation has been taking place, changes in the alar plate have begun which lead to the formation of terminal nuclei of peripheral afferent nerves, as well as terminal nuclei of other tracts, all of which send fiber bundles to suprasegmental structures.
The formation of the receptive nuclei of the afferent nerves of peripheral (segmental) structures is complicated by the fact that the central continuations of the peripheral afferent nerves are not confined to their own respective segments but form longitudinal tracts which continue to grow upward (columns of Goll and Burdach) or downward (descending solitary, vestibular and trigeminal tracts) passing into other segments and overlapping externally structures already in process of formation there. In each segment, then, the terminal nuclei of the afferent nerves of that segment must be distinguished from the terminal nuclei of afferent elements from other segments. The latter are external or added to the former and are differentiated from additional proliferations of neuroblasts of the alar plate. In addition to these nuclei, there are certain nuclei forming links between the two great suprasegmental structures, the pallium and cerebellum. These nuclei are the olive* and pons nudei, both of which form afferent cerebellar bundles and which are differentiatec. by still further proliferations and migrations of alar plate neuroblasts.
It has already been seen that the afferent peripheral nerves (IX and X) of the visceral segment form (together with descending fibers of the VII) the tractus solitarius. This is at first (5th week) short, but in six weeks has reached the cord. The terminal nucleus of the tractus solitarius is differentiated irom the neuroblasts of the medial portion of the alar plate. The course of the axones of this nucleus is not known. Judging from comparative anatomical grounds, they would not follow the fillet pathway (C. J. Herrick). The most caudal part of this nucleus is the nucleus commissuralis at the lower apex of the fourth ventricle.
The formation of the other terminal nuclei lying in the region of this segment is begun by the further developments of the alar plate already alluded to. These are initiated by an expansion and consequent folding of its border (formation of the rhombic lip, p. 483). followed by further cell-proliferation, leading to fusion of these folds and copious formation of neuroblasts in this region. These neuroblasts represent fresh accessions to the neuroblasts already formed in the mantle layer of the more medial part of the alar plate. This latest development of the border portions of the alar plate is the last step in the progressive development of the neural tube from the medial portion (basal plate) to the lateral (dorsal) border of the lateral walls of the tube where further development ceases at the attachment to the roof plate (taenia). (Fig. 414)
Many of the neuroblasts of the rhombic lip region migrate ventrally.t Some of those from the medial part of the swelling produced by the fusion of the rhombic lip folds (p. 483, migrate along the inner side of the tractus solitarius, while those from the lateral part of the swelling pass outside the tractus, which becomes thereby enclosed in the mantle layer (Fig. 415). Many of these neuroblasts continue their journey, passing along the outer side of the differentiating formatio reticularis, until they are arrested at the septal marginal layer (Figs. 416 and 417).
- This is conjectural. The origin of fibers to the inferior olivary nuclei is not known. The most conspicuous tract to the olive is von Bechterew's central tegmenlal trad. Purely a priori considerations might be adduced in favor of this being considered a descending tract from thalamic nuclei which in turn receive pallio-thalamic fibers. It may, however, arise from lower optic centers.
fit is, perhaps, an open question whether the formation of the lip is a fundamental feature in this last proliferation and invasion of neuroblasts from the border of the alar plate. The prominence of the rhombic lip in man is the early embryological expression of the future great development of parts subsequently formed from this portion of the neural wall, especially the cerebellum and neurone groups in connection with it.
From these neuroblasts which remain in situ near the dorsal border are developed the nucleus gracilis and nucleus cuneatus. The axones of these nuclei form internal arcuate fibers which decussate and form a bundle of longitudinal fibers in the opposite septal marginal layer ventral to the reticularis alba. This tract is the medial fillet whose fibers appear during the second month and is one of the afferent paths to suprasegmental structures (mid-brain roof and pallium). Other neuroblasts, which probably migrate further, form the substantia gelatinosa of Rolando. Axones of this group also form tracts representing afferent paths to suprasegmental structures (pallium). Neuroblasts which migrate further form, as already mentioned, afferent cerebellar connections. Those migrating to the septal marginal layer form there an L-shaped mass mesial to the root fibers of the XII cranial nerve (Fig. 417). This is the medial accessory olive. Fresh groups of neuroblasts, added laterally to these in streaks, form the inferior olivary nucleus, while others which have not advanced so far form the lateral nucleus. Axones of the olivary neuroblasts (olivo-cerebellar fibers) pass across the median line (seventh or eighth week) to the opposite dorsal border where they, together with axones from the lateral nuclei and the continuation from the cord of Flechsig's tract, form (end of the second month) the bulk of the restiform body (Fig. 417). At three months the olives have acquired their characteristic folded appearance.
Fig. 414. Half of a transverse section of the medulla of a 9.1 mm human embryo (during the fifth week). His. The arrow is in the inner median sulcus. F. r. a., beginning of white reticular formation.
Owing to the later development and ventral migration of the alar plate neuroblasts, there are thus formed the various nuclei which lie external to the reticular formation in the adult. The continuations of ascending spinal cord tracts (Flechsig and Gowers) occupy the most external position on the lateral surface, and other cord continuations (medial fillets) the most external mesial positions. Later, however (fifth month), there is added ventral to the fillets the descending cortico-spinal fibers (pyramids). Their decussation takes place at the cervical flexure.
Fig. 415. Half of a transverse section of the medulla of a 10.5 mm human embryo (end of fifth week). His.
By the external accessions from the alar plate above described, forming terminal nuclei of overlapping tracts from above (especially the nucleus of the spinal V) , the tractus solitarius becomes buried, as it were, hence its deep position in the adult. The great development of the reticular formation may contribute to this result. As the trigeminus is the most cephalic rhombic segment, its descending fibers are not overlapped by fibers from above and therefore occupy the most external position of all these descending peripheral systems.
Fig. 416. Half of a transverse section through the medulla of a 13.6 mm. human embryo (beginning of sixth week). His. F. r. a., Beginning of white reticular formation in dorsal part of septal marginal layer. Another bundle has formed more ventrally (F. r. a. v.) .
Fig. 417. Transverse section through the medulla of an 8 weeks' human embryo. His.
The terminal nuclei belonging to the auditory (acustico-facialis-abducens) segment are those of the vestibular and cochlear portions of the VIII nerve.
The development of these nuclei is not fully known, but they are derived from the alar plate, except possibly Deiters' nucleus (see p. 487), the nuclei of the later formed cochlear nerve occupying the more external position. The vestibular nuclei apparently send axones both to cerebellum and reticular formation. The cerebellum itself may be regarded as primitively a receptive vestibular structure (p. 436) and probably receives vestibular root fibers. The axones of the cochlear nuclei pass across the median line, along the ventral border of the reticular formation (second half of second month), forming the trapez'um. On the lateral boundary of the opposite reticular formation they ascend, forming the lateral fillet, to the suprasegmental posterior corpus quadrigeminum. Accessions are received from the superior olive, in which some of the trapezium fibers terminate.
The alar plate of this segment also forms the substantia gelatinosa and the anterior portions of the olivary nuclei in this region. The various remaining tracts assume the same positions as further caudally.
Later, the pyramids are added ventrally to the fillet, and the great development of the pons leads to it's covering the ventral surface of part of this region. Owing to the late development of the pons and pyramids, the trapezium is thus uncovered and lies on the ventral surface of the rhombic brain during the third month. It is permanently uncovered in the dog and cat.
In the trigeminus segment, the terminal nucleus of the afferent portion of this nerve is probably similarly formed from the alar plate. Its axones decussate, probably joining the fillet, and proceed to the thalamus, which is connected with the pallium. Descending axones from cells in the mid-brain roof form part of the trigeminus known as its descending or mesencephalic root. The view has been advanced (Meyer, Johnston) that these are afferent neurones equivalent to certain dorsal horn cells found in some adult and embryonic Vertebrates and representing spinal ganglion cells which have become included in the neural tube instead of becoming detached with the rest of the neural crest (compare p. 422).
In front of the lateral recess another extensive development of the alar plate occurs, evidenced by the large rhombic lip of this region. The neuroblasts thus differentiated form the enormously developed pontile nuclei whose axones pass across the median line (fifth month) to the opposite cerebellar hemisphere, forming the middle cerebellar peduncle or brachium pontis. The pons extends over the ventral surface of the cephalic part of the medulla and over the ventral surface of part of the mid-brain. It receives fibres from various parts of the neopallium, which form a great part of the pes pedunculi or crusta. A still greater development of the alar plate forms the cerebellum.
In the mid-brain region, the reticular formation already described (p. 487) is enveloped ventrally and laterally by the upward extension of the medial and lateral fillets, the whole comprising the tegmentum. Ventral to this are added later the pons and the descending cortico-pontile, cortico-bulbar and corticospinal bundles forming here the pes pedunculi or crusta (probably during the fifth month).
The alar plate of the mid-brain region forms the corpora quadrigemina (mid-brain roof).
The further changes in the gross morphology of the medulla are due mainly to further growth of structures already present. The nuclei of the dorsal columns by their increase cause the swellings on the surface of the medulla known as the clava and cuneus, and likewise by their increase in size cause a secondary dorsal closing in of the caudal apex of the fourth ventricle which formerly extended to the cervical flexure. The tuber culum of Rolando is produced by the growth of the terminal nucleus of the spinal V, and the restiform body largely by the development of the afferent cerebellar fibers (Fig. 419).
