Molecular Development - Genetics
| Embryology - 1 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Genetics (Greek, genetikos = “origin”) and embryology have merged to such an extent that the two cannot now be separated from each other. The strong evolutionary conservation of developmental mechanisms has been astounding. Currently, much of modern medicine is a search for a disease gene and having found it, embryology is usually employed in understanding its mechanism. Embryological development begins with meiosis and is after all the opportunity for a specific genome to be expressed, regulated and utilized as it will never be again in the adult animal.
Humans have genetic information stored within the nucleus and within mitochondria. The nuclear chromosomes consist of 22 autosome pairs and 2 gonadosomes (sex chromosomes; X, Y). The mitochondrial genome consists of multiple copies of circular DNA 16,569 base pairs in length.
In combination with our statistical understanding of congenital abnormalities there now exist a large number of clinical tests for inherited abnormalities. This particular section of the notes is a link to bind our understanding of genetics with its relevance to development. There are several pages on DNA and links from the computer activities below to relevant sections. You can jump right in and look through the DNA database for a gene of interest using a keyword, or browse through the human genome by chromosome or by genetic diseases that have been identified. Or you can work through an exercise in using genetic databases for diseases.
See also the list of mouse gene knockouts which has made the geneticists not only use embryological tools, but also go back and learn some embryology. Recent research has also focussed on the new science of Epigenetics, the inheritance mechanisms that lie outside the actual DNA sequence of our genes.
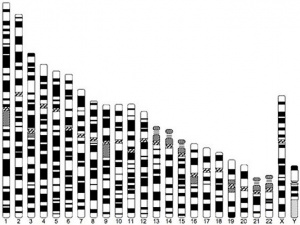
| Human Chromosomes: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | X | Y |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Developmental Genetics | Embryology Genetics | Mitochondrial Genetics | trisomy |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page. |
Diploid Chromosome Number
| Species | Chromosome Number |
|---|---|
| Human (Homo sapiens) | 46 |
| Mouse (Mus musculus) | 40 |
| Fruit fly (Drosophila melanogaster) | 8 |
| Worm (Caenorhabditis elegans) | 12 |
| Frog (Xenopus laevis) | 36 |
| Dog (Canis familiaris) | 78 |
| Chicken (Gallus gallus) | 78 |
| Echidna (Tachyglossus) | 63 male 64 female |
| Cow (Bos primigenius) | 60 |
| Species Chromosome Number | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
| ||||||||||||||||||||
| Links: Genetics |
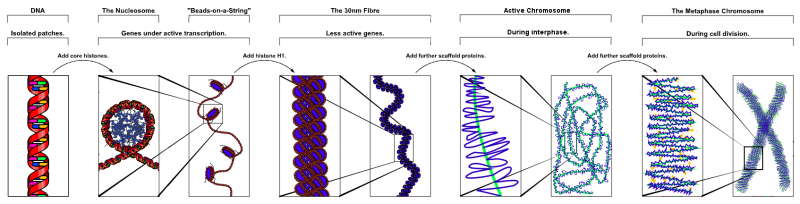
Chromatin Structure
| Cells not undergoing cell division have their DNA dispersed throughout the nucleus in what are know as "chromosomal domains". There is also a peripheral nuclear rim area that does not contain gene-rich regions of DNA, which tend to be located in the core of the nucleus. |  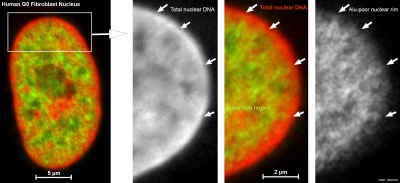
Adult G0 fibroblast DNA and gene localization.[3] |
Cells undergoing division have their DNA compacted into chromosomes with a short arm (p), a long arm (q), and a mid-section (centromere). The duplicated chromosomes are also joined together as pairs at the centromere. These chromosomal arms are only seen when the chromosome is folded for cell division.
