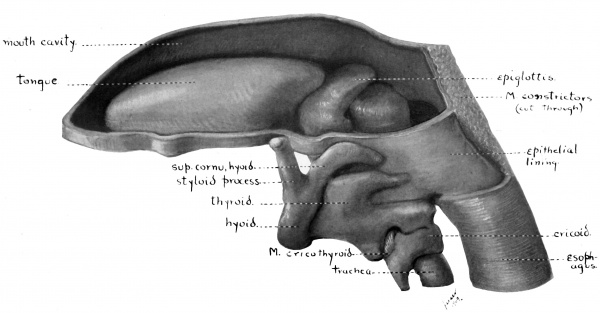
Paper - Studies on the development of the human larynx (1911)
| Embryology - 26 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Lisser H. Studies on the development of the human larynx. (1911) Amer. J Anat. 12: 27-66.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
On the Development of the Human Larynx
H. Lisser
From the Anatomical Laboratory of the Johns Hopkins University
Thirty-Nine Figures
Introduction
The investigation of the embryonic larynx has been by no means neglected. It has received the attention of many investigators resulting in several valuable contributions. Either by reason of limited material or of especial interest in particular features, these researches have usually been directed into the consideration of only parts of this complex field. There is still lacking a well rounded comprehensive review of the whole subject. Nor does this paper propose to accomplish this. The scope has been limited to a study of the cartilages, muscles, and nerves, during that period of embryonic life, when the most active development occurs. This comprises those stages where these respective elements are first definitely recognizable, to where they assume more or less, their adult relationship, namely, from the 10.5 mm. (5 weeks?) human embryo to the 20 mm. (7.5 weeks?) human embryo. Investigations on earlier stages although perhaps somewhat problematic in results have been made, and excellent accounts of still later development can be found in the literature.
Materials and Methods
The material at my disposal was Dr. Mall's collection of human embryos, for the use of which I am sincerely grateful. Likewise at this time, I desire to express my appreciation of the interest and advice of Dr. Warren H. Lewis, under whose direction these studies were undertaken, and to Mr. Max Broedel for valuable suggestions regarding some of the illustrations.
I have included a large number of drawings in the hope that they would help to make lucid the descriptions of the various structures involved. Accuracy and fidelity to the original sections has always been the main object. However, it must be said, that in reproducing embryonic tissue, peculiar difficulties are encountered. Condensed mesenchyma is not as a rule clearly outlined, but shades gradually into the surrounding tissue, so that some exaggeration is permissible, and in fact necessary in giving sharp clear outlines to such structures for proper interpretations and the construction of models.
The embryos were cut in various planes, sagittal, transverse, and frontal, thereby affording better opportunities for study. They include the following:
| Study Embryos from the Carnegie Collection | ||||
|---|---|---|---|---|
| Embryo | Carnegie stage | Length (mm) | Thickness (μm) | Sections |
| 109 | 18 | 10.5 | 20 | Transverse |
| 317 | 18 | 12.5 | 50 | Frontal |
| 144 | 18 | 14 | 50 | Sagittal |
| 43 | 19 | 16 | 50 | Sagittal |
| 128 | 21 | 19.5 | 50 | Frontal |
| 22 | 21 | 20 | 50 | Transverse |
| Online Editor - The column "Carnegie stage" has been added to the original paper's table. | ||||
Each section in the laryngeal region was carefully studied, projected to sufficient magnification, and the different structures outlined and identified. Recourse was then had to wax model reconstruction, and to the several well known graphic methods of reconstruction.[1]
I have purposely omitted any consideration of the vascular development of this region, as reconstruction methods in embryos are somewhat hazardous for such purposes. The modern injection methods are far more accurate. Much remains to be done in this section.
In considering the musculature of the larynx, it soon became evident, after dissections and studies of the adult larynx, that there was by no means unanimity of opinion among different authorities as to just what constituted the muscles of the larynx. Of course, every one recognized and mentioned the cricothyreoideus, the cricoarytaenoideus posterior and the cricoarytaenoideus lateralis. But some spoke of an inter artyaenoideus muscle, others divided this into an arytaenoideus transversus and artyaenoideus obliquus. Some added an aryepiglotticus and a thyreoepiglotticus ; others merely pictured one of these, and some, neither of them. Others again, divide the thyreo-arytaenoid into an internus and externus, and Sewell Seymour ('05) has dissected a 'small or superficial thyreo-arytaenoid muscle' which, moreover, he differentiates into four tj^pes. It is therefore apparent that there is not yet a clear cut conception of the exact musculature of the normal adult larynx. Certainly, if such difficulties present themselves in the adult fully formed larynx, how much more confusion would there be in attempting to isolate these varieties in a human embryo. So that I arbitrarily selected the following nomenclature for the
laryngeal musculature, as being the simplest and most reliable:
M. m. cricothyreoideus, cricoarytaenoideus posterior, cricoaryeotaenoideus lateralis, thyreoartyaenoideus, interarytaenoideus, aryepiglotticus, and thyreoepiglotticus. I have purposely regarded the thyreoarytaenoideus as a single muscle, because any demarcation between an externus and internus and a small or superficial, though interesting, is quite artificial, not conclusively demonstrated, and not warranted by the existence of separate distinct action of such subdivisions. The advantage of this classification has been borne out by the following embryological studies, where each of the above enumerated muscles has been clearly found, and none of the other varieties has been represented, though carefully searched for.
Embryo 109
Carnegie Embryo 109 10.5 mm. Transverse sections, 20 micron
(Embryo 109 measures V. B. 10.5 mm and N. B. 11 mm in length and is about 5 weeks old.)
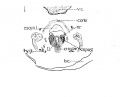
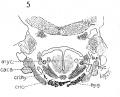
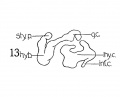
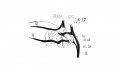
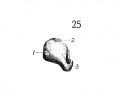
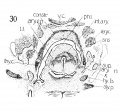
Fig 1 Cross section of human Embryo no. 109 (10.5.) to show cricoid cartilage and M. criooarytaenoideus posterior.
Fig 2 Cross section of human Embryo no. 109 (10.5mm.) to show thyreoid cartilage, M. criooarytaenoideus lateralis,
The Cartilages

The cartilages of the larynx, or better what become the cartilages of the larynx, are at this stage but very imperfectly formed, as is to be expected, since they reveal themselves for the first time in a 10.5 mm. human embryo. Consequently their appearance is introduced by condensations of mesenchyma, very well termed "pre cartilage." No true cartilage formation, whatsoever, has occurred at this time in any of the laryngeal cartilages, nor yet in the hyoid bone and styloid process. I have investigated the sections of 8, 8.5, 9, and 10 mm. embryos for earlier traces of these structures, but have been consistently unsuccessful, so that I place the first recognizable stage of the larynx skeleton (if one may be permitted to use such an expression) at 10.5 mm. in man. Of course, it is well known that all the structures of the larynx have their ultimate foundation in the gill arches, and I refer those interested in this very early stage of the subject to Frazaer's interesting studies ; but I would be skeptical of finding, wdth the present means of study at our disposal, actual structural status, as regards separate cartilage and muscle masses, earlier* than 10.5 mm. in man.
The cricoid-cartilage
{Figs. 1-2.) Contrary to expectations, the cricoid cartilage does not appear to develop from two lateral portions of condensed mesenchyma, separate and independent of each other, which later grow together ventrally and dorsally about the lumen of the larynx; nor as one might be led to anticipate, form a large area posterior or dorsal to the larnyx lumen (this portion ater becoming more prominent by far than the anterior arcus). But at this stage, there is a predominance of condensed mesenchyma about the ventral portion or arcus, which fades of laterally, and then becomes more emphasized again, by greater compactness and deeper stain, dorsal or posterior to the lumen, but not so extensively nor so well marked as ventrally. This is true provided one considers the deeper staining, more strikingly isolated portions of condensed mesenchyma, as the anlage. So that it seems reasonable to assume that the cricoid cartilage originates from an anlage primarily ventral in the position of what later becomes the anterior arcus, and also, though less prominently from a posterior portion, of itself perhaps, originating from two slightly separated posteriorly lateral portions. The lateral portions then, develop by a welding of the anterior and posterior portions lateralward. At this stage then, the ventral portion of the cricoid cartilage is appreciably advanced in development over the rest of this structure. It does seem difficult to reconcile this with the appearance, for instance, in the 20 mm. stage, when the ventral portion still persists as condensed mesenchyma, while the lateral parts have undergone considerable chondrification.