The growth of the olivary nuclei produces the swellings known as the olives. The above mentioned accession of the descending cerebrospinal tracts to the ventral surface is indicated by the pyramids.
In the floor of the ventricle there is a longitudinal ridge each side of the median line occupied by swellings produced by the nucleus of the XII and, further forward, the nucleus of the VI, together with other nuclei (intercalatus, funiculus teres and incertus, Streeter) which are not well understood. The furrow forming the lateral boundary of this area is usually taken to be the representative of the sulcus limitans and consequently the area in question would be the basal plate. Lateral to it is a triangular area with depressed edges the ala cinerea. It represents a region where portions of the vagoglossopharyngeal nuclei (dorsal efferent and terminal nuclei of fasciculus solitarius) lie near the surface. Possibly a secondary invasion by surrounding more recently differentiated nuclei may account for their apparent partial retreat from the surface. It is possible that the ala cinerea may be regarded not so much as a part of the alar plate, but that it or rather the branchial nuclei involved in its formation represents an independent intermediate region corresponding to the intermediate region in the cord (J. T. Wilson). The remaining portion of the alar plate, in -the floor, is apparently represented principally by the acoustic, especially the vestibular, field.
In the development of the segmental brain there are thus the following overlapping stages: (i) The differentiation of the inner, mantle and marginal layers. (2) The primary neural apparatus, consisting of (a) the peripheral segmental neurones, the central processes of the afferent neurones entering the alar or receptive plate, the efferent neurone bodies forming two main series of nuclei in the basal plate, and (b) intersegmental neurones composing the reticular formation in which the long tracts occupy external positions.
The further differentiation, from the alar plate, of terminal nuclei for the afferent peripheral segmental neurones, the axones of the terminal nuclei forming afferent tracts to suprasegmental structures. These tracts and other later forming afferent suprasegmental tracts with their nuclei are laid down external to the. reticular formation. (4) Formation of efferent (chiefly thalamic(?) mid-brain and cerebellar) suprasegmental tracts which act upon the intersegmental neurones or reticular formation. (5) Accession at a late stage of development of a descending system of fibres from the neopallium. These lie ventral to the preceding structures.
The Cerebellum
It has already been pointed out that at an early period (three weeks) the anterior boundaries of the thin expanded roof plate of the rhombic brain form two lines converging anteriorly to the median line where the roof plate is represented by the usual narrow portion connecting the two alar plates (Fig. 418). It has also been pointed out that the pontine flexure produces on the dorsal surface a deep transverse fold in this thin roof, into which vascular tissue grows later forming the chorioid plexus (Fig. 410). At this stage, the continuations of the alar plates of the medulla form two transverse bands which, when viewed laterally, are vertical to the general longitudinal axis of this part of the brain (Fig. 448). At the same time, the rhombic lips are formed along the caudal border of these bands and the latter become musm thickened into the two rudiments of the cerebellum, a H IB considerable portion of which may be derived from the
I: fj lips. These rudiments are thus two transverse and vertical swellings and are connected across the median line by the roof plate. The attachment (taenia) of the
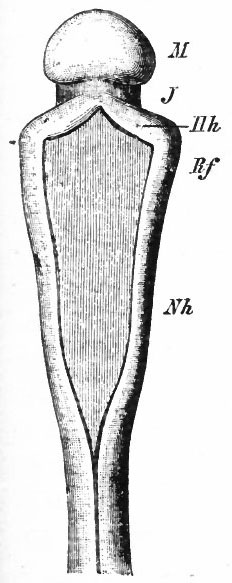
Fig. 418. Dorsal view of that part of the brain caudal to the cephalic flexure (human embryo of 3d week, 2.15 mm). Hh. Cerebellum; i, isthmus; M, mid-brain; Rf, Nh, medulla. Compare with Fig. 416. His.
While the structure thus formed expands enormously in a lateral direction, in its subsequent development its greatest growth is in a longitudinal direction. The effect of this is that the continuations of the cerebellum forward (velum medullare anterius) and backward (velum medullare posterius) into the adjoining, brain walls of the isthmus and medulla are comparatively fixed points and are completely overlapped by the spreading cerebellum, producing an appearance in sagittal section as though they were rolled in under the latter structure (comp. Fig. 370, F). Another result of this longitudinal growth is the formation of fissures running across the organ, transversely to the longitudinal brain axis. First, lateral incisures separate two caudal lateral portions, the flocculi (Fig. 419), the median continuation of which, the nodule, is finally rolled in on the under side of the cerebellum as explained above. Another transverse fissure, the primary fissure, beginning in the median part and extending laterally, separates an anterior lobe from a middle lobe, the former comprising the future lingula, centralis and culmen and their lateral extensions. The anterior portion is rolled forward under the anterior part of the cerebellum. Another transverse fissure next appears in the median part (secondary fissure} which later extends (peritonsillar) to the floccular incisure, and thereby completes the demarcation of a posterior lobe, including not only the flocculus and nodule, but also the tonsilla and uvula, which are also rolled backward and under. The result of this transverse fissuration would be the production of a cerebellum resembling that of certain forms below Mammals where the cerebellum is well developed (Selachians, Birds). A complicating factor, however, is the great growth of certain lateral portions of the middle lobe, forming the future cerebellar hemispheres (Fig. 419), which causes also a lateral overlapping and rolling inward of adjoining parts. This growth is the chief factor in the division of the cerebellum into vermis and hemispheres and is correlated with the development of the neopallium (p. 436 and Fig. 371).
Fig. 419. Dorsal view of the cerebellum and medulla of a 5 months' human fetus. Kollmann.
The early histological development of the cerebellum has been most closely studied in Bony Fishes (Schaper) and there is every reason to suppose that the processes taking place in the human cerebellum are essentially the same. In that part of the alar plate forming the rudiment above described, the cells proliferate, forming first a nuclear layer with the dividing cells along its ventricular surface, and a non-nucleated outer or marginal layer. Later, owing to beginning migration and differentiation, there is formed the usual mantle layer, representing a differentiation of part of the original nuclear layer and thereby forming the three layers: an inner, a mantle and a marginal. The outer cells of the mantle layer increase in size and differentiate into the cells of Purkinje, snaller cells within forming the granular layer. The earliest stage of differentiation of the Purkinje cells has not been accurately described, but the axones of the neuroblasts evidently proceed (end of fifth month) toward the ventricular surface instead of entering the marginal layer. In this way the fibrous layer (white matter) comes to lie within instead of on the outer surface as in the cord, and, to some extent, in the medulla. There is thus formed the outer gray matter or cortex. The axones of the Purkinje cells form the great bulk of the centrifugal fibers of the cerebellar cortex. The marginal layer becomes ultimately the outer or molecular (plexiform) layer of the adult cerebellum.
Fig. 420. Diagram representing the differentiation and migration of the cerebellar cells in a teleost. The arrows indicate the migration of cells from the borders of the cerebellar rudiment into the marginal layer; these cells probably all differentiate into nerve cells. Clear circles, indif ferent cells; circles with dots, neuroglia cells (except in marginal layer); shaded cells, epithelia* cells; circles with crosses, epithelial cells in mitosis (germinal cells); black cells, neuroblasts; Z* lateral recess; M, median furrow, above which is roof plate; R, floor of 4th ventricle (IV), Schaper.
It has been seen that in the other parts of the tube development begins in the medial parts of the lateral plates and thence advances toward their dorsal borders, which actively develop after the corresponding stages have ceased in the medial portions. The same is true of the cerebellar rudiment. In this, the edges which border on the thin roof plate, i.e., those parts adjoining the lateral recesses, the main roof of the fourth ventricle and the roof plate interposed between the two original lateral cerebellar rudiments, are the last to proliferate. The cells thus formed spread into the marginal layer of the earlier developed parts and by further proliferation form a nucleated layer of considerable thickness (Fig. 420). This complication is apparently essentially similar to that described above in the development of the medulla. From the cells of this invasion are formed a part, at least, of the granule cells, as well as the basket cells and other cells which remain in the marginal (molecular) layer. These are all association cells of the cerebellum.
Fig. 421. Scheme showing the various stages of position and form in the differentiation of granule cells from the outer granular layer. Cajal.
- A, Layer of undifferentiated cells; B, layer of cells in horizontal bipolar stage; C, partly formed molecular (plexiform) layer; D, granular layer; b, beginning differentiation of granule cells; c, cells in mo no polar stage; d, cells in bipolar stage; e,f, beginning of descending dendrite and of unipolarization of cell; g,h, i, different stages of unipolarization or formation of single process connecting with the original two processes; j, cell showing differentiating and completed dendrites; k, fully formed granule cell.
The cerebellum reaches its full histological development very late; after birth in many Mammals. These last postnatal stages of development naturally involve principally those cells proliferated last and which lie in the marginal layer. These have been studied by means of the Golgi method in new-born Mammals by Cajal and others. The majority of these cells form granule cells by means of a progressive migration and differentiation, as shown in the accompanying Fig. 421. Each cell first develops a single horizontal process, then another, thus becoming a horizontal bipolar cell. Following this, the cell body migrates past the Purkinje cells into the granular layer, remaining in connection with the original processes by a single process. There are thus formed the axone of the granule cell with its bifurcation into two horizontal processes, the parallel fibers of the molecular layer. This mode of formation is thus similar to the unipolarization of the cerebrospinal ganglion cell. The dendrites begin to be formed during the migration, branch when the cell body reaches the granular layer and there finally attain the adult form. Other undifferentiated cells in the marginal layer send out horizontal processes the collaterals of which envelop the Purkinje cell bodies, and form the baskets. The place vacated, so to speak, by the migrating granules, is filled at the same time by the developing dendrites of the Purkinje cells. These at first show no regularity of branching, but subsequently differentiate into the definite branches of the adult condition, at the same time advancing toward the periphery (Fig. 422). When they reach this, the migration of the granules is completed and the molecular layer is definitely formed. This condition, evidenced by the disappearance of the outer granular layer, is usually reached in Mammals within two months after birth, but in man not until the sixth or seventh year. There are observations indicating that animals possessing completely developed powers of locomotion and balancing at birth have more completely differentiated cerebella at that time. The axones of the Purkinje cells form many embryonic collaterals which are afterward reduced in number.