These letters prefixed by the chromosome number and followed by the chromosome band number, indicate gene location. |
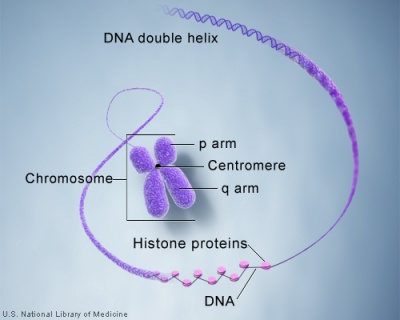
Chromosome pair structure |
Chromosome Banding
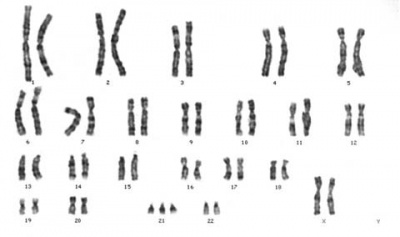
The term refers to the light and dark pattern, seen after staining with a dye, of individual chromosomes identified in metaphase. It is only in meiosis and mitosis during metaphase that chromosomes can be easily identified, during the normal cell life (interphase) the chromosomes are unravelled and distributed within the nucleus in chromosome territories. A band is that part of a chromosome which is clearly distinguishable from nearby regions by appearing darker or brighter with one or more banding techniques.
Depending on the type of stain used a number of different banding patterns can be seen:
- G-banding - banding pattern seen by treating with trypsin and then staining with the dye giemsa.
- R-banding - banding pattern seen as a of reverse giemsa chromosome banding, producing bands complementary to G-bands often used to determine whether there are deletions. Can be fluorescent using the dye acridine orange.
- Q-banding - banding pattern seen by treating with a fluorochrome or the fluorescent dye quinacrin.
- C-banding - banding pattern seen for centromeric or constitutive heterochromatin, the centromere appears as a stained band compared to other regions.
Metaphase is a cell division term referring to the third mitotic stage, mitotic spindle kinetochore microtubules align chromosomes in one midpoint plane. Metaphase ends when sister kinetochores separate. Originally based on light microscopy of living cells and electron microscopy of fixed and stained cells. A light microscope analysis called a "metaphase spread" was originally used to detect chromosomal abnormalities in cells.
- Links: Histology Stains
Human Genome
Human Genome Length 3,101,788,170 bp. Mitochondrial Genome 16,568 bp.
| Human Chromosomes: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | X | Y |
- Links: Human Genome Project (HGP) | NCBI Human Genome Resources | History of the Human Genome Project | Project Timeline
Nuclear Genome
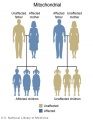
Mitochondrial Genome
- In humans this genome is maternally inherited.
- Exists as multiple copies within the matrix of each mitochondrion within the cytoplasm of cells.
- In 1981 the human mitochondrial genome was sequenced.
- The genome is a small circular DNA molecule 16,568 bp in length containing 37 genes.
- 24 genes specify RNA molecules involved in protein synthesis (22 transfer RNAs (tRNA) and 2 ribosomal RNAs (rRNA))
- 13 genes encode proteins required for the biochemical reactions that make up respiration.
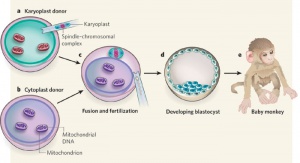
Swapping mitochondrial DNA mammalian oocytes[4] Assisted Reproductive Technology
Chromosome Regions
Nucleolar Organizer Regions
On the p-arms of acrocentric human chromosomes (13, 14, 15, 21, and 22) are located the nucleolar organizer regions (NORs), that contain the ribosomal gene (rDNA) arrays.
Chromosome Abnormalities
Ploidy
| ICD-11 LD42 Polyploidies |
|---|
| LD42.0 Triploidy - A disease caused by one additional set of chromosomes, for a total of 69 chromosomes. Triploidy can present with albuminuria, edema, or hypertension in the mother. The fetus may present with microcephaly and a placenta that is enlarged and filled with cysts in the case of extra maternally inherited chromosomes, while extra paternally inherited chromosomes cause severe growth problems, an enlarged head, and a small placenta that does not have cysts. Non-mosaic triploidy is highly lethal, and is rarely observed in live births. Confirmation is through observation of an additional set of chromosomes by karyotyping.
LD42.1 Tetraploidy - A disease caused by two additional sets of chromosomes, for a total of 92 chromosomes. This disease commonly results in spontaneous abortion during the first trimester. Live births of tetraploidy individuals are very rare. These cases are characterized by facial dysmorphism, severely delayed growth and developmental delay. Confirmation is through observation of two additional set of chromosomes by karyotyping. |
Ploidy refers to the chromosomal genetic content of cells. Euploidy (euploid) is the genetic term used to describe the normal cell genome chromosomal set (n, 2n, 3n) or complement for a species, in humans this is diploid (2n).