The arytenoid cartilages
It is doubtful whether these structures can be determined at this early period in development; probably not. There are two faint, indefinite masses which suggest beginning condensation, but as there is a possibility of these being a part of the superior portion of the cricoid, no positive assertions can be made as to their independence.
Thyreoid cartilage
This cartilage like the cricoid, makes its initial appearance at this stage. I have looked in vain for its rudiments in earlier human embryos. There has been quite a lengthy discussion, during most of the nineteenth century, in which many have participated, as to whether the thryeoid cartilage develops from two lateral anlages which grow around and fuse ventrally, or whether there is in addition to these lateral anlages, a third one a pars intermedia. Nicolas, in his excellent resume of the subject treats this very fully. My observations inclined to the former view, as in all the stages studied, the lateral portions depict a decidedly more advanced stage of development; yet, I must add that I have found no stage, where the lateral halves alone were present, and in which there was no indication whatsoever of a ventral condensation. Fig. 2 shows the thjTCoid cartilage as it exists in a 10.5 mm. embryo. It will be noticed that there is no interruption in the continuity between the two lateral portions via the ventral part ; but it will also be seen that the lateral parts exhibit a denser condensation. There is no suggestion of an inferior or superior cornu. The thryeoid cartilage merely looks like a horse shoe mass of condensed mesenchyma.
The hyoid bone and styloid process
These bodies are easily made out existing as pure condensed mesenchyma. The styloid process is more advanced than the greater cornu of the hyoid at this time.
The epiglottis. This cartilage can be discerned, much flattened in comparison with the adult type. It shows as a beginning area of condensation.
The Musculature
The eight muscles are first visible at this period of development, not all as individual muscle masses however, although it is true that one or two of the muscles can be clearly differentiated from the rest. It is curious to note that the laryngeal musculature shows more advanced differentiation than the pharyngeal constrictors, as shown by clearer outline and more extensive fibrillation. The intrinsic oesophageal musculature however is farther developed. The sphincter formation, to which attention has been called by so many writers, can be recognized at this stage, and in a few sections there is some tendency to continuity between the fibres of the outer pharyngeal ring and the inner laryngeal one. Probably too much stress has been laid on this structure, perhaps by reason of the fact that an analogous muscle has been found in lower animals. At any rate, the muscles of the larynx differentiate themselves much earlier than has been previously believed. For instance, the crico artyaenoideus posterior (fig. 1) is unmistakably isolated at this stage, well defined of good size, and abundant fibrillation ; nor does it include any other laryngeal muscle. There is also a muscle mass with some fibrillation, not so large, but plainly evident, which apparently includes the crico aryteanoideus lateralis and thyreo arytaenoideus (fig. 2), principally the former. It is placed on the lateral surface of the cricoid cartilage. The inter-arytaenoideus; if it exists at all, does not appear as one muscle. In the position where one would expect to find it, there is continuity of the larynx and pharynx lumina. But just at the point where these join there are muscle fibres on either side, but which do not unite. These may later bridge across to form the m, interarytaenoideus, but of that point, I am by no means certain. No trace was found of the m. aryepiglotticus or the m. thyreoepiglotticus. The m. cricothyroideus » is fairly well developed, though not nearly so far advanced in form and size as the m. cricoary taenoideus posterior. It is the only one that shows any tendency to relation with the pharyngeal musculature, and Frazaer considers them to have the same origin. In general the musculature of the larynx is rather better defined than the cartilages at this period. Strazza in 1888, completed the only really valuable work done on the development of the human laryngeal musculature. Nothing of importance has been added since his paper. He thinks that the laryngeal, tongue and pharyngeal musculature develop out of one and the same muscle mass, which in the early embryo develops from an isolated 'muscle island,' which exists independently of the muscle plates. And that the premuscle tissue of the tongue and larynx is a continuous one, the latter merely lying inside the former. In the region of the epiglottis and larynx, he thinks, is also contained the premuscles masses, though he cannot differentiate them at all in his youngest embryo (12 to 13 mm). He associates the simultaneous development of the tongue and larynx musculature from the same source, with the fact of the union in speech between the muscles of the tongue and larynx. This is an attractive theory, but my observations cannot substantiate his statement. There is no indication that the larynx muscles develop from the myotomes, on the contrary, they apparently arise from the ventral visceral mesenchyme which continues up into the floor of the mouth. Bvt in this 10.5 mm. embryo in which even certain larynx muscles can he isolated, there is no association with the tongue musculature, and hut little with the pharyngeal set. In earlier stages the cells which are to form the premuscle masses cannot be distinguished by our present methods from other cells of the condensed mesenchyme of this region.
The Nerves
The n. laryngeus superior can be traced to the vicinity of the greater cornu of the hyoid, and the wing of the thyreoid, but I could not follow either the motor branch to its inervation of the crico thyreoideus muscle, or the sensory portion, within the larynx. Bits of tissue, were seen that might be nerve tissue, but I cannot be certain of this branch of the vagus any further than to its proximity to the hyoid and thyreoid. The nerve Recurrens, later the nerve laryngeus inferior (fig. 1) is better developed and can be followed clearly to its innervation of the crico artyaenoideus posterior ; but I cannot trace it to the other muscles, nor to any anastomosis with the n. laryngeus superior.
Embryo 317
Carnegie Embryo 317 - 12.5 mm. Frontal sections - 50ix
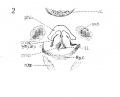
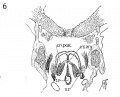
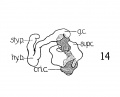
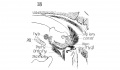
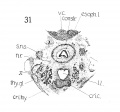
Fig 3 Frontal section of human Embryo no. 317 (12.5 mm.) to show epiglottis thyreoid cartilage, and hyoid bone. Ant. Card., anterior cardinal vein.
Fig 4 Frontal section of human Embryo no. 317 (12.5 mm.) to show superior laryngeal nerve, sup. /ary., superior laryngeal nerve; car. a., carotid artery.
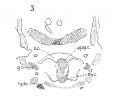
Fig 5 Frontal section of human Embryo no. 317(12.5 mm.) to show M. crico-thyreoideus and arytaenoid masses.
Fig 6 Frontal section of human Embryo no. 317 (12.5 mm.) to show M. cricoary taenoideus posterior, interarytaenoideus and nerve recurrens.
The Cartilages
The thyreoid cartilage still consists purely of precartilage, - condensed mesenchyma, and has not changed greatly in appearance from the one in the 10.5 mm. human embryo. However, rudiments of a superior cornu and inferior cornu are just discernable, and the lateral halves are not quite so rounded as a part of the horseshoe arrangement, but rather more vertical as in fig. 3. The ventral condensation is again in perfect continuity with the lateral parts. This ventral portion is at a lower level than in the adult, coming in close contact with the body of the cricoid, especially its anterior arcus. The lateral wings are well removed from the lateral portions of the cricoid, and between them is ample room for the lateral larynx muscles; the circothyreoideus, circoarytaenoideus lateralis, and perhaps thyreoarytaenoideus.
The cricoid cartilage consists likewise of condensed mesenchyma but is somewhat ahead of the thyreoid in assuming definite shape; certainly the intensity of the condensation is greater, as determined by deeper staining and clearer outline; the ventral arcus maintains its lead over the dorsal and lateral portions although the latter show definite increase in the size of the condensed masses and assume a clearer outline. Figs. 4 and 5, show two sections
of this cartilage. These drawings of course, are of necessity, exaggerated, as no such absolutely isolated areas exist for they fade
off imperceptibly into the surrounding tissue, and outlines must
be somewhat arbitrarily decided upon. The lumen is still narrow
and slit-like, so that the circular, ring-like appearance of the cricoid is not yet established. The sides seem compressed more or
less one upon the other.
The artaenoid masses, (fig. 4), make their appearance at this
time, and although rather intimateh' related to the cricoid mass,
nevertheless permit of recognition. They are roughly of oval
shape and bear little resemblance to their adult appearance.
They are of course composed purely of precartilage ; they develop
more slowly than do the cricoid or thyreoid, but keep abreast
of the epiglottis in their growth. Even at the 20 mm. stage when
the cricoid and thyreoid show a predominance of chondrification,
the arytaenoids and epiglottis are still represented only by condensed mesenchyma.
The epiglottis. The epiglottis is shown in fig. 3 ; it is situated at a lower level than in the adult.
There is a crowding together of laryngeal structures at this
period in development. In the adult the length or height of the
epiglottis is much greater than its breadth while in the embryo
the length and breadth are about equal. This congestion of the
cartilage is very likely due, in great part, to the general ventral
curvature of the entire embryo, especially the way the head is bent
upon the body and with the subsequent lengthening out and
straightening out of the whole body, the laryngeal cartilages
naturally assume their adult relationship. Such changes, though
partial, have already occurred by the 20 mm. stage, as seen in fig.