Fig. 422. Section through cerebellar cortex of a dog a few days after birth Showing the partial development of the dendrites of two cells of Purkinje. Cajal.
- A, external limiting membrane; B, external (embryonic) granule layer; C, partly formed molecular (plexiform) layer; D, granular layer; a, body of cell of Purkinje; &, its axone; c, and d, collaterals with terminal arborizations (e).
Of the centripetal fibers to the cerebellum, those from the inferior olives cross the median line of the medulla about the seventh or eighth week, and thence advance to the vermis, reaching their final destination during the third month. The fibers from the pontile nuclei (middle peduncle) do not develop until considerably later (end of the fourth month), the time of their reaching their destination in the cerebellar hemispheres not being definitely known. Many at least of the centripetal fibers do not reach their full development in Mammals till birth or after. Some of these fibers (climbing fibers) form arborizations around the inferior (axone) surface of the Purkinje cell bodies and later creep upward, enveloping the upper surface instead, and finally the dendritic branches. Other centripetal fibers (mossy fibers) ramifying in the granular layer are varicose fibers, at first otherwise smooth. From the varicosities a number of branches are given off which later become abbreviated and modified into the shorter processes of the adult condition. This final differentiation occurs simultaneously with the final differentiation of the dendrites of the granule cells with which they come into connection. The glia elements apparently develop in a manner essentially similar to their development elsewhere.
The development of the internal nuclei of the cerebellum has not been thoroughly investigated. The nucleus dentatus is well developed at the end of the sixth foetal month. Eminences passing forward and ventrally along the sides of the isthmus are the earliest indications of the superior peduncles* formed later by the axones of the cells of these nuclei.
Corpora Quadrigemina
The mid-brain roof is an expansion of the alar plate of the mid-brain. Later this differentiates into the anterior and posterior corpora quadrigemina. In the former, by the usual ventricular mitoses (germinal cells), a nuclear layer is formed with a non-nucleated marginal layer external to it which becomes the outer or zonal layer. Still later the neuroblast or mantle layer is differentiated, there being an unusually thick inner layer. The further development has not been closely studied in man. Owing to the diminished importance of the anterior corpora quadrigemina (p. 437) the neuroblasts do not differentiate into the well marked "spread out" layers characteristic of the optic lobes of many Vertebrates. This is probably due to a lack of development of their association neurones.
The fibers of the optic tracts grow toward the anterior corpora quadrigemina in the marginal layer forming the anterior brachia. When they reach the anterior corpora quadrigemina, they leave the marginal layer and penetrate the gray matter forming the most external fiber layer. The medial (and some lateral) lemniscus fibers enter more deeply than the optic. Neuroblast axones grow toward the ventricle, turn internally to the lemniscus fibers, cross (Meynert's decussation) , and proceed as the predorsal tracts to the segmental brain and cord, lying ventral to the medial longitudinal fasciculi.
The Diencephalon
The stage of development of the diencephalon at four weeks has already been mentioned (p. 448). (Figs. 423, 433 and 434.) In the lateral walls the principal feature is the presence of a furrow, the sulcus hypothalamicus, which beg : ns ventrally as an extension of the optic recess and extends dorsally and caudally toward the mid-brain. A branch of it extends to the posterior part of the foramen of Monro. This is the sulcus Monroi. The sulcus hypothalamicus is sometimes regarded as the representative in this region of the sulcus limitans. It is doubtful whether it has the same morphological value as the latter. Such a comparison is seen a priori to be difficult when it is considered that this region is in the most highly modified part of the brain tube, lacking motor peripheral apparatus, and that it is also the end region of the tube where all longitudinal divisions would naturally merge. The sulcus deepens till the end of the second month (Fig. 429). Later it becomes shallower, but appears to persist till adult life. The region of the diencephalon ventral to the sulcus, as already mentioned, is termed the pars subthalamica or hypothalamus. The ventral part of the optic stalk forms a transverse groove in the floor, the preoptic recess, caudal to which is a ridge or fold, the chiasma swelling, in which the fibers of the optic chiasma later appear.* Caudal to this is the recess or invagination of the floor, representing the postoptic recess and the beginning of the infundibulum (Figs. 424 and 425) . Its extremity later becomes extended into the infundibular process, the posterior part of which in the fifth week comes into contact with the hypophyseal (Rathke's) pouch. This is a structure formed from the stomodaeal epithelium and is connected with the latter by a stalk. The pouch, which is at first a flat structure, develops two horns which envelop the infundibulum. The cavity of the end of the infundibular process becomes nearly shut off from the rest of the infundibular cavity. The process penetrates the upper part of the pouch and then bending reaches its posterior surface and ends blindly. In the second half of the second month epithelial sprouts, which become very vascular, begin to appear, first in the lateral parts of the pouch, next the brain, and then extending through the pouch and finally nearly obliterating its cavity (third month). The shape of the organ (the hypophysis) formed by the union of these two parts is subsequently changed by its relations to surrounding parts. Its posterior lobe is derived from the infundibular portion, its anterior lobe from the pouch.
Fig. 423. Transverse section through the diencephalon of a 5 weeks' human embryo. Dp., Roof plate; Ma., mammillary recess; P.s. hypothalamus; S.M., sulcus hypothalamicus; Th., thalamus. His.
- According to Johnston, the chiasma is formed in front of the optic recess which would then be represented by the postoptic recess. In this case the chiasma would be regarded as falling in the region of the telencephalon instead of forming the optic part of the hypothalamus (comp. Figs. 364 and 433).
Fig. 424. Lateral view of a model of the brain of a 10.2 mm human embryo (middle of 5th week). His.
Diencephalon Thalamus Pineal region
Fig. 425. Median view of the right half of a model of the brain of a 10.2 mm human embryo (middle of 5th week). Compare Fig. 424. His.
An expansion of the floor of the brain caudal to the infundibulum has been mentioned as the mammillary region. Subsequently there is formed from its cephalic part another evagination, the tuber cinereum. The mammillary region forms the mammillary bodies. The region caudal to the mammillary region later receives many blood vessels, thereby becoming the posterior perforated space.
At the end of the fourth week the roof plate of the diencephalon is smooth. At about this time the greater part of the roof expands, forming a median longitudinal ridge (Fig. 426). This ridge, which remains epithelial throughout life, is broader at its anterior end where it passes between the beginning pallial hemispheres. As the roof plate expands further, the anterior part is next thrown into longitudinal folds. The ridge forms the epithelial lining of the tela chorioidea of the third ventricle (diatela). By further growth and vascularization of its mesodermal covering at the beginning of the third month, there is formed the chorioid plexus of the third ventricle (diaplexus). Lateral extensions of the tela form the chorioid plexuses of the lateral ventricles (see p. 5 17) . In the fifth week a protrusion appears at the caudal end of the median ridge which is the beginning of the epiphysis. Soon after this, the furrow which forms its caudal boundary extends forward along the upper part of the sides of the walls, marking off a fold which is the lateral continuation of the median protrusion. From the median protrusion is later formed the pineal body, while from the lateral folds are formed the pineal stalk, and in front the habenula, with its contained nucleus (ganglion) habenulce, and the stria medullaris. Still further caudally, the anterior part of the mid-brain forms a horseshoe-shaped fold the arms of which extend forward over the diencephalon, ventral to the pineal folds. The median part of this fold forms the anterior corpora quadrigemina. From its lateral extensions are formed the anterior brachia of the anterior corpora quadrigemina, the pulmnar and the lateral and medial geniculate bodies, all of which (pulvinar ?) later receive optic fibers. The transverse furrow which forms the boundary between the rudiments of the pineal body and of the anterior corpora quadrigemina marks the location of the future posterior commissure (Figs. 426, 427 and 428).
The part of the roof anterior to the pineal fold, as already stated, forms the tela chorioidea of the third ventricle. Certain folds appear in it, however, which are more clearly indicated in later stages of embryonic development than in the adult and which probably represent structures already mentioned as common to the vertebrate brain ("cushion" of the epiphysis, velum transversum, paraphysis?) (p. 4 2 4 and Fig. 364).
From the above it is evident that at the close of the fifth week the rudiments of the various parts of the diencephalon are already well marked. These rudiments are principally indicated by foldings of the walls, there being no very strongly marked differences of thickness except the early differentiation between the median and lateral plates. From this time on, both general and local thickenings of the lateral walls occur. This indicates a rapid proliferation of the cells, especially a differentiation of the nerve cells and consequent formation of masses of gray and white matter. Another factor affecting the diencephalon is the subsequent growth backward over it of the cerebral hemispheres.
Fig. 426. Dorsal view of a model of the brain of a 13.6 mm human embryo (beginning of 6th week). The dorsal part of the pallium on each side has been removed. Compare with Figs. 427 and 428 . His.