The terms used to describe the classes of numerical chromosomal abnormalities include:
- Aneuploidy are chromosome mutations in which chromosome number is abnormal (increased or reduced), nondisjunction in meiosis or mitosis (anaphase of meiosis I, sister chromatids fail to disjoin at either meiosis II or at mitosis) is the cause of most aneuploids.
- Polyploidy includes triploidy, usually due to two sperm fertilizing a single egg.
- Mixoploidy includes mosaicism, where there are two or more genetically different cell lines in an individual.
Trisomy
Can occur in autosomes and sex chromosomes. Presence of one extra chromosome, for a total of three. The most common form is with teh autosome, Trisomy 21.
- Links: Abnormal Development - Genetic | Trisomy 21 | Trisomy 18 | Trisomy 13
Autosome Deletions
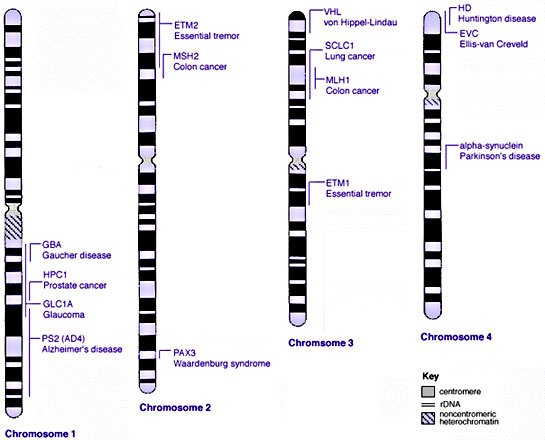
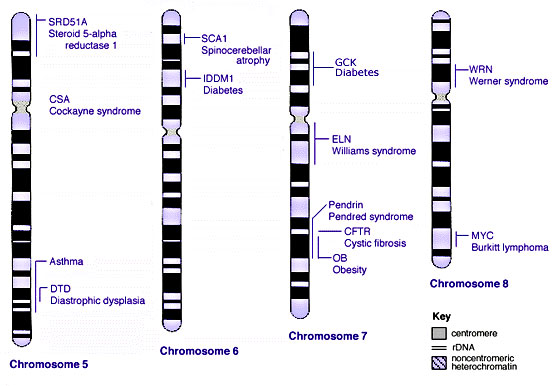
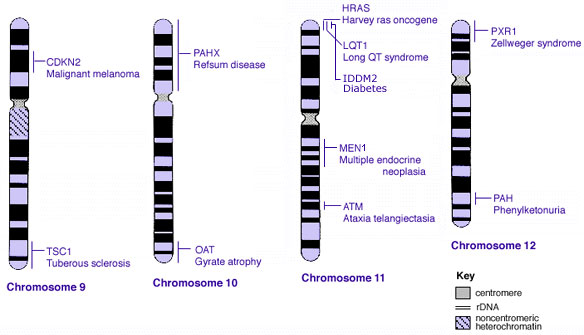
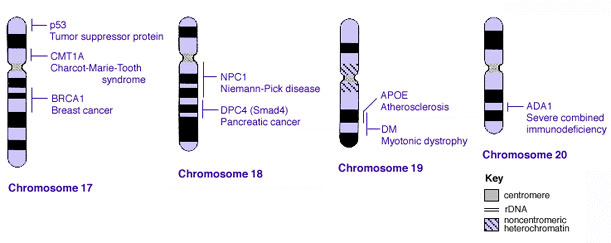
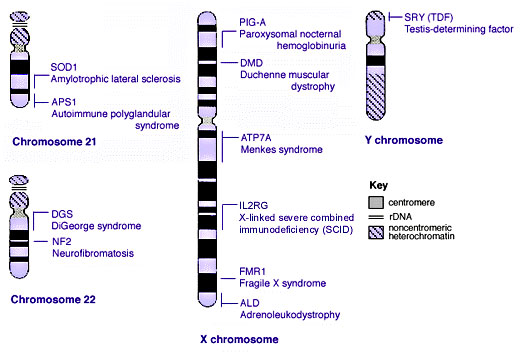
Some Human Disease Gene Locations
Inheritance Genetics
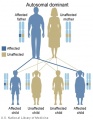
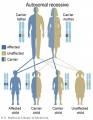
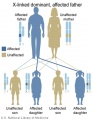
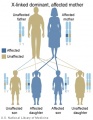
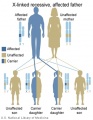
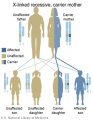
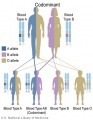
- Inheritance Pattern images: Genetic Abnormalities | autosomal dominant | autosomal recessive | X-linked dominant (affected father) | X-Linked dominant (affected mother) | X-Linked recessive (affected father) | X-Linked recessive (carrier mother) | mitochondrial inheritance | Codominant inheritance | Genogram symbols | Genetics
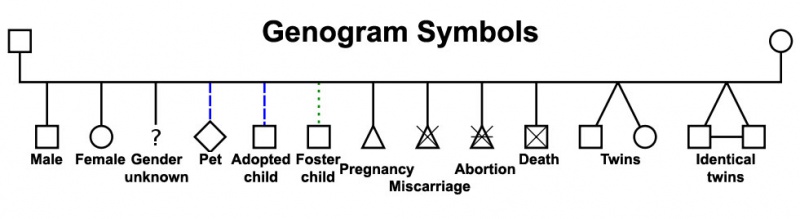
Genogram