38 of the model and illustrate the tendency, which is fulfilled more markedly later.
The hyoid hone and styloid process are composed of condensed mesenchyma and are very clearly outlined. Fig. 3 shows this. The greater cornu is developing rapidly and has attained large proportions.
The Muscles
The figs. 4 and 5 which are intended to illustrate, among other things, some of the musculature of the larynx, may give a wrong impression, which possibility therefore, I hasten to avoid. There is by no means the fibrillation in these rimscles, as shown in fig. 6. The muscles, with the exception of the circoartyaenoideus posterior, which is faithfully drawn - are merely condensed mesenchyme, with the barest suggestions of fibrillation, and the character given them in the drawings are merely for the sake of clarity.
M. cricoarytaenoideus posterior is very well developed, as shown in fig. 8, of large size, and well advanced to fairly complete fibrillation. The muscle is somewhat more laterally situated than in the adult, but otherwise conforms closely to the later stages. It is plentifully innervated by the recurrent laryngeal nerve.
M. cricoarytaenoideus lateralis (fig. 4) though not nearly so precisely outlined, this muscle mass, nevertheless, assumes considerable proportions at this stage. It is difficult, as it was in the case of the 10.5 mm. embryo, to decide whether the premuscle tissue represents only the cricoarytaenoideus lateralis, or whether it also includes what is to become the thyreoarytaenoideus m. No fibers originating from the mesial surface of the lateral portions of the thyreoid cartilage were found, not even a slight trend of the
condensed mesenchyma. So that the muscle may not appear at all till later in development. The thyreoepyglotticus and aryepiglotticus are both absent.
The inter arty aenoideus (fig. 6) has made its appearance, but is partly attached to the insertion fibers of the circoarytaenoideus posterior. It is fibrillated.
The cricothyreoideus muscle (fig. 5) can be made out at this time, sending its fibers from the ventrolateral portion of the cricoid to the mesial surface of the thyreoid. It cannot be entirely separated from the cricoarytaenoideus lateralis mass, but its innervation by the superior laryngeal nerve helps in the identification.
Nerves
(Figs. 4, 5, 6)
The N. laryngeus superior (fig. 4) and inferior (fig. 6) can be followed perfectly in these sections, from the point where they leave the vagus, to their ultimate endings in the various muscles. No reconstruction was made to prove positively an anastomosis between the two, although a study of the sections suggested this strongly. The innervations of the various muscle masses were definitely seen.
Fig 4 Frontal section of human Embryo no. 317 (12.5 mm.) to show superior laryngeal nerve, sup. /ary., superior laryngeal nerve; car. a., carotid artery.
Fig 5 Frontal section of human Embryo no. 317 (12.5 mm.) to show M. crico-thyreoideus and arytaenoid masses.
Fig 6 Frontal section of human Embryo no. 317 (12.5 mm.) to show M. cricoary taenoideus posterior, interarytaenoideus and nerve recurrent.
Embryo 144
Embryo no. 144 - Sagittal sections - 50 micron
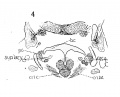
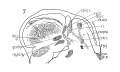
Fig 7 Sagittal section of human Embryo no. 144 (14 mm.) to show, especially, M. interarytaenoideus.
Fig 8 Sagittal section of human Embryo no. 144(14 mm.) to show hyoid bone and thyreoid cartilage, M. cricothyreoideus, and tongue region
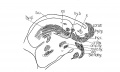
Fig 9 Sagittal section of human Embryo no. 144 (14 mm.)
Both the embryo under consideration and the following one of 16 mm. were cut sagittally and this circumstance affords excellent opportunity for comparison. But the conclusion forced upon one is, that there is very little disparity between the two, - practically no changes of sufficient significance to warrant recording. This, of course, may be due to the fact that the shorter embryo, may be older than its length would indicate, or vice versa, as regards the longer embryo. Yet it is not unlikely that little progress is made in the various laryngeal structures during this period of development. There is an indication of beginning chondrification in the 16 mm. embryo, whereas there is none whatsoever in the 14 mm. embryo, but otherwise the differences are negligible. A few drawings were included of some sections (figs. 7, 8, 9), in the 14 mm. embryo, which happen to show the musculature a little more distinctly than in the 15 mm. sections - and they will also serve the purpose of visualizing the similarity mentioned above. Accordingly, a detailed description of the larynx in this embryo will be omitted, as the description of the 16 mm. one will suffice. And moreover, more complete studies, such as graphic reconstructions, were made on the older embryo.
Embryo 43
Embryo no. 43- 16 mm Sagittal sections
Embryo no. 43 measures 16 mm V.B. and 4 mm N.B. about six weeks old.
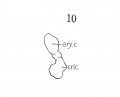
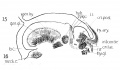
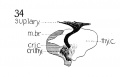
Fig 10 Graphic reconstruction of cricoid and arytaenoid cartilages in human Embryo no. 43 (16 mm.).
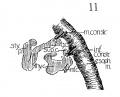
Fig 11 Graphic reconstructions of pharyngeal constrictors in human Embryo no. 43 (16 mm.)
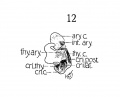
Fig 12 Graphic reconstruction of laryngeal musculature in human Embryo no. 43 (16 mm.).
Fig 13 Graphic reconstruction of thyreoid cartilage hyoid bone, and styloid process in human Embryo no. 43 (16 ram.).
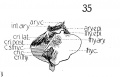
Fig 14 Graphic reconstructions of larynx cartilages in human Embryo no. 43 (16 mm.).
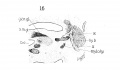
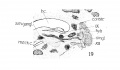
Fig. 15 Sagittal section of human Embryo no. 43 (16 mm.) to show laryngeal musculature and nerve recurrens. Meek, c, Meckel's cartilage.
Fig. 16 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region, s. m. gl., submaxillary gland.
Fig. 17 Graphic reconstruction of 9th, 10th, and 12th cranial nerves in larynx region of human Embryo no. 43 (16 mm.), ana., anastomosis between superior laryngeal and inferior laryngeal nerves; m br., motor branch of superior laryngeal nerve.
Fig. 18 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region.
Fig. 19 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region, s. m. gangl., submaxillary ganglion.
Fig. 20 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region.
The Cartilages
The thyreoid cartilage is a peculiar structure at this stage, still consisting purely of condensed mesenchyma. The lateral alae are united ventrally, but it is to the odd shape of the lateral masses, that I would call attention. Fig. 13 shows a graphic reconstruction of this cartilage from the side view. The superior cornu, fig. 13, is in evidence, and is in correct relation to the greater cornu of the hyoid bone, between which develops the thyreohyoid ligament. Another point in favor of this being the superior cornu of the thyreoid, is the attachment to it of the inferior constrictor pharyngis, as seen in fig. 11. Posterior, and below them, protrudes a curious cylindrical mass of condensed mesenchyma, which undoubtedly forms the rudiment of the inferior cornu of the thyreoid. It overlaps the cricoid and is in close apposition to it as shown in fig. 14. Probably there is no actual articular facet at this stage. Anteriorly, there is a strange projection, without apparent attachment to anything ; it seems to be evidence of greater activity of growth in the ventral part of this lateral mass, just as the superior and inferior cornua are the results of active growth in the posterior portion of this lateral mass. Apparently then, the directly lateral part lags behind temporarily, and the peculiar gap between the anterior cornu (as I call this odd projection) and the superior cornu, is filled in during the next week or so.
In the 20 mm. stage, there appears to be a slight tendency to condensation in this area, not marked enough to be included in the reconstruction of this stage.
The cricoid cartilage (fig. 10) consists of pure condensed mesenchyma, with no evidence as yei of chondrification. Although rather crude in outline, yet it begins to suggest roughly the maturer form. Its relation to the thyreoid cartilage resembles the adult rather closely, and the continued ring, ventral and dorsal, is now complete. Also, the posterior portion is enlarging and begins to show advances over the relatively slower growth of the anterior arcus, conforming with the adult type. Certainly, it is further advanced than the thyreoid cartilage.
The aryiaenoids are still represented by a roughly oval mass of condensed mesenchyma, with no accuracy of form or outline.
The aryiaenoids are still represented by a roughly oval mass of condensed mesenchyma, with no accuracy of form or outline. Fig. 10 is a reconstruction of one of them from a lateral view. The mass is continuous with the cricoid mass, as indicated by the cross lined area, (fig. 14) but its main arytaenoid portion is stained deeply enough to differentiate it absolutely from being a part of the cricoid mass.