During the second month, the lateral walls become thickened, forming a prominence on the inner surface of each side. This reduces much of the cavity of the third ventricle to a cleft and in the third or fourth month a fusion of a portion of these two projections takes place, forming the commissura mollis or massa intermedia. The condition at this stage is shown in Fig. 429. Later this protrusion thrusts the lateral structures above described (the pulvinar, geniculate bodies and brachia) to the side, the cavity of the lateral geniculate body being obliterated. The prominence itself extends to the tegmental swelling (see Figs. 4 2 9-30) and there thus arises the possibility of direct connections between these two structures. There can, then, be distinguished in the diencephalon three regions, a hypothalamic region, as already described, an epithalamic region comprising the pineal body, ganglia habenulae and related structures, and finally the thalamus proper. In the latter, the geniculate bodies already mentioned constitute a metathalamic portion, while the portion derived from the thickened part, which is continuous anteriorly with the corpus striatum, differentiates various nuclei, especially those which receive the general somatic sensory fibers (medial lemniscus or fillet), and other nuclei in relation to definite centers of the pallium. The thalamus is thus strongly developed, owing to its containing the nuclei which receive the general sensory (ventro-lateral nuclei), acoustic (medial geniculate bodies), and optic (lateral geniculate bodies) systems of fibers and which in turn send fibers (thalamic radiations) to the pallium. These thalamic nuclei do not receive fibers probably until after the middle of the. second month. About this time the thalamic radiations begin to be formed from the thalamic nuclei and grow toward the corpus striatum which they reach toward the end of the second month. With the first appearance of the cortical layer of the developing neopallium (see p. 512) they penetrate the corpus striatum and pass to the cortex, forming the beginning of the internal capsule, and corona radiata. It has already been pointed out (p. 437) that the great development of the thalamus and its radiations is more recent phylogenetically and is due to the newly acquired connections with the neopallium.
Fig. 427. Lateral view of the model of the brain of a 13.6 mm. human embryo (beginning of 6th week). F, Beginning of frontal lobe; T, beginning of temporal lobe. His.
Fig. 428. From a model of the brain of a 13.6 mm human embryo, right half, seen from the left side. His, Spalt'eholz,
Fig. 429. Median sagittal section of the brain of a 7.5 weeks' human embryo. Aq. S., Aquaeductus Sylvii; C. c., fold between mid- and interbrain; C.m., commissura mollis; C. s., corpus striatum; H. b., tegmental swelling; R.g., geniculate recess; R. i., recessus infundibuli; R. o., recessus (prae-?) opticus; S.h., habenular evagination; 5. M. } sulcus hypothalamicus; S.p., pineal evagination; T. T., thalamus. His.
Fig. 430. Brain of a human foetus 'in the 3d month, right half, seen from the left. His, Spalteholz,
Fig. 431. Adult human brain, right half, seen from the left, partly schematic. Spalteholz.
Before the development of these neopallial connections, other tracts have begun to appear which represent older epithalamic and hypothalamic connections existing practically throughout the Vertebrates (pp. 437 and 438) . Some of the hypothalamic connections are the mammillo-tegmental fasciculus which appears early in the second month, the thalamomammillary fasciculus (Vicq d'Azyr's bundle), which appears later, and the bundles from the rhinencephalon (p. 475) and archipallium (columns of the jornioc, middle of fourth month, p. 521). In the hypothalamic region is also differentiated the corpus Luysii, connected by fiber bundles with the corpus striatum and tegmentum. Epithalamic connections are represented by bundles from anterior olfactory regions (stria medullaris, seventh week), by the commissure, habenularis, and by bundles to caudal regions (fasciculus retroflexus of Meynert to the interpeduncular ganglion, middle of second month), (pp. 437 and 475.) The posterior commissure fibers are formed early in the second month in the fold between mid- and inter-brain (Fig. 429). (Fig. 432 )
Fig. 432. Construction of the brain of a 19 mm. human embryo (7.5 weeks). Showing the stage of development of some of the principal fiber-systems. His.
- C.C., posterior commissure; F. s., tractus solitarius; F. t., fasciculus spinalis trigemini (spinal V); K, nuclei of dorsa! funiculi of cord; L., medial longitudinal fasciculus; M., fasciculus retroflexus of Meynert; Ma., mammillary bundle; n. i., nervus intermedius; O., olive; Ol., olfactory nerve; S., fillet; St., stria medullaris thalami; T., thalamic radiation; T. o., tractus opticus; V, Gasserian ganglion; VII, facial nerve and geniculate ganglion; VIII, ganglia of acoustic nerve; IX, N. glossopharyngeus; X, N. vagus.
The Telencephalon (Rhinencephalon, Corpora Striata and Pallium)
To understand the development of this part of the brain it is necessary to keep firmly in mind certain relations which are laid down at a comparatively early stage. Some of these relations are shown in the diagram of the inner surface of a model of a brain of four weeks. At this stage the pallium is unpaired, i.e., there is no median furrow separating the two halves of the pallial expansion. The various boundaries of the pallium in one side are (i) the median line uniting the two halves of the pallial expansion (Fig. 433, be) ; (2) the boundary line or line of union with the thalamus lying caudally (pallio-thalamic boundary) (Fig- 433> cd) ; (3) the boundary between pallium and corpus striatum (striopallial boundary) (Fig. 433, bd) . The boundaries of the future corpus striatum are (i) the median (Fig. 433, ab), (2) the strio-pallial (Fig. 433, bd), (3) the strio-thalamic or peduncular (Fig. 433, de) and (4) the strio-hypothalamic (Fig. 433, a<0- The internal prominence which is the rudiment of the corpus striatum, has three limbs or crura, (i) a ridge proceeding forward (anterior crus), which corresponds externally to the furrow (external rhinal furrow) foiming the lateral boundary of the anterior olfactory lobe, (2) a middle crus corresponding to the constriction separating the two olfactory lobes, and (3) a posterior crus corresponding to the posterior boundary of the posterior olfactory lobe. This latter is merged with the earlier furrow separating the telencephalon from the thalamus and hypothalamus (peduncular furrow). What may be called the main body of the corpus striatum, from which these limbs radiate, soon becomes expressed externally by a shallow depression in the lateral surface of the hemispheres immediately dorsal to the olfactory lobes. This depression is the first indication of the fossa Sylvii (Fig. 427).
Fig. 433. From a model of the brain of a human embryo at the end of the first month, right half, seen from the left. His, Spalteholz.
The boundaries of the pallial hemisphere above indicated are identical with the boundaries of the future foramen of Monro.
The median lamina uniting the two halves of the pallium and the two corpora striata may be termed the lamina terminalis and represents the roof plate and floor plate of this region. The point of meeting of the roof plate and floca plate at the end of the tube is often taken to be at the recessus neuroporicus ; and the lamina terminalis or end wall of the neural tube, more strictly speaking, is limited to the median wall ventral to this point. Here it will be understood as including the median wall to the point where the pa'llio-thalamic boundary begins, marked later |py the angulus prathalamicus of His (see p. 517 and Fig. 442).
Rhinencephalon
The term rhinencephalon is a convenient one for those basal structures of the fore-brain which are in most intimate connection with the olfactory nerve. The term has been extended by some to include the pallial olfactory structures. For descriptive purposes it is here used in the more limited sense.
Fig. 434. Lateral view of outside of brain shown in Fig. 433. His.
At the fourth week, as already indicated (p. 5 16 . Fig. 434) , there is a slight longitudinal furrow on the external surface, marking the ventral limit of the pallial eminence. The part of the brain ventral to this furrow is the rhinencephalon, Somewhat later the latter becomes better marked off, the fissure forming its boundary on the lateral surface being the external rhinal fissure (Fig. 424). Later the mesial side is also marked off by an extension of the fissure around on the mesial side (medial rhinal fissure) and also by a notch, the incisura prima, a continuation of which later ascends along the middle part of the median surface of the hemispheres and is known as the (interior arcuate fissure (fissura prima of His). (Fig. 442.) The existence of a fissura prima in early stages, however, is doubtful. At about this time, the rhinencephalon shows a beginning division into anterior and posterior portions, the anterior and posterior olfactory lobes, the whole structure assuming a bean-shape (comp. p. 512) (Fig. 427). On the lateral surface immediately above this constriction is the beginning concavity in the lateral surface of the hemispheres which marks the earliest appearance of the fossa Sylvii. The external rhinal fissure, as it becomes more pronounced, may be regarded as an extension forward of the fossa (anterior crus of the corpus striatum) . On the mesial surface the incisura prima marks this constriction. With the further curvature of the hemispheres, the anterior lobe becomes bent back under the posterior (third month), but later is again directed forward. It contains a diverticulum of the fore-brain "cavity. The cavity of the posterior lobe is not so well marked off and is bounded by the corpus striatum and the inward projection of the incisura prima. (Figs. 424, 425, 427, 428 and 442.)
Fig. 435. Ventral view of the brain of human foetus at the beginning of the 4th month. Kollmann.
The olfactory nerve at the end of five weeks has reached the anterior lobe on its ventral and posterior side. The lobe develops into the receptive centei 5 for the nerve the olfactory bulb; into the stalk in which the secondary olfactory tract proceeds; and also into a triangular area where the tract divides the trigonum. The posterior olfactory lobe develops into the anterior perforated space and an eminence known as the lobus pyriformis which becomes reduced later (comp. Fig. 3 70, G and H). From it is developed the gyms olfactorius lateralis, connected with the lateral division of the olfactory tract and thegyri ambiens and semilunaris (Fig. 435). On the mesial wall, the posterior lobe is especially connected with the region between the anterior arcuate fissure and the lamina terminalis (trapezoid area of His, parolfactory or preterminal area of G. Elliot Smith) (Fig. 442). Part of this mesial region represents the anterior portion of the archipallium (comp. Fig. 370, G and H and p. 482).