A clinical diagram constructed to show an individual's family relationships and medical history, more detailed than a pedigree chart including non-genetic factors such as family emotional and social relationships. Additional colour coded symbols and connectors are used to show these relationships and interactions.
DNA Sequencing
Changes in genes can occur by a number of different methods including mutations, deletions and epigenetic modifications. Specific changes in DNA sequences can also be detected by a range of techniques, including direct sequencing of the DNA.
Recent technological developments have improved how DNA sequencing occurs and we are said to now be up to the "third generation" of sequencing techniques.[5]
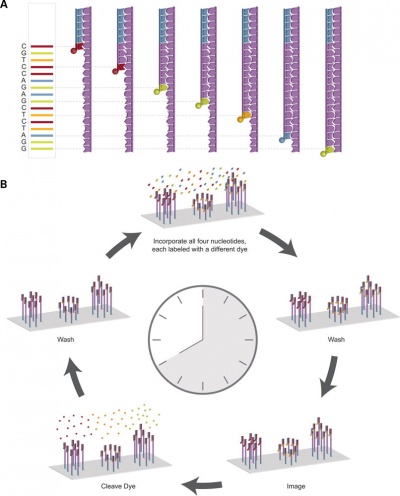
|
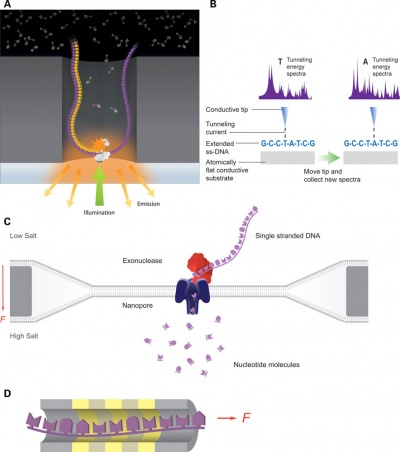
|
| First and second generation sequencing[5] | Third generation sequencing[5] |
Using Genetic Databases
This exercise is an exploration of the available WWW and other database resources that relate to Human genetic diseases. The exercise is to explore the Human genome and its relationship to examples of know human genetic diseases that affect development and impact on health.
To start with, think of a specific Human disease and see what you can find out about:
- Known gene?
- What are the major effects of the disorder?
- Does it have an effect/role in development?
- Known mutations?
- Likely hood of mortality?
- History of the disease?
- Who discovered the cause of the disease?
- Most recent published data relationg to the disease?
- What therapies are being explored?
- Where to next?
Genetic Editing
Originally a more general approach was used to study the fly model of development, where random genome mutation (random mutagenesis) was carried out to identify a specific fly phenotype and then researchers would go back and find the gene that had been altered.