The epiglottis shows but little advance over its condition in the earlier embryos, except for some gain in length over breadth, but the mass out of which it assumes its adult shape, is easily recognized.
The hyoid hone and styloid process begin to show small areas of chondrification, and their appearance is seen in a reconstruction fig. 13 and 14. The attachment of the middle constrictor to the greater cornu is shown in fig. 11.
The Muscles
In a 14 mm. embryo Strazza says that a muscle mass, of spindle shaped formation can be made out laterally in cross sections, but that no distinct muscles can be isolated at this stage, and that the mass simply represents laryngeal musculature. And at 16 mm. he distinguishes a muscle band, bending around with the posterior convexity, which in its upper portions he calls the arytaenoideus transversus (interarytaenoideus), but considers this band a continuous muscle mass, only differentiated later by the development of the cartilages. Further down he thinks it to be the cricoary-taenoideus lateralis and thyreoarytaenoideus, but says it is con- tinuous with the above, only spread over a greater area. So he calls the larynx musculature an arch, which however, is not entirely horizontal, but goes from above and behind, to below and in front. Now it is true that considerable interlacing of fibres exists at this stage, but not much more than occurs in careful dissections of the adult larynx. It is also true that further separation and development of the cartilages will bring about clearer differentiation of the muscles. But that a continuous muscle band, which cannot be differentiated into individual muscles, exists at this stage is not in accord with the results shown, especially in fig. 12, a reconstruction of the 16 mm. stage, and in figs. 2, 8, 9, faithful drawings of the sections of the 14 mm. embryo, and fig. 15 of the 16 mm. embryo.
The cricothyreoideus, the cricoarytaenoideus posterior, and the
interarytaenoideus are definitely isolated, while the cricoarytaenoideus lateralis and thyreoarytaenoideus are clearly separated from the
others aiid are about as much separated from each other as they
are in the adult. There is no need of describing them any further.
The figures show them all in sufficient detail.
The constrictor muscles of the pharynx and oesophagus have
been reconstructed and the middle and inferior constrictors stand
out rather clearly. Nicolas states that the pharynx musculature
only unites from two independent lateral halves at 3 cm. I have
found perfect continuity at 10.5 mm. in the lower pharyngeal
portion, more union at 12.5 mm. and complete union at 14 nun.
Several of the tongue and pharynx muscles have been included
in the illustrations, and it will be seen that they are very clearly
isolated and well developed even at the 14 mm. stage (figs. 7-9).
For the identity of these muscles, I am under obligations to Dr.
Lewis, who very kindly gave me his own sketches, from which
figs. 7, 8 and 9 were developed.
The Nerves
The nerves, are reconstructed in fig. 14 and in addition to showing the superior laryngeus and n. recurrens and n. laryngeus inferior, which can be followed to their respective innervations and to their anastomosis, there is included the relations of these, to the glossopharyngeal and to the hypoglossus (also figs. 15, 16, 19, 20).
Embryo 22
Embryo no. 22, 20 mm Transverse sections
Embryo no. 22 measures 20 mm. V. B. and 18 mm. N. B., about 11 weeks old.
The Cartilages
Thyreoid
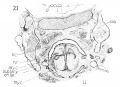
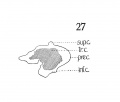
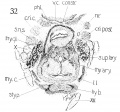
This cartilage is easily recognized at this stage and has a definite outline. Although it has not attained the adult shape, in all particulars, it has, nevertheless, developed into the adult type. Not all of the tissue representing this structure, is composed of true cartilage, and fig. 27 gives, from a lateral aspect, some conception of the relative portions of chondrification and pre-cartilage. Very little, if any, of the anterior, ventral, median portion, between the two wings is represented by cartilage, this being almost entirely condensed mesenchyme. This is well illustrated in figs. 29 and 30, which likewise depict the broad generous curve (convex throughout) uniting the two wings ventrally. The notched appearance with the prominent ventral ridge is not evident as yet. This ventral portion, in a vertical direction (caudo-cephalad) is not very extensive, being appreciably smaller in height than in the adult (relatively).
The wings of the thyreoid cartilage are quite well developed indeed, as can be seen in figs. 29 and 32, showing considerable chondrification.
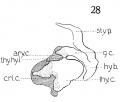
The upper cornu is prominent, but it does not present the upward curve, as strikingly as in the adult. Its attachment to the greater cornu of the hyoid by the thyrohyoid ligament is well seen, - the latter appearing as condensed mesenchyme. Just where the thyreoid leaves off, and the ligament begins, and where the latter establishes connection with the hyoid, cannot be definitely ascertained, but the continuity of the parts is easily demonstrated, by graphic reconstruction, as shown in fig. 28.
The inferior cornu of the thryeoid is of a more exaggerated type than in the adult, projecting from the wings over the lateral posterior portion of the cricoid. The relations of these two cartilages are shown in fig. 28. A study, in cross sections, of these two cartilages reveals an ummistakable apposition - not approximate, but in close contact. Whether there is an actual articular facet present at this stage, is difficult to determine. The wax model suggests such a possibility very strongly but the appearance is within the limits of error, unavoidable in the construction of such a model. Nicolas ('94), in his excellent studies on the thyreoid cartilage, insists that the articulations crico-thyreoid and cricoarytaenoid appear very late. I am inclined to disagree with him on that score. Certainly the rudiments of such an articulation are present, at least of the crico-thyreoid. However I would not make a positive assertion as to the completeness of the articulation, because of the possibilities of error as mentioned above.
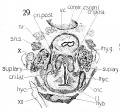
The cricoid (fig. 25). This structure is likewise very well developed at this stage, corresponding with the adult tj^ even more closely than does the thyreoid. Its seal ring appearance is quite characteristic. Its shape is almost round and accordingly shows a corresponding development in the larynx lumen, - for the two are interrelated in their growth. Fig. 26 illustrates the extent of chondrification in this cartilage. The ventral arcus is, however, condensed mesenchyme (pre-cartilage) . It is quite definite, low in front, gradually higher behind to the posterior arcus, which is very large. The latter's posterior surface seems to possess already the two flat fossae for the origin of the mm. crico-arytenoidei posteriores. Again, there is typical overlapping of the arytaenoid cartilage over the cricoid, in its superior portion, and the typical apposition again strongly pictures the possibility of a definite articulation. The relation of the two cartilages is seen in fig. 28.
The arytaenoids. These cartilages are rather behind the thyreoid and cricoid in development, certainly as regards chondrification, which has scarcely begun, as they are almost entirely composed of condensed mesenchyme. Their shape approaches that of the adult type - but the fovea triangularis and fovea oblonga on the ventral surface are not at all clear. There is no definite processus muscularis, but the presence of all the muscles in characteristic position, and with characteristic attachment, indicates no doubt the place where this process will appear.
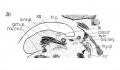
The cartilages of Wrisberg and Santorini, Siccording to Nicolas, *'do not appear until the other cartilages have taken definite form, not becoming cartilagenous until the epiglottis is fully formed at 6.5 months." The first part of the statement is rather indefinite. I should say that all the other cartilages had definite form even before this stage, but surely at the 20 mm. stage. Certainly, the cartilages of Wrisberg and Santorini are not present at this period as definite separate structures, but there is a suggestion of the latter in the smaller model of this stage — an appendage of the aryaenoids - fig. 36,
The Hyoid hone and styloid process need not be described in detail. A very good idea of them can be obtained from a study of figs. 28, 29 and 32. The drawings of the model, figs. 37, 38 and 39, show the general shape, and relation to contiguous structures, and the section drawings give some idea of the extent of chondrification.
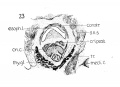
The Muscles
At this stage the musculature is quite distinct and all the muscles permit of clear differentiation. Fibrillation is fairly extensive, especially in the cricothyreoideus and cricoarytaenoideus posterior. The muscles have attained their adult relationship and reconstructions reveal their character with considerable precision. Thus I cannot agree with Kanthack ('92), who says that at four months the cricothyreoid and constrictor are in close contact and cannot be separated one from the other ; that it is impos- sible at two months to separate the interarytaenoideus and cricoarytaenoideus posterior. That up to the fourth month the fibres have the same direction and that the muscles cannot be really differentiated until the fourth month. He adds that it is impossible to separate the thyreoarytaenoideus and cricoarytaenoideus lateralis, which holds also for the child and adult. Although 'impossible' is expressing it a bit strongly yet the latter part of the statement is well taken, as even in the child and adult the differentiation is not always clearly manifest.