Corpora Striata and Pallium
The leading feature of the development of this part of the brain is the great expansion of the pallial hemispheres. That part of the brain wall marked externally by the fossa Sylvii and internally by the body of the corpus striatum, and especially that part where the corpus striatum is continuous with the thalamus (peduncular part) , may be considered as a fixed point from which the pallial walls expand in all directions, anteriorly, dorsally and posteriorly, i.e., in both transverse and longitudinal directions. At first, this expansion causes the pallial hemispheres to assume a bean-shape with the hilum at the fixed point (Fig. 427). The anterior end curves downward and forms the frontal lobe with its enclosed cavity (anterior horn of the lateral ventricle). The posterior end curves downward caudally and forms the temporal lobe with the descending horn of the lateral ventricle. At the same time, owing to the great expansion in a transverse plane of each pallial eminence, the median lamina uniting them (Figs. 425 and 426) not sharing in this growth, there are formed the hemispheres with their cavities, the lateral ventricles, and the great longitudinal fissure between the hemispheres. Later, vascular mesodermal tissue fills this fissure, forming the falx cerebri. The paired cavities of the pallium are connected with the unpaired end-brain cavity (aula) by the foramina of Monro, the boundaries of which are the same as those of the pallium described above (p. 508).
At first the walls of the telencephalon, like those of other parts of the tube, are epithelial in character and nearly uniform in thickness. By proliferation there is formed a several-layered epithelium differentiated into an inner nuclear layer and an outer marginal layer. Later a mantle layer is differentiated. The hemispheres are late in development and until the end of the second month the walls are thin and simply show the above three layers. Toward the end of the first month a greater activity in cell proliferation takes place in the basal portion of the telencephalon which thickens into the corpus striatum. At eight weeks there first appears on the external surface of the corpus striatum, a cortical layer of cells lying next the marginal layer and separated from the inner layer by an intermediate layer comparatively free of cells and known as the fibrous or medullary layer (see p. 524). The differentiation thus begun extends gradually around the circumference of the hemispheres until the mesial surface is reached. This differentiation permanently ceases at the medial pallial margin. The cortical layer does not extend as far as the medullary layer, thus leaving an uncovered medullary layer on the mesial hemisphere wall. As a result of this, there is in this region, passing toward the median line, (i) a region covered with a cortical layer (limbus corticalis of His); (2) an uncovered medullary layer (limbus medullaris); (3) a fibrous transitional zone (the t&nia) passing into (4) a membranous zone, the roof plate.
This process resembles that taking place in other parts of the neural tube, in which there is the same progressive development from the ventral portion of the lateral wall to the dorsal border of the same, where the latter passes into the roof plate which is either ependymal or expanded into a thin membrane.
The longitudinal growth of the hemispheres naturally affects the form of a number of its structures. As already mentioned, this growth consists in an elongation around a fixed point, which may be regarded as located on its ventral border, the result of this being a curving down in front and behind this point. This is especially marked in the caudal half which thereby becomes curled first ventrally and then forward, thus forming a spiral. This growth in length is interstitial, i. e., due to expansion of the intermediate parts, and pari passu with it there is an elongation not only of the corpus striatum and structures in the mesial hemisphere wall (hippocampal formation, corpus callosum, chorioid plexus of lateral ventricle), but also of adjacent thalamic structures (stria terminalis or semicircularis), as described later.
Fig. 436. View of the inside of the lateral wall of anterior part of fore-brain. Human embryo of about 4.5 weeks. His.
- C, Corpus striatum; H, pallium; h. R, posterior olfactory lobe; L, lamina terminalis; O, recessus (prae-?) opticus; R. i., recessus infundibuli; S. M.. sulcus hypothalamicus; St, hypothalamus; T, thalamus; v. R., anterior olfactory lobe.
The early divisions of the corpus striatum have been mentioned, and also the relations of its parts with the rhinencephalon. The anterior end of the corpus striatum at this period and later shows a longitudinal division into three portions, a lateral, a middle and a medial, due to the original division into three limbs described above (p. 508). (Figs. 436, 437, and 438.) With the elongation backward of the hemisphere the corpus striatum also becomeselongated, being drawn out and curled around the peduncle or stalk of the hemisphere and forming a thickening along the elongated wall. This caudal prolongation of the striatum is its cauda (tail) and extends to the tip of the inferior horn (Figs. 437 and 438). The medial portion of the corpus striatum forms a triangular projection (Figs. 426 and 428) the edge of which is directed toward the foramen of Monro.
Fig. 437. View of inside of the lateral wall of lateral ventricle of a human foetus at beginning of third month. His.
- Bb, bulbus olfactorius; C. L, lateral limb of corpus striatum; C.m., medial segment (consisting of the middle and inner limbs) of the corpus striatum. The furrow between these two parts opens into the anterior olfactory lobe; hRl., posterior olfactory lobe; L./., frontal lobe; L. o. y occipital lobe; Og. } olfactory nerve; R. i., recessus infundibuli; R. o., recessus (prae-?) opticus; St., stalk of hemisphere (strio-thalamic junction); V.I., lateral ventricle; v.Rl.+Bb, anterior olfactory lobe.
to some, there is a, fusion of the striatum, the medial wall of the hemisphere and the anterior part of the thalamus. According to others, the increase in bulk of this region is produced by a simple thickening of the walls, thus causing a flattening out or shallowing of the grooves marking the junctions of striatum and thalamus on the ventricular surface, and between medial hemisphere wall and thalamus externally (Fig, 439). The effect is much the same whether accom plished by apposition and fusion or by interstitial thickening, massive connections being formed which consist mainly of fibers connecting hemispheres and thalamus, the foramen of Monro at the same time being changed in form to a slit. From the metathalamic region the fibers of the optic and acoustic pathways grow forward into the hemispheres (see also p. 507) . entering more caudally and forming the retro- and sub-lenticular portions of the internal capsule (comp. p. 507). That part of the thalamic radiation from the anterior portion of the thalamus (fillet pathway) also forms a part of the internal capsule as described on p. 507. Later, the internal capsule is completed by the growth from the pallium of descending fibers from the neopallial cortex, through the striatum to the pes. By these various traversing fibers the striatum is divided into the nucleus lenticularis or lentiformis and the nucleus caudatus. The posterior arm of the internal capsule is formed by fibers passing between and thus separating thalamus and lenticularis (Figs. 439 and 440).
Fig. 438. Dorsal view of the brain of a 3 months' (45 mm) human foetus. The dorsal part of each cerebral hemisphere has been removed. Kollmann.
Fig. 439. 1, 2 and 3, Schematic horizontal sections through human embryonic fore-brains at different stages of development; 4, vertical section through fore-brain at about same stage as 1. Goldstein.
- a, That part of the lateral ventricle lying between the corpus striatum and the junction of medial hemisphere wall and thalamus (leading into the inferior horn); b, furrow or trough between mesial hemisphere wall and thalamus, produced by backward extension of hemisphere; c. /., internal capsule; P.M., foramen of Monro; &, external surface at junction of mesial hemisphere wall and thalamus; Str., corpus striatum; Th., thalamus; U, place where mesial hemisphere wall continues into the thalamus wall (junction of hemisphere wall and thalamus) ; U 1 , place where mesial hemisphere wall is continuous with lateral hemisphere wall.
- In 1, owing to the thickening of U and growth of the corpus striatum, these two are brought into apposition, as indicated by the dotted lines on the right, and apparently fuse, obliterating a and producing the condition shown in 2 and 3. In 2 and 3 the position of the former space a is indicated by the dotted lines a a' By comparison with 4, it will be seen that this obliteration by apparent fusion is actually produced by a filling up from the bottom of a (indicated faintly by dotted lines on the right in 4). The thickening of thfe walls at this region also produces a shallowing of b (indicated by dotted lines on the right in i). The principal cause of this general thickening is the passage of the fibers of the thalamic radiation to the hemispheres and, later, of fibers from hemisphere to pes, forming the internal capsule (4, 2 and 3).
Fig. 440. Lateral view of the brain of a 3 months' (42 mm) human foetus. The lateral wall of the left cerebral hemisphere has been removed. His, Kollmann.
The Archipallium
During the fifth week, following the stage shown in Figs. 433 and 434, the pal Hal evaginations or hemispheres have become much more pronounced and consequently the foramina of Monro much better defined. A comparison will show that the boundaries of the foramen of Monro are essentially unaltered. Anteriorly it is bounded by the medial wall connecting the two hemispheres, posteriorly by the boundary between pallium and thalamus, ventrally by the corpus striatum and junction of it and thalamus (Figs. 425 and 441).