More recently in vertebrate models of development, mainly in mice, genetic editing was carried to to "knock out" (KO mice) a specific gene and then to look for a specific phenotype. Generally targeting known genetic disorder genes, but later a range of genes involved in signalling, proliferation, migration, cell cytoskeleton. This technology has developed to the stage where we can now not only "knock out" but also "knock in" as well as "transiently knock out" (at a specific stage) a specific gene of interest. This KO technology was complex and required long term projects to generate these knock out animal models.
CRISPR
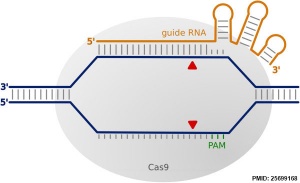
More recently a new technology called CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated nuclease 9) has allowed more accurate and faster genetic editing. There has been concern in the scientific community that this technique may be applied to the human genome, and lead to "germ line" changes in the human genome. See also review.[6]
| CRISPR History |
|---|
Like any great discovery, some contention as to who writes the history...
|
- Links: Nature News 12 March 2015
Other Gene Editing Mechanisms
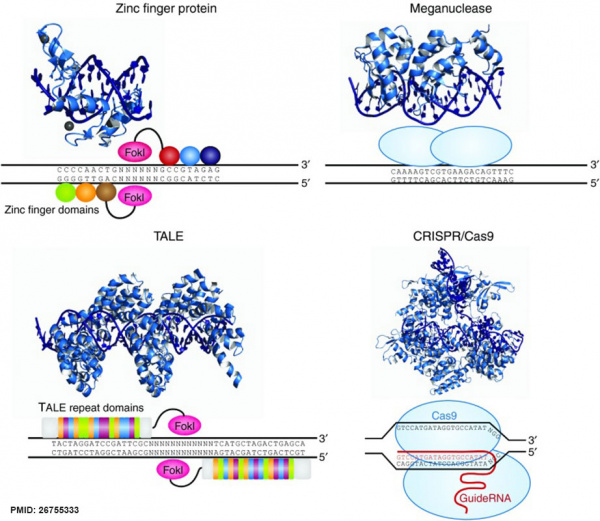
DNA Targeting Platforms for Genome Editing[9]
| Zinc finger nucleases | Meganucleases |
|---|---|
| Zinc finger (ZF) proteins are the most abundant class of transcription factors and the Cys2-His2 zinc finger domain is one of the most common DNA-binding domains encoded in the human genome. The crystal structure of Zif268 has served as the basis for understanding DNA recognition by zinc fingers. In the presence of a zinc atom, the zinc finger domain forms a compact ββα structure with the α-helical portion of each finger making contact with 3 or 4 bp in the major groove of the DNA. Tandem fingers in a zinc finger array wrap around the DNA to bind extended target sequences such that a three-finger protein binds a 9 bp target site. | Meganuclease technology involves re-engineering the DNA-binding specificity of naturally occurring homing endonucleases. The largest class of homing endonucleases is the LAGLIDADG family, which includes the well-characterized and commonly used I-CreI and I-SceI enzymes.140 Through a combination of rational design and selection, these homing endonucleases can be re-engineered to target novel sequences. |
| TALENs | CRISPR/Cas nucleases |
| The discovery of a simple one-to-one code dictating the DNA-binding specificity of TALE proteins from the plant pathogen Xanthomonas again raised the exciting possibility for modular design of novel DNA-binding proteins.114,115 Highly conserved 33–35 amino acid TALE repeats each bind a single base pair of DNA with specificity dictated by two hypervariable residues. Crystal structures of TALEs bound to DNA revealed that each repeat forms a two-helix structure connected by a loop which presents the hypervariable residue into the major groove as the protein wraps around the DNA in a superhelical structure. These modular TALE repeats can be linked together to build long arrays with custom DNA-binding specificities. | CRISPR-Cas RNA-guided nucleases are derived from an adaptive immune system that evolved in bacteria to defend against invading plasmids and viruses. Decades of work investigating CRISPR systems in various microbial species has elucidated a mechanism by which short sequences of invading nucleic acids are incorporated into CRISPR loci. They are then transcribed and processed into CRISPR RNAs (crRNAs) which, together with a trans-activating crRNAs (tracrRNAs), complex with CRISPR-associated (Cas) proteins to dictate specificity of DNA cleavage by Cas nucleases through Watson-Crick base pairing between nucleic acids. Building off of two studies showing that the three components required for the type II CRISPR nuclease system are the Cas9 protein, the mature crRNA and the tracrRNA, Doudna, Charpentier