The m. cricothyreoideus. Figs. 29 and 31 picture this muscle in
section, and figs. 34 and 38 give the appearance in reconstruction,
both wax models and graphic representation. Its origin on the
external surface of the arcus cartilaginis cricoideae is as in the
adult. It inserts on the medial surface of the inferior margin of
the lamina cartilagins thyreoideae. It is a well developed muscle with numerous evidences of fibrillations. However there is
no division into a pars recta and pars obliqua as in the adult, nor does the muscle extend as far posteriorly in its insertion on the thyreoid. So that it would appear that the pars obliqua is a later
manifestation, and owes its development to a more complete
growth along the inferior cornu, along which it inserts more extensively later.
The m. cricoarytaenoideus posterior is a large conspicuous muscle
as seen in fig. 36 of the wax model. It is in its adult position,
arising from the medial inferior part of the lamina cartilaginis
cricoideae and converging upward to insert on the apex of the processus muscularis cartilaginis arytaenoideae. It is closely applied
to the cricoid cartilage as can be seen from a study of the sections
and extends somewhat more lateralward on the cricoid cartilage
than in the adult. Otherwise it conforms perfectly with the adult
type.
The ni. cricoarytaenoideus lateralis is a considerable muscle mass on the lateral surface of the cricoid cartilage, which from a study of the sections alone, would seem to merge in with the mass that by nature of position and relation should become the thyreoarytaenoideus muscle, but careful graphic reconstruction reveals the picture seen in fig. 35 where the independence of the muscle is plainly evident. Its fibrillation is not as extensive as that of the cricoarytaenoideus posterior. Its origin on the upper lateral surface of the cricoid and its insertion on the processus muscularis cartilaginis arytaenoideae is well marked.
M. thyreoarytaenoideus is likewise well marked at this stage and fig. 35 shows its relation to the median surface of the thyreoid and its insertion on the processus muscularis cartilaginis arytaenoideae. Undoubtedly some of its fibres and those of the cricoarytaenoideus laterahs interlace, just as they do in the adult, but the origin of the majority of the fibres from the thyreoid cartilage is distinct enough to justify a clear differentiation of the two muscle masses. Fiirbringer ('75), maintains that the secondary interlacement of these muscles as exists in adults becomes apparent at 48 mm., and does not believe that the sphincter formation of adults is related to the primary sphincter. I have not studied embryos later than this stage, so that I am in no position to question the accuracy of this statement; yet I can hardly see why it is necessary to suppose that the interlacement, which is surely present according to my findings in an embryo of 20 mm., should disappear and reappear again at 48 mm. ; for surely no very radical changes occur in development, as regards the larynx, after 20 mm.
M. inter arytaenoideus. The fibres of this muscle show up beautifully in cross sections, and are unmistakably present in abundance in this stage. The muscle is well defined and quite thick.
Figs. 30 and 36 give an idea of it, both in section and reconstruction.
I hardly see why there should have been any difficulty in separating it from the cricoarytaenoideus posterior or thyreoarytaenoideus as was reported by Kanthack. The fibres run in a different direction and reconstruction shows it quite as sharply defined as in the adult.
M. aryepiglotticus. (figs. 35 and 36.) There is some doubt in
mind as to whether the fibres which seem to make up this muscle
can be clearly isolated from the interarytaenoideus ; whether perhaps, the adult appearance is not due to the later development
upward of the epiglottica. Primarily no doubt, these two muscles originate as. one mass and at this stage cannot as yet be definitely isolated. The fibres which make up the aryepiglotticus
seem to be continuous with the interarytaenoideus, merely having
extended upward along the lateral surface of the epiglottis.
M. thyreoepiglotticus. At this stage and in this embryo, there
seems to be an indication of this muscle as brought out in the reconstruction, figs. 35 and 36. It seems reasonable to assume
that this muscle is originally unrecognizable from the thyreoarytaenoideus, and is again dependent for its differentiation upon the
development upward of the epiglottica, which draws these fibres
away from the other muscle mass. This has apparently occurred at this stage.
The constrictors. The esophageal musculature is very well advanced by this time, and by nature of the attachments to the superior cornu of the thyreoid and the greater cornu of the hyoid bone, a middle and inferior constrictor mass may be recognized. Fibrillation[2] is extensive.
In comparison with muscular development elsewhere in the
body, and even in the neighborhood of the larynx, it is noticed that
the laryngeal musculature is backward, especially in the richness
and thickness of fibrillation; but I think that a study of the vari-
ous illustrations will justify the statement that in a 20 mm, human
embryo sufficient differentiation has occurred to admit of definite
recognition of all the extrinsic and intrinsic muscles of the larynx.
Considerable emphasis has been laid by most authorities on the
sphincter formation of the larynx and pharnyx musculature; the
continuity of the two, one sphincter within another, as it were.
Notably the excellent contributions of Strazzer, Fiirbringer and
Kanthack lay stress on this. The existence of such a structure,
is suggested in the earlier stages, but that this sphincter formation
and horizontal fibre arrangement is the predominate feature at the
20 mm. stage, is I feel, not true, but capable of explanation. A
study of cross sections, certainly inclines to such an impression;
and fig. 30 suggests this especially; but reconstructions at this
period of development do not bear this out, nor do the study of
frontal sections as in embryo 19.5. mm.; and these studies surely
suggest that the muscles are too clearly differentiated at this time
to exemplify a sphincter arrangement, except in the same sense
as such a general structure exists in the adult. Of course reconstructions, expecially wax models are by no means infallible, and
not nearly as accurate as, for instance, the injection method for
the study of blood vascular development; but I do consider the
results of these reconstructions, both wax and graphic, to be well inside the limits of error.
The Nerves
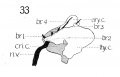
A, laryngeus superior (figs. 33, 34, and 36) is very distinct, of considerable proportion, and easily made out. It divides into two portions, the ra7nus externus and internus at about the level of the superior cornu of the hyoid. Ramus externus is by no means small in calibre, as seen in figs. 34 and 36, it descends almost vertically upon the outside of the muscle constrictor pharyngis inferior to the m. cricothyreoideus, in whose substance it can be readily traced, as seen in the graphic reconstruction fig. 34. The ramus internus extends externally to the level of the thyro-hyoid membrane, and together with the a. laryngea superior perforates this, and divides into several branches, the main mass however passing downward along the median surface of the thyreoid cartilage and over the lateral internal muscles of the larynx to anastomose with the n. laryngeus inferior of the n. recurrens. This anastomosis is perfectly demonstrated at this stage by the wax model reconstruction. Some authorities state that in the adult this ramus internus is not entirely sensorj, but sends some motor twigs to the m. interarytaenoideus. Others do not mention this. I cannot satisfy myself absolutely on this point. The anastomosis between the n. laryngeus inferior and superior is a large one, and it is difficult to say where one nerve leaves off and the other begins, and accordingly what distribution belongs to each.
N. laryngeus inferior. The chief resultant portion of the n. recurrens ascends along the medial surface of the lateral lobe of the thyreoid gland, under and medial to the m. constrictor pharyngis inferior, to the level of the articulatio cricothyreoidea, where it divides into a lateral and posterior branch ; the former anastomosing with the n. laryngeus superior (ramus internus) and innervating the mm. cricoarytaenoideus lateralis, thyreoarytaenoideus, aryepiglotticus, and thyreoepiglotticus; the latter innervating the cricoarytaenoideus posterior and interarytaenoideus (figs. 33 and 36).
Nicolas ('94) describes a ganglion on the superior laryngeal nerve after it enters through the thyrohyoid membrane at the level of the arytaenoid eminences. In a couple of sections I have noticed tissue somewhat suggestive of such a structure, but have not investigated the matter carefully enough to add any thing further.
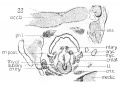
Embryo No. 128
Embryo no. 128, 19.5 mm. Frontal sections
The larynx shows about the same conditions found in the preceding 20 mm. embryo. It was selected for study, before wax reconstructions were undertaken, and it was thought that a study of the various laryngeal structures, when cut in a different plane, would shed further light on some of the more doubtful features.
The cartilages are developed to practically the same extent as in Embryo 22 (20 mm.) and an idea of their appearance in frontal section, can be obtained by reference to figs. 21, 22, 23. There are no differences in the cartilagenous growth of sufficient importance to warrant a detailed description. The high degree of chon-drification can be noticed in figs. 21, 22, 23.
The nerves likewise offer no difficulty for study, and can be followed to their respective terminations. For further details reference is made to the results obtained from a study of Embryo 22 (20 mm.).