At the beginning of the sixth week the foramen of Monro has changed somewhat in shape. The pallio-thalamic part of its boundary passes forward and forms the above-mentioned (p. 5 10) acute angle (angulus praethalamicus) with that part of the wall uniting the two hemispheres (lamina terminalis). The latter wall descends to the region of the optic recess. The inferior part of the foramen is partly closed by the medial part of the corpus striatum as already described. (Comp. Figs. 441, 426 and 428.) In the ependymal mesial wall of the hemispheres just below the taenia, described above, there arises a folding inward, which begins anteriorly near the angulus praethalamicus and proceeds caudally along the upper (pallio-thalamic) border of the foramen of Monro. This infolding is the chorioid fissure. In the ependymal mesial wall there are now the following: limbus chorioideus (the infolded part) and a small strip of the ependyma wall below the fold, the lamina infrachorioidea (Fig. 442). This invagination soon becomes very deep, resulting in the formation of a doublelayered ependymal fold (the chorioid fold, plica chorioidea) lying in the lateral ventricle over the corpus striatum (Figs. 441, 426 and 444). Later, vascular mesodermal tissue passes in from the falx between the lips of this fold and thereby forms the chorioid plexus of the lateral ventricles. The chorioid fissure is at first quite short, but becomes elongated (Fig. 443) with the above-described posterior elongation of the hemisphere of which it is a part, and thus extends into the inferior horn of the temporal lobe. (Figs. 443 and 444.)
Fig. 441. Transverse section through fore-brain of a 16 mm embryo (six to seven weeks). His.
Toward the end of the second month, according to some authorities (His) , but not until considerably later, according to others (Hochstetter, Goldstein), another furrow appears in the limbus corticalis above and parallel to the chorioid fissure, and known as the posterior arcuate fissure. This fissure does not extend at first as far forward as the chorioid, but extends farther caudally, arching downward in the temporal lobe around the caudal end of the chorioid fissure (Fig. 443) . The posterior arcuate fissure is a total fissure, involving the whole wall and producing a fold on the inner surface of the medial hemisphere wall (plica arcuata). The temporal or caudal part of this whole formation persists in the adult without much further change. The fissure here becomes the hippocampal fissure separating the fascia dentata from the gyrus hippocampus; the part rolled in by the hippocampal fissure produces the eminence in the lateral ventricle known as the cornu ammonis or hippocampus major; the edge of the limbus corticalis forms the fascia dentata; the limbus medullaris or exposed fibrous part is thefimbria which is continued by its thinning edge or tania fimbria into the ependymal or epithelial portion (lamina chorioidea) of the chorioid plexus of the lateral ventricle. The chorioid plexus is attached by the taenia chorioidea and lamina infrachorioidea (here the lamina affixa) to the brain wall, usually near the junction of corpus striatum and thalamus, thereby forming a part of the wall of the inferior horn of the lateral ventricle. At this line of junction of thalamus and hemisphere wall is formed the stria terminalis. The fimbria is continuous anteriorly with the posterior pillar of the fornix. (Fig. 444.)
Fig. 442. Diagram of a graphic reconstruction of the mesial hemisphere wall of a 16 mm human embryo (about six weeks). His, Ziehen. Cavities are dotted, cut surfaces are lined.
- Apt, Angulus praethalamicus; Atr, preterminal area; Fpr, anterior arcuate fissure (fissura prima); Frhl, mesial termination of lateral rhinal fissure; hRh, posterior olfactory lobe (tuberculum olfactorium + substantia perforata anterior) ; Lt, lamina terminalis (lined) ; Vmr, depression between the two olfactory lobes; vRh, anterior olfactory lobe (bulbus olfactorius + tractus olfactorius + trigonum olfactorium).
The anterior part of the hippocampal formation above described undergoes further modifications, due principally to the development of commissural fibers in this region. Some of these commissural fibers connect the representatives on each side of the hippocampus (limbus corticalis) of this region, forming the fornix commissure, but most of them (corpus callosum) connect the rest of the cortical areas (neopallial areas) of the two hemispheres.
Fig. 443. Graphic reconstruction of the mesial hemisphere wall of a human foetus (fourth month). His, from Quain's Anatomy.
- c and v, Anterior and posterior parts of pre terminal area; li, lamina infrachorioidea; km, limbus or border of mesial hemisphere wall (gyrus dentatus and fimbria) between hippocampal and chorioid fissures; P, " stalk " of hemisphere.
Fig. 444. Diagram of a transverse section through the fore-brain of a human foetus (fourth month). To show the relations of the margins of the mesial walls of the hemispheres. His t from Quain's Anatomy.
- Cs., corpus striatum; fi., limbus medullaris (fimbria); /a., limbus corticalis (gyrus dentatus); h.f. t hippocampal fissure; Th., thalamus
There are two views regarding the formation of these commissures. According to one view, the first commissural fibers appear in the upper (dorsal) part of the lamina terminalis. The latter subsequently expands pari passu with the expansion of the corpus callosum. The commissural fibers are thus confined to the original walls connecting the two hemispheres. According to the other view, there is a secondary fusion of the mesial hemisphere walls and in these fusions the fibers cross. The first fibers appear during the third month and form at first a small band in the upper part of the lamina terminalis (Fig. 443) . These fibers come partly from the limbus corticalis (fornix commissural fibers) and partly from other parts of the cortex (callosal fibers), in either case traveling along the intermediate layer. According to the fusion view, the exposed intermediate layers (limbi medullares) fuse where the fibers cross. This fusion can easily be imagined by conceiving the opposite surfaces in question to be brought together in the upper part of Fig. 444. It is more probable, though, that not only the first fibers cross in the lamina terminalis, but that the later ones also cross in extensions of the latter. There are three views regarding the further development of the corpus callosum. The first is that all parts are represented at this stage, future growth being by intussusception of fibers; the second is that the part first formed represents the genu, the rest being added caudally; the third (His) is that this first formed part represents the middle portion of the callosum, both anterior (genu and rostrum) and posterior (splenium) portions being subsequently added (Figs. 443 and 445). This latter view is indicated in Fig. 445, the later additions being shaded darker.
Fig. 445. Graphic reconstruction of the mesial hemisphere wall of a 120 mm foetus (end of four months). His, from Quain's Anatomy. 6, Fimbria; cs , cavity of septum pellucidum ("fifth" ventricle, ventricle of Verga); Icm, limbus corticalis (gyrus dentatus); P, stalk of hemisphere; v, outline of cavity of hemisphere (lateral ventricle).
As the callosal fibers connect the limbi medullares, the limbus corticalis and the arcuate fissure, corresponding to the gyrus dentatus and hippocarr pal fissure of the temporal lobe, lie dorsal to the callosum. The limbus corticalis is reduced to a mere vestige (indusium griseum and strict Lancisi) on the dorsal surface of the corpus callosum the fissure becoming the callosal fissure. The part of the limbus medullaris ventral to the corpus callosum, corresponding to the fimbria of the temporal lobe, forms the posterior pillars and body of the fornioc.
++++++++++++++++++++++++++++++++++ These relations are shown in the following table from His (slightly modified):
++++++++++++++++++++++++++++++++++
Fibers from the hippocampus enter the fimbria and pass forward in the posterior pillars and body of the fornix. In or near the lamina terminalis these fibers of the fornix descend, forming the anterior pillars of the fornix, and thence pass back of the anterior commissure and caudally to the mammillary region.
They are joined by fibers from the dorsal surface of the callosum (fornioc longus), i.e., from the vestigial hippocampal formation, many of which also descend in front of the anterior commissure to the rhinencephalon. The triangular mesial area (septum pellucidum) included between callosum and fornix probably represents an extended part of the lamina terminalis or "commissure-bed," in which a cavity is formed, the so-called fifth ventricle and ventricle of Verga. A remnant of the hippocampal formation at the anterior end of the callosum is represented by the gyms subcallosus (Fig. 445).
The Neopallium
The hippocampal or cornu ammonis formation and preterminal area represent the older part of the pallium (archipallium) comp. pp. 438 and 439. This part of the pallium is olfactory in character, being mainly a higher center for the reception of secondary and tertiary olfactory tracts. In its extension backward and partial obliteration by the corpus callosum, its embryologic presents a striking similarity to its phylogenetic development (compare p. 438). The rest of the pallial hemispheres (neopallium) are occupied by the nonolfactory higher centers.
The further growth of the neopallial hemispheres leads to their extension backward, overlapping the caudal portions of the brain tube. In the course of this extension the occipital lobe and its cavity, the posterior horn of the lateral ventricle, are formed. The growth of various portions of the hemisphere surface is unequal, producing folds (convolutions) and fissures. This folding may be partly due to growth in a confined space, but especially important is the relation between gray and white matter. The gray matter, containing not only fibers but also neurone bodies, remains spread out in a comparatively thin layer, probably to accommodate associative connections. The white matter, on the other hand, increases in thickness. This leads to a folding of the outer layer. The position of these folds is probably partly determined by the local histological differentiation and growth of various cortical areas (p. 527). Only some of the earliest and most important of these folds will be mentioned here.
It has been seen (p. 509) that early in the development of the pallium a shallow depression appears on the external lateral surface of each hemisphere, the fossa Sylvii (Fig. 446). The bottom of this is the future insula. It is external to the corpus striatum and does not grow as rapidly as the parts bounding it, which consequently overlap it, forming its opercula. These bounding walls are formed by the fronto-parietal lobe on its upper side, by the temporal on its lower, and by the orbital on its anterior. The temporal and frontoparietal opercula begin about the end of the fifth month, the temporal at first growing more rapidly but later the fronto-parietal, thereby changing the direction of the Sylvian fissure from an oblique to the more horizontal angle characteristic of man as compared with the ape. In the meanwhile the development of the frontal lobe leads to its also overlapping the insula. If the frontal lobe fully develops, it forms a U-shaped operculum between the frontoparietal and the orbital, if it does not so fully develop it forms a V-shaped operculum, and a still less developed condition is shown by a Y-shaped arrangement in which the frontal lobe does not completely separate the fronto-parietal and orbital opercula. The opercula cover the fore-part of the Sylvian fossa during the first year. Conditions of arrested development are thus indicated by the Y-shaped anterior ascending branch of the Sylvian fissure coupled with an absence of the pars triangularis and also by a partial exposure of the island of Reil. In the ape the frontal operculum is absent and the island of Reil partly exposed.