A word might be said about the muscles, since the cutting into frontal sections presents at least this feature of interest. The conception of the laryngeal musculature as a sphincter laryngeus, as Strazza expressed it; with a definite horizontal direction to its fibers, expecially emphasized by Kanthack, is not borne out at all by a study of this embryo. The muscles like the cricoarytaenoideus lateralis and thyreoarytaenoideus have a frontal direction to their fibers, as seen in fig. 21 and 22 and by no means any horizontal trend. Moreover the muscles are all clearly differentiated, as much so as they are in the adult, and give no indication whatsoever of being related or connected with the pharyngeal constrictors.
Conclusions
- Towards the end of the fifth week (?) (10.5 mm.) of embryonic life, the integral structures of the larynx, under which I include the precartilage masses and the premuscles masses, first make their appearance. The laryngeal nerves can be recognized but have not yet entered the interior of the larynx, although the recurrent laryngeal has pushed upward as far as the lower portion of the cricoarytaenoideus muscle. The rudiments of the cricoid and thyreoid and epiglottic cartilages can be identified. The arytaenoids have not made their appearance. Four premuscles masses are present, two on each side of the larynx; all are independent of each other. The upper group contains the cricothyreoideus, cricoarytaenoideus lateraHs and thyrearytaenoideus. The lower mass, the cricoarytaenoideus posterior is well differentiated. The interarytaenoideus has not yet appeared nor the aryepiglotticus nor the thyreoepiglotticus. There is apparently no relation with the pharyngeal constrictors.
- During the following week, the sixth week of embryonic life (14 and 16 mm.) the cartilages though still existing purely as condensed mesenchj^ma, have developed into distinctly outlined masses; the arytaenoids have made their appearance during this time. The muscles have grown with considerable rapidity, so that by the end of the sixth week, they can be well differentiated from each other; have received all their innervation, and have developed abundant fibrillation. The thyreoepiglotticus and aryepiglotticus are still absent however. The nerves have advanced correspondingly and can be traced to their final terminations, and the anastomosis between the inferior and superior laryngeal nerves has been accomplished. In comparison to the adult form, the larynx at this stage gives an impression of being crowded upon itself.
- By the end of the seventh week (?) (20 mm.) the larynx has assumed its adult relationships, both externally with regard to neighboring organs and internally with respect to its various constituents. All the cartilages with the exception of those of Wrisberg and Santorini are well developed. All the muscles are clearly represented, capable of absolute differentiation; and the gross nerve supply of the region is likewise complete.
- The existence of such a sphincter as Strazza describes seems very doubtful indeed, as there are no clear indications of it in the specimens studied, and the very first indication of the laryngeal musculature shows several independent masses.
- Finally I desire to record my strong impression that the human larynx develops as a unit ; that its cartilages and muscles are dependent or interrelated with the pharynx or the tongue in their development, in no further degree, than is the natural association of one part of the body with a contiguous portion.
Footnotes
- ↑ 24th Session. Amer. Assoc, of Anat., Baltimore, Md., December 1908-January 1909. Lisser: Models to show development of human larynx.
- ↑ Folio. viiiR I lie ilea of Dr. Lewis, I have used the word fibrillation, not with the view of indicatinfi; fibrillae. but with reference to the definite direction of the muscle fibres.
Bibliography
Chronologically arranged
Fleischmann 1820 De chondrogenesi asperae arteriae Erlangae.
Theile, F. G. 1825 De musculis nervigque laryngis 4° Jenae.
Cavasse 1833 vSur les fractures traumatiques du larynx, These de Paris.
Henle 1839 Vergleichend — anatomische Beschreibung desKehlkopfsmitbesondere Beriicksichtigung des Kehlkopfs der Reptilien. Leipzig.
Remak 1844 Neurologische Erlaiiterungen - Muller's Archiv f. Anatomie p. 463.
Arnold 1851 Handbuch der Anatomie des Menschen. Bd. 2, p. 1317.
Halbertsma 1860 De lamina mediana cartilaginis thyreoid. Verslagen Mededeelingen d.k. Akad van Wetenschappen Natuurkunde — Diel xi (Analyse dans Henles' Bericht f. 1860.
Rambaud et Renault 1864 Origine et developpement des os Paris, p. 245.
Henle 1866 Handbuch d syst. Anatomie d. Menschen, Bd. 2, p. 234.
Verson 1871 Der Kehlkopf und Trachea — Strieker's Handbuch, Bd. 1, p. 461.
LuscHKA 1871 Kehlkopf des Menschen — Tiibingen, p. 56 et p. 67.
FtJRBRiNGER M. 1875 Beitrag zur Kenntniss der Kehlkopfumskulatur, Jena.
Roth 1878 Der Ivehldeckel und die Stimmritze im Embryo Schenks Mittheilungen. Heft. 2, p. 245.
ScHOTTELius 1879 Die Kehlkopf — Knorpel Wiesbaden, p. 7.
Sappey 1879 Traite' d'anatomie, T. IV.
Ganghofner, F. 1880 Beitrage zur Entwickelungsgeschichte des kehlkopfes Zeitschrift f. Heilkunde Bd. 1, p. 187, Prag. ii 400 2 pi.
Kolliker1882 Embryologie de I'homme - Trad Schneider, Paris, p. 905.
Chievitz 1882 Untersuchungen iiber die Verknocherung der menschlichen Kehlknorpel — Archiv fur Anat. u. Physiol. (Anat. Abth.) p. 302.
Ledouble, A. 1884 Des muscles anomaux et des diucis modes de conformation des muscles normaux de larynx dans c' espice humaine et de lens homologues dans la serie animale - A - internat. de laryngol Amee' 7 N2, p. 1-40.
Tourneux 1885 Sur le developpment de 1' epithelium et des glandes du larynx et de la trachee chez I'homme. Comptes rendus de la Soc de biol. Aout. 1885,
Dubois 1886 Zur Morphologic des Larynx Anat. Anz. Bd. 1, p. 178.
Kain 1887 Zur Morphologie des Wrisberg'schen Knorpels Mittheikmgen des Vereins der Aerzte in Steiermark 23 Vereinsjahr, 1886 Graz. 188f.
Strazza 1889 Zur lehre von der Entwicklung der Kehlkopf Muskuhitur. Schenk'sMitteil a. d. embryol Inst. d'Universitat Wien. Jalirg. 1888.
Gegenbaur Traite'd'anatomie Humaine Trad. Julin, p. 615.
Bland Sutton On the nature of ligaments, part 6 — Jour. Anat. Physiol, vol 23, part 2, p. 256.
Gegenbaur 1892 Die Epiglottis, Leipzig.
Wilder 1892 Studies in the phylogenesis of the larynx. Anat. Anz. Jahrg. 7, p. 570.
Kanthack, a. a. 1892 The myology of the larynx. Jour. Anat. Physiol. V26, p. 279-294.
Merkel, Fr. 1894 Handbuch der topographischen Anatomie Bd. 2, Heft. 1, p. 55.
GoppERT 1894 Ueber die Herkunft des Wrisberg'schen Knorpels. Ein Beitrag zur vergleichender Anatomie des Saugethierkehlkopf's - Morphol. Jahrbuch, Bd. 21, Heft, i, p. 68.
Nicolas, A. 1894 Recherches sur le developpement des quelques elements du larynx humaine. Bibliog. anat. no. 5.
Kollmann, J. 1898 Lehrbuch der Entwicklungsgeschichte des Menschen-Jena - Verlag von Gustav Fischer, p. 297.
KuTTNER A, Katzenstein, J. 1901 Ueber den Musculus cricothyreoideus Monatisch Ehrenheilk. Jhrg. 35, no. 5, p. 212-213.
Muller, Jorgen and Fischer, J. F. 1903 Ueber die Wirkung des Mm. cricothyreoideus and thyreo-arytaenodeus internus — Arch. Laryngol u Rhinol. Bd. 15, 70-76.
Sewell, R. B., Seymour 1905 Small or superficial thyroarytaenoideus muscle. Jour. Anat. Physiol., vol. 39, p. 301-307.
McMuRRiCH, J. Playfair 1907 The development of the human body, 3rd edition, Phila., p. 215-216.
Frazear, J. Ernest 1910 The development of the larynx, Jour. Anat. Physiol., Jan.
Figures
Fig 1 Cross section of human Embryo no. 109 (10.5.) to show cricoid cartilage and M. criooarytaenoideus posterior.
Fig 2 Cross section of human Embryo no. 109 (10.5mm.) to show thyreoid cartilage, M. criooarytaenoideus lateralis,
Fig 3 Frontal section of human Embryo no. 317 (12.5 mm.) to show epiglottis thyreoid cartilage, and hyoid bone. Ant. Card., anterior cardinal vein.
Fig 4 Frontal section of human Embryo no. 317 (12.5 mm.) to show superior laryngeal nerve, sup. /ary., superior laryngeal nerve; car. a., carotid artery.
Fig 5 Frontal section of human Embryo no. 317(12.5 mm.) to show M. crico-thyreoideus and arytaenoid masses.
Fig 6 Frontal section of human Embryo no. 317 (12.5 mm.) to show M. cricoary taenoideus posterior, interarytaenoideus and nerve recurrens.
Fig 7 Sagittal section of human Embryo no. 144 (14 mm.) to show, especially, M. interarytaenoideus.
Fig 8 Sagittal section of human Embryo no. 144(14 mm.) to show hyoid bone and thyreoid cartilage, M. cricothyreoideus, and tongue region
Fig 9 Sagittal section of human Embryo no. 144 (14 mm.)
Fig 10 Graphic reconstruction of cricoid and arytaenoid cartilages in human Embryo no. 43 (16 mm.).
Fig 11 Graphic reconstructions of pharyngeal constrictors in human Embryo no. 43 (16 mm.)
Fig 12 Graphic reconstruction of laryngeal musculature in human Embryo no. 43 (16 mm.).
Fig 13 Graphic reconstruction of thyreoid cartilage hyoid bone, and styloid process in human Embryo no. 43 (16 ram.).
Fig 14 Graphic reconstructions of larynx cartilages in human Embryo no. 43 (16 mm.).
Fig. 15 Sagittal section of human Embryo no. 43 (16 mm.) to show laryngeal musculature and nerve recurrens. Meek, c, Meckel's cartilage.
Fig. 16 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region, s. m. gl., submaxillary gland.
Fig. 17 Graphic reconstruction of 9th, 10th, and 12th cranial nerves in larynx region of human Embryo no. 43 (16 mm.), ana., anastomosis between superior laryngeal and inferior laryngeal nerves; m br., motor branch of superior laryngeal nerve.
Fig. 18 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region.
Fig. 19 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region, s. m. gangl., submaxillary ganglion.
Fig. 20 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region.
Fig. 21 Frontal section of human Embryo no. 128 (19.5 mm.) to show thyreoid cartihige and M. cricoarytaenoideus lateralis, occ. h., occipital bone.
Fig. 22 Frontal section of human Embryo no. 128 (19.5 mm.) laryngeal muscles and cartilages.
Fig. 23 Frontal section of human Embryo no. 128 (19.5 mm.) to show cricoid cartilage, M. cricoarytaenoideus posterior, and thyreoid gland.
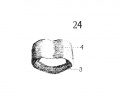
Fig. 24 Graphic reconstruction of cricoid cartilage, posterior veiw, in human Embryo no. 22 (20 mm.) 3, anterior arch; J!^, posterior arch.
Fig. 25 Graphic reconstruction of cricoid cartilage, lateral veiw in human Embryo no. 22 (20 mm.). /, articular facet for thyreoid cartilage; 2, articular facet for arytaenoid cartilage.
Fig. 26 Embryo no. 22 same as fig. 25, showing extent of choudrification. tr. c, true cartilage; pre. c, pre-cartilage (condensed mesenchyma).
Fig. 27 Graphic reconstruction thyreoid cartilage in human Embryo no. 22(20 mm.) showing extent of chodrification.
Fig. 28 Graphic reconstruction of laryngeal cartilages in human Embryo no. 22 20 mm.) thy. hy., 1. thyreohyoid ligament.
Fig. 29 Cross section of human Embryo no. 22 (20 mm.) to show especially superior laryngeal nerve and nerve recurrens.
Fig. 30 Cross section of human Embryo no. 22 (20 mm.) to show, especially, M. interarytaenoideus and aryepiglotticus. This section shows at the mark (x) the tendency to continuity between the laryngeal and pharj'ngeal musculature, as - mentioned by Strazza.
Fig. 31 Cross section of human Embryo no. 22 (20 mm.) (very low in laryngeal region).
Fig. 32 Cross section of human Embryo no. 22 (20 mm. ) to show thyreoid cartilage, cricoid cartilage, and hyoid bone. Also M's cricoarytaenoideus posterior and thyreorarytaenoideus.
Fig. 33 Graphic reconstruction of nerve recurrens and its branches in relation to the laryngeal muscles and cartilages in humanEmbryo no. 22
Fig. 34 Graphic reconstruction of motor branch of superior laryngeal nerve in human Embryo no. 22 (20 mm)
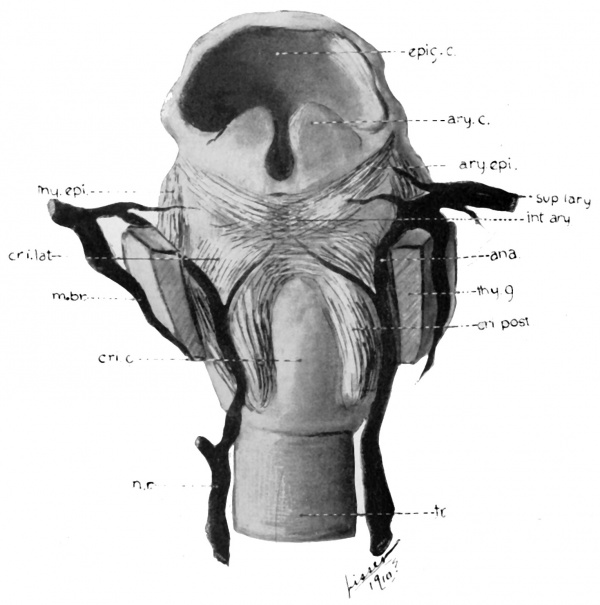
Fig. 35 Graphic reconstructions of laryngeal muscles, and cartilages, and their relations in human Embryo no. 22 (20 mm.) ary. epi., M. aryepiglotticus; thy. epi., M. thyreoepiglotticus; c. s., thy. c, cut surface thyreoid cartilage.
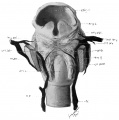
Fig. 36 Wax model of laryngeal muscles and nerves in human Embryo no. 22
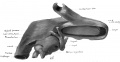
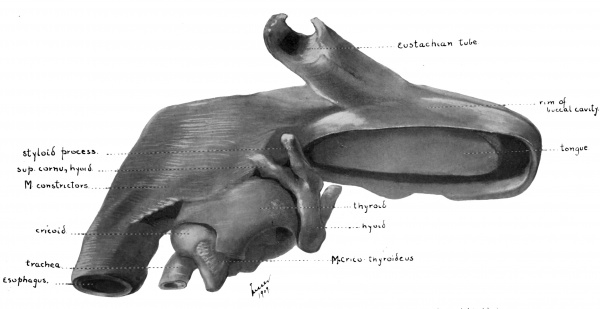
Fig. 37 Wax model of laryngeal region in human Embryo no. 22(20 mm.). (seen from below)
Fig. 38 Wax model of laryngeal region in human Embryo no. 22 (20 mm.). (drawn from the left side)
Fig. 39 Wax model of laryngeal region in human Embryo no. 22 (20 mm.). (drawn from below)
Fig. 1 Cross section of human Embryo no. 109 (10.5.) to show cricoid cartilage and M. criooarytaenoideus posterior. V. C, vertebral cohimn; Esoph. L., Lumen of esophagus; constr., constrictor muscle; cri. post., M. criooarytaenoideus posterior; n. r., nerve recurrens; cri. c, cricoid cartilage; thy. gl., thyreoid gland; B. C, buccal cavity; L. L., larynx lumen.
Fig. 2 Cross section of human Embryo no. 109 (10.5mm.) to show thyreoid cartilage, M. criooarytaenoideus lateralis, M. thyreoarytaenoideus. Ph. L., Pharynx lumen; S. N. S., sympathetic nervous system; thy. c, thyreoid cartilage; n. XII, n. hypoglossus; cri. lat., M. cricoarytaenoideus lateralis; thy. ary., M. thyreoarytaenoideus.
Fig. 3 Frontal section of human Embryo no. 317 (12.5 mm.) to show epiglottis thyreoid cartilage, and hyoid bone. Ant. Card., anterior cardinal vein.
Fig. 4 Frontal section of human Embryo no. 317 (12.5 mm.) to show superior laryngeal nerve, sup. /ary., superior laryngeal nerve; car. a., carotid artery. (The cricoarytaenoideus lateralis mass {eri. lat.) includes whatever there is of the thyreoarytaenoideus muscle.)