Fig. 446. Lateral view of the brain of a human foetus at the beginning of the 4th month. Kollmann.
Fig. 447. Median view of the left half of the brain of a human foetus at the end of the 7th month. Kollmann.
Toward the end of the third month the calcarine fissure appears, producing on the ventricular surface the eminence known as the calcar avis. At the beginning of the fourth month the parieto-occipital fissure unites with it forming the cuneus. The parieto-occipital reaches the superior border of the hemispheres by the sixth or seventh month. At the sixth month the fissure of Rolando (central fissure) appears. The condition of the surface of the hemisphere at the end of the seventh month is shown in Figs. 447 to 450.
The early histogenetic development of the pallial wall, resulting in the differentiation into the usual ependymal, mantle and marginal layers, has been mentioned. (Fig. 451). The next stage, already alluded to (p. 519), marks a difference in development between the pallium, as well as other suprasegmental structures, and the rest of the walls of the neural tube. This stage consists apparently in a further migration outward of the neuroblasts and their accumulation under the marginal layer, forming, at eight weeks, a definite layer of closely packed cells, the beginning of the cortex (Fig. 452). Later neuroblast migrations probably add to this layer. It has already been mentioned that the fibers of the thalamic radiation appear in the pallial walls about this time. They proceed internally to the cortical layer and thus mark the beginning of the fiber layer (medullary layer) which by later myelination becomes the white matter of the hemispheres.
Fig. 448. Dorsal view of the cerebral hemispheres of a human foetus at the end of the 5th month. Kollmann.
The extension of the process of differentiation of the cortical layer from the region of the corpus striatum over the rest of the pallium has also been mentioned (p. 512). It is probable that the afferent pallial fibers (thalamic radiation) in their growth keep pace with this process. Those fibers from the lateral geniculate bodies proceed to the occipital region, those from the medial geniculate bodies to the temporal, and those from the ventro-lateral thalamic nuclei (continuation of the medial fillet) to the future postcentral region. The afferent pallial fibers are often termed the afferent or ascending projection fibers.
Fig. 449. Lateral view of the right cerebral hemisphere of a human foetus at the end of the 5th month. Kollmann.
The axones of the neuroblasts of the cortical layer grow inward, entering the medullary layer. Their peripherally directed processes become the apical dendrites of the pyramid cells into which most of the cortical cells differentiate. According to Mall and Paton, this change of direction in the growth of the axone is due to a turning of the cell axis during its outward migration. It would seem more probable that the cells retain an original bipolar character and that the inner processes differentiate into axones instead of the cells going through a monopolar stage (pp. 454 and 455 and Fi s - 3^6 and 387). The axones of the cortical cells form either efferent or descending projection fibers, proceeding to other parts of the nervous system, or crossed (callosal) and uncrossed association fibers, connecting various cortical areas of the hemispheres. The basilar dendritic processes of the pyramid cells and the axone collaterals develop last. Many details of development of the cells in Mammals are not completed until afterbirth (Fig. 453).
Fig. 450. Ventral view of the brain of a human foetus at the beginning of the sixth month. Retzius, Kollmann.
Fig. 451. Section through the pallial wall of a two months' human foetus. His, Cajal. a, Layer of germinal cells; b, nuclear layer; c, mantle layer; d,' marginal layer; e, germinal cell
Fig. 452. Section through the pallial wall of a human foetus at the beginning of the third month. His, Cajal.
- a, Layer containing germinal cells; b, fibrous (medullary) layer (rudimentary white matter) ; c, layer of neuroblasts forming rudimentary cortical gray matter; d, marginal layer (future molecular layer) ; e, germinal cell; /, g, neuroblasts with radial processes. Spongioblasts and myelospongium are shown on the right side.
During the fourth and fifth fcetal months the cortical layer shows a differentiation into a denser outer and an inner layer. During the sixth and seventh months a differentiation and grouping of the nerve cells begins which results in the formation of six cortical layers (Brodmann). These are: (1) the zonal layer (marginal layer, molecular layer of adult), (2) the external granular layer (layer of small pyramid cells of adult), (3) pyramid layer (medium and large pyramid cells), (4) internal granular layer, (5) ganglionic layer (internal pyramid cells), (6) multiform layer (polymorphous cells). By various local modifications of this six-layered cortex the differentiation of the various histological areas of the adult cortex is brought about. In the calcarine region of the occipital lobe, in the sixth month, the internal granular layer differentiates into two layers between which is formed the line of Gennari which contains terminations of the fibers from the lateral geniculate bodies, representing the visual pathway. This area is the visual cortex. In the temporal (future transverse gyri) and postcentral regions, areas are differentiated which mark the reception of the terminations of the fibers of the acoustic and somaesthetic (medial fillet) pathways. These areas are thus, respectively, the auditory cortex and the somcesthetic (general bodily sensation) cortex. (Cf. Fig. 371.)
Fig. 453. Section through cortex of a mouse foetus before birth. Showing later stages of differentiation of pyramid cells. Golgi method. Cajal. a, large pyramid cells; b, c. medium-sized and small pyramid cells; d, beginning collaterals of, e y axis-cylinders or axones; /, horizontal cell of molecular layer. Basal dendrites of pyramid cells are beginning to appear.
In the precentral region, the internal granular layer becomes merged with the adjoining layers and practically disappears, the two inner layers become more or less fused and in them certain cells develop to a great size forming the layer of giant pyramid cells. It is the axones of these cells, in all probability, which proceed as the pyramidal tracts through the middle part of the internal capsule and pes to the epichordal segmental brain and cord. The area in which these cells lie is the motor cortex (cf. Fig. 371). Descending axones develop similarly from cells in the calcarine area, possibly here also from large pyramidal cells of the fifth and sixth layers (solitary cells of Meynert), which probably pass to the anterior colliculus (operating there upon reflex eye mechanisms) .
In the whole pallium there are thus four great projection fields, differentiated both by their histological structure and their connections. These are (i) the archipallial olfactory area with mesial ascending and descending connections ; (2) the visual; (3) the acoustic; (4) the somatic. The systems of projection fibers of the three neopallial fields are lateral. The visual and acoustic fields represent certain specialized and concentrated groups of receptors (rods and cones, hair cells of organ of Corti) upon which stimuli of a certain definite nature (light and sound waves), from distant objects, are focussed by means of accessory apparatus (eye, ear). The somatic area represents receptors scattered over the whole organism. In the visual and acoustic mechanisms, the efferent element is small or lacking in both peripheral apparatus and cortical areas, in the somatic the efferent element is large and is represented cortically by an area (motor, precentral area) distinct from that of the receptive portion (somaesthetic, postcentral area). Gustatory and other visceral areas have not been well determined (vicinity of archipallium ?) .
These four primary sensori-motor fields are probably the first differentiated of the various pallial cortical areas. This is evidenced by the myelination (comp. p. 464) which first involves the projection fibers of these areas (at or soon after birth, Flechsig), the afferent projection fibers probably myelinating before the efferent (Figs. 454 and 455).
The process of myelination next spreads over areas adjoining the primary areas, the intermediate areas of Flechsig. Descending projection fibers from these areas in the frontal, temporal and occipital lobes are probably represented by the cortico-pontile systems of fibers, securing cerebellar regulation of pallial reactions. The presence of other fibers connecting with thalamic nuclei is probable, but knowledge of their develoDment and connections is very incomplete.
The cells whose axones form descending or efferent projection fibers constitute only a small fraction of the cortical cells. The great majority are association cells whose axones, or collaterals, pass across the median line in the lamina terminalis as the callosal fibers already mentioned (p. 520) or pass to distant or near parts of the same hemisphere. In general, these develop later than the projection neurones and the completion of their development is carried to a much later period. Variations which arise in their differentiation and arrangement probably contribute largely to the formation of various histological areas which develop at different periods. These local inequalities of growth probably constitute a factor in the production of the convolutions appearing later than those already mentioned in connection with the primary areas. The last areas to myelinate, the terminal areas of Flechsig, are poor in projection fibers and are thus composed largely (entirely ?, Flechsig) of association cells. It is the extent of these last developing areas which constitutes the principal difference between the human cortex and that of related forms. These pallial areas are those which continue to grow in human development. Myelination in the cortical areas may continue for twenty years or so. It is a significant fact that the last areas to develop are comparatively poor, even when completely developed, in both cells and fibers (Campbell). The association neurones thus probably follow the same order of development as the projection systems. As their development spreads from the primary receptive areas (perceptions?), the incoming stimuli receive a more and more extended associative "setting" (psychologically, the "meaning" or "significance" of perceptions?), extensive associations between the various areas being provided by the extension of their development to the terminal areas (rendering possible the association of symbols: mental processes?).
Fig. 454. Diagram of cortical areas of mesial surface of pallium as determined by the myelogenetic method. Flechsig, from Quain's Anatomy. For explanation see Fig. 455.