Fig. 5 Frontal section of human Embryo no. 317 (12.5 mm.) to show M. crico-thyreoideus and arytaenoid masses. Ary. m., arytaenoid masses; cri. thy., M. cricothyreoideus.
Fig. 6 Frontal section of human Embryo no. 317 (12.5 mm.) to show M. cricoary taenoideus posterior, interarytaenoideus and nerve recurrens. tr., trachea; int. ary.. M. interarytaenoideus.
Fig. 7 Sagittal section of human Embryo no. 144 (14 mm.) to show, especially, M. interarytaenoideus.
Fig. 8 Sagittal section of human Embryo no. 144 (14 mm.) to show hyoid bone and thyreoid cartilage, M. cricothyreoideus, and tongue region, thy. hy., M. thyreohyoidcu.s; umo. hy., M. omohyoideus; mylo. hy., M. mylohyoideus; sterno. thy., M. sternothyreoideus; sterno. hy., M. sternohyoideus" dig., M. digastricus; gen. gl., M. genioglossus; X, N. vagus.
Fig. 9 Sagittal section of human Embryo no. 144 (14 mm.) to show M. Cricoarytaenoideus lateralis, thyreoarytaenoideus, cricoarytaenoideus posterior, and nerve recurrens. ary. c, arytaenoid cartilage; esoph. m., esophageal musculature; gen. hy., M. geniohyoideus.
Fig. 10 Graphic reconstruction of cricoid and arytaenoid cartilages in human Embryo no. 43 (16 mm.).
Fig. 11 Graphic reconstructions of pharyngeal constrictors in human Embryo no. 43 (16 mm.), m. constr., middle constrictor muscle; inf. constr., inferior constrictor muscle.
Fig. 12 Graphic reconstruction of laryngeal musculature in human Embryo no. 43 (16 mm.). (Thyreoid cartilage partly removed).
Fig. 13 Graphic reconstruction of thyreoid cartilage hyoid bone, and styloid process in human Embryo no. 43 (16 ram.).
Fig. 14 Graphic reconstructions of larynx cartilages in human Embryo no. 43 (16 mm.). .s»/). c, superior cornu (thyreoid cart.) ; inf. c, inferior cornu (thyreoid cart.).
Fig. 15 Sagittal section of human Embryo no. 43 (16 mm.) to show laryngeal musculature and nerve recurrens. Meek, c, Meckel's cartilage.
Fig. 16 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region, s. m. gl., submaxillary gland.
Fig. 17 Graphic reconstruction of 9th, 10th, and 12th cranial nerves in larynx region of human Embryo no. 43 (16 mm.), ana., anastomosis between superior laryngeal and inferior laryngeal nerves; m br., motor branch of superior laryngeal nerve.
Fig. 18 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region.
Fig. 19 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region, s. m. gangl., submaxillary ganglion.
Fig. 20 Sagittal section of human Embryo no. 43 (16 mm.) laryngeal region.
Fig. 21 Frontal section of human Embryo no. 128 (19.5 mm.) to show thyreoid cartihige and M. cricoarytaenoideus lateralis, occ. h., occipital bone.
Fig. 22 Frontal section of human Embryo no. 128 (19.5 mm.) laryngeal muscles and cartilages.
Fig. 23 Frontal section of human Embryo no. 128(19.5 mm.) to show cricoid cartilage, M. cricoarytaenoideus posterior, and thyreoid gland.
Fig. 24 Graphic reconstruction of cricoid cartilage, posterior veiw, in human Embryo no. 22 (20 mm.) 3, anterior arch; J!^, posterior arch.
Fig. 25 Graphic reconstruction of cricoid cartilage, lateral veiw in human Embryo no. 22 (20 mm.). /, articular facet for thyreoid cartilage; 2, articular facet for arytaenoid cartilage.
Fig. 26 Embryo no. 22 same as fig. 25, showing extent of choudrification. tr. c, true cartilage; pre. c, pre-cartilage (condensed mesenchyma).
Fig. 27 Graphic reconstruction thyreoid cartilage in human Embryo no. 22 (20 mm.) showing extent of chodrification.
Fig. 28 Graphic reconstruction of laryngeal cartilages in human Embryo no. 22 20 mm.) thy. hy., 1. thyreohyoid ligament.
Fig. 29 Cross section of human Embryo no. 22 (20 mm.) to show especially superior laryngeal nerve and nerve recurrens.
Fig. 30 Cross section of human Embryo no. 22 (20 mm.) to show, especially, M. interarytaenoideus and aryepiglotticus. This section shows at the mark (x) the tendency to continuity between the laryngeal and pharj'ngeal musculature, as - mentioned by Strazza.
Fig. 31 Cross section of human Embryo no. 22 (20 mm.) (very low in laryngeal region).
Fig. 32 Cross section of human Embryo no. 22 (20 mm. ) to show thyreoid cartilage, cricoid cartilage, and hyoid bone. Also M's cricoarytaenoideus posterior and thyreorarytaenoideus.
Fig. 33 Graphic reconstruction of nerve recurrens and its branches in relation to the laryngeal muscles and cartilages in human Embryo no. 22 (20 mm.) br. 1, Branch of n. recurrens, to M. cricoarytaenoideus posterior; hr. 2, branch on n. recurrens to M.'s cricoarytaenoideus lateralis and thyreoarytaenoideus; br. 3, branch of n. recurrens to M. thyreoepiglotticus; hr. 4, branch of n. recurrens to M.'s interarytaenoideus and aryepiglotticus.
Fig. 34 Graphic reconstruction of motor branch of superior laryngeal nerve in human Embryo no. 22 (20 mm. ) .
Fig. 35 Graphic reconstructions of laryngeal muscles, and cartilages, and their relations in human Embryo no. 22 (20 mm.) ary. epi., M. aryepiglotticus; thy. epi., M. thyreoepiglotticus; c. s., thy. c, cut surface thyreoid cartilage.
Fig. 36 Wax model of laryngeal muscles and nerves in human Embryo no. 22 (20 mm.). Drawn from the posterior aspect; the thyreoid cartilage has been partly removed above and posteriorly to admit of clearer view of muscles and nerves.
Fig. 37 Wax model of laryngeal region in human Embryo no. 22 (20 mm.). (seen from below)
Fig. 38 Wax model of laryngeal region in human Embryo no. 22 (20 mm.). (seen from below)
Fig. 39 Wax model of laryngeal region in human Embryo no. 22 (20 mm.). (seen from below)
List of Abbreviations
V. C, vertebral column
Esoph. L., lumen of esophagus
L. L., larynx lumen
B. C, buccal cavity
Ph. L., pharynx lumen
- S. N. S., sympathetic nervous system
Tr., trachea
constr., constrictor muscle
m. constr., middle constrictor muscle
inf. constr., inferior constrictor muscle
cri. post., M. cricoarytaenoideus posterior
cri. lat., M. cricoarytaenoideus lateralis
thy. ary., M. thyreoarytaenoideus
cri. thy., M. cricothyreoideus
int. ary., M. interarytaenoideus
ary. epi., M. aryepiglotticus
thy. epi., M. thyreoepiglotticus
thy. hy., M. thyreohyoideus
omo. hy., M. omohyoideus
mylo. hy., M. mylohyoideus
sternothy., M. sternothyreoideus
sternohy., M. sternohyoideus
dig., M. digastricus
gen. gl., M. genioglossus
gen. hy., M. geniohyoideus
esoph. TO., esophageal musculature
ary. m., arytaenoid masses
cri.c, cricoid cartilage
thy. c, thyreoid cartilage
hy. b., hyoid bone
g. c, greater cornu, hyoid bone
sty. p., styloid process
epig., epiglottis condensation
ary. c, arytaenoid cartilage
sup. c, superior cornu, thyreoid cartilage
inf. c, inferior cornu, thyreoid cartilage
Meek, c, Meckel's cartilage
occ. b., occipital bone
tr. c, true cartilage
pre. c, pre-cartilage
c. s. thy. c, cut surface thyreoid cartilage
n. v., nerve recurrens
n. XII., N. hypoglossus
sup. lary., N. laryngeus superior
n. X., n. vagus
ana., anastomosis between superior laryngeal and inferior laryngeal nerves
TO. br., motor branch of superior laryngeal nerve
thy. gl., thyreoid gland
s. TO. gl., submaxillary gland
s. TO. gangl., submaxillary ganglion
p. thy., parathyreoid gland
thy. hy. 1, thyreohyoid ligament
car. a., carotid artery.
ant. card., anterior cardinal vein
Cite this page: Hill, M.A. (2024, April 26) Embryology Paper - Studies on the development of the human larynx (1911). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_Studies_on_the_development_of_the_human_larynx_(1911)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G