The general biological significance of this late development of the pallium and especially of its associative mechanisms has already been alluded to. These "added" parts of the nervous system are the most modifiable mechanisms of the human organism; they are those mechanisms which perform its newest and most highly adaptive adjustments. The other parts of the nervous system are fixed at birth, but the cerebral hemispheres are still plastic for the reception and recording of individual experience. Such experience symbolized and formulated (spoken, written, etc.) is transmitted to the next generation, as already pointed out (p. 440)- An example of the far-reaching consequences of this capacity of the pallium is the prolonged period of infancy and education of man.
Fig. 455. Diagram of cortical areas of lateral surface of pallium as determined by the myelogenetic method. Flechsig, from Quain's Anatomy. The numerals indicate, in a general way. the order of myelination. The primary areas (1-10) are indicated by dots, the intermediate areas (11-31) by oblique lines and the terminal or final areas (32-36) by clear spaces.
Anomalies
Those anomalies of the nervous system involving more general developmental anomalies (cyclopia, anencephaly, cranioschisis, spina bifida, etc.) are dealt with in the chapter on Teratogenesis (XX) . Owing to the fact that the nervous system consists of parts which are more or less separated, and yet connected and interdependent, it is in certain respects affected differently from the other organs when portions of it are injured or inhibited in development. Thus an injury or inhibition in development of one part of the nervous system may, because of the dependence upon this part of other perhaps distant parts, affect the development of the latter. Even in the adult, injury of an axone leads to the disappearance of that portion of the axone distal to the point of injury; it may also lead to the disappearance of the entire neurone where regeneration is not possible. Such an injury during development will not only cause a disappearance of the whole neurone, but it may also lead to the disappearance of other neurones forming links in the same functional pathway. Thus a developmental defect involving the central area will not only lead to absence of the pyramidal tract, but also to partial atrophy of the corresponding fillet bundles. When one cerebellar hemisphere fails to develop, there results a correlated defect in its centripetal and centrifugal pathways. The opposite inferior olive is practically absent, as is also the central tegmental tract leading to that olive. The pontile nuclei of the opposite side, the middle peduncle leading from them to the affected cerebellar hemisphere, and the fibers in the pes which pass to the pontile nuclei in question are likewise suppressed, and the superior peduncle and red nucleus are absent or reduced. In this case it is evident that the correlated atrophy affects at least two neurones in the pathways leading to and from the cerebellum. This illustrates the far-reaching character of correlated developmental defects in the nervous system arising from the nature of the connections between various portions of the system.
- Next: Special Sense
References for Further Study
BARDEEN, C. R.: The Growth and Histogenesis of the Cerebrospinal Nerves in Mammals. Am. Jour, of Anat., Vol. II, No. 2, 1903.
DEJERINE, J.: Anatomic des centres nerveux. Tome I, Ch. 2 and 3.
EDINGER, L.: Vorlesungen iiber den Bau der nervosen Zentralorgane. Seventh Ed.
EDINGER, L. The Relations of Comparative Anatomy to Comparative Psychology. Jour. ofComp. N enrol, and Psychol., Vol. XVIII, No. 5, Nov., 1908.
FLECHSIG, P. : Einige Bemerkungen iiber die Untersuchungsmethoden der Grosshirnrinde insbesondere des Menschen. Berichten der math.-phys. Klasse d. Konigl. -Sachs. Gesellsch. d. Wissensch. zu Leipzig. 1904. See also Johns Hopkins Hosp. Bull, Vol. XVI, 1905, pp 45-49.
HARDESTY, I. : On the Development and Nature of the Neuroglia. Am. Jour, of Anat., Vol. Ill, No. 3, July, 1904.
HARRISON, R. G. : Further Experiments on the Development of Peripheral Nerves. Am. Jour, of Anat., Vol. V, No. 2, May, 1906.
HARRISON, R. G.: Observations on the Living Developing Nerve Fiber. Anat. Record. Vol. I, No. 5, 1907.
HARRISON, R. G.: Embryonic Transplantation and Development of the Nervous System. Anat. Record, Vol. II, No. 9, 1908.
HERRICK, C. J.: The Morphological Subdivision of the Brain. Jour, of Comp. Neurol. and Psychol., Vol. XVIII, No. 4, ipo 8
His, W.: Zur Geschichte des menschlichen Riickenmarkes und der Nervenwurzeln. Abhandl. der math.-phys. Klasse der Konig. -Sachs. Gesellsch. d. Wissensch., Bd. XIII, 1887.
His W Zur Geschichte des Gehirns, sowie der centralen und periphenschen Nerven bahnen' beim menschlichen Embryo. Abhandl. d. math.-phys. Klasse d. Konig.-Sachs. Gesellsch. d. Wissensch., Bd. XIV, 1888.
His, W.: Die Neuroblasten und deren Entstehung im embryonalen Mark. Abhandl. d. math.-phys. Klasse d. Konig. -Sachs, d. Wissensch., Bd. XV, 1890. Also Arch. f. Anat. u. Physiol., Anat. Abth., 1889.
His, W.: Ueber.die Entwickelung des Riechlappens und des Riechganglions und liber diejenige des verlangerten Markes. Verhandl. d. Anat. Gesellsch. zu Berlin, 1889. Also Abhandl. d. math.-phys. Klasse d. Konig.-Sdchs. Gesellsch. d. Wissensch., Bd. XV, 1889.
His, W.: Die Entwickelung des menschlichen Rautenhirns vom Ende des ersten bis zum Beginndesdritten Monats. I. verlangertesMark. Abhandl. d. math.-phys. Klasse d. Konig.Sachs. Gesellsch. d. Wissensch., Bd. XVII, 1891.
His, W.: Die Entwickelung des menschlichen Gehirns wahrend der ersten Monate. Leipzig, 1904.
JOHNSTON, J. B.: The Nervous System of Vertebrates. 1906.
KOLLMANN, J.: Handatlas der Entwickelungsgeschichte des Menschen. Bd. II, 1907.
VON KUPPFER, K. : Die Morphogenie des Centralnervensystems. In Hertwig 's Handbuch d. vergleich. u. experiment. Entwickelungslehre der Wirbeltiere. Bd. II, Teil III, Kap. 8, 1905.
MARBURG, O.: Mikroskopisch-topographischer Atlas des menschlichen Zentralnervensystems, 1904*
MEYER, A.: Critical Review of the Data and General Methods and Deductions of Modern Neurology. Jour. ofComp. Neurol., Vol. VIII, Nos. 3 and 4, 1898.
NEUMAYER, L.: Histo- und Morphogenese des peripheren Nervensystems, der Spinalganglien und des Nervus sympathicus. In Hertwig's Handbuch der vergleich. und experiment. Entwickelungslehre der Wirbeltiere, Bd. II, Teil III, Kap. 10, 1906.
RAMON Y CAJAL, S. : Sur 1'origine et les ramifications des fibres nerveuses de la moelle embryonnaire. Anat. Anz., Bd. V, Nos. 3 and 4, 1890.
RAMON Y CAJAL, S. : A quelle epoque apparaissent les expansions des cellules nerveuses de la moelle epiniere du poulet? Anat. Anz., Bd. V, Nos. 21 and 22, 1890.
RAMON Y CAJAL, S.: Textura del sistema nervioso del hombre y de los vertebrados. Madrid, 1899-1904. Also translation into French by Azoulay, 1910-11.
RAMON Y CAJAL, S.: Nouvelles observations sur 1'evolution des neuroblasts, avec quelques rernarques sur 1'hypothese neurogenetique de Hensen-Held. Anat. Anz., Bd. XXXII, Nos. i, 2, 3 and 4, 1908.
SCHAPER, A.: Die morphologische und histologische Entwickelung des Kleinhirns der Teleostier. Morph.Jahrbuch, Bd. XXI, 1894.
SCHAPER, A.: Die friihesten Differenzierungsvorgange im Centralnervensystems. Arch f. Entw.-Mechan., Bd. V, 1897.
SMITH, G. E.: On the Morphology of the Cerebral Commissures in the Vertebrata, etc. Trans. Linnoean Soc. of London, 2d Ser. Zoology, Vol. VIII, Part 12, 1903. See also articles by same author in Jour, of Anat. and Physiol.
Streeter GL. The development of the cranial and spinal nerves in the occipital region of the human embryo. (1905) Amer. J Anat. 4(1):83–116.
Streeter GL. The peripheral nervous system in the human embryo at the end of the first month (10 mm) (1908) Amer. J Anat. 8(1): 285–302.
ZIEHEN, TH.: Die Morphogenie des Centralnervensystems der Saugetiere. In Hertwig's Handbuch der vergleich. u. experiment. Entwickelungslehre der Wirbeltiere. Bd. IL Teil III, Kap. 8, 1905.
ZIEHEN, TH.: Die Histogenese von Him- und Riickenmark. Entwickelung der Leitungsbahnen und der Nervenkerne bei den Wirbeltierer-. In Hertwig's Handbuch der vergleich. u. experiment Entwickelungslehre der Wirbeltiere, Bd. II, Teil III, Kap. IX, 1905,
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Text-Book of Embryology: Germ cells | Maturation | Fertilization | Amphioxus | Frog | Chick | Mammalian | External body form | Connective tissues and skeletal | Vascular | Muscular | Alimentary tube and organs | Respiratory | Coelom, Diaphragm and Mesenteries | Urogenital | Integumentary | Nervous System | Special Sense | Foetal Membranes | Teratogenesis | Figures
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Book - Text-Book of Embryology 17. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Text-Book_of_Embryology_17
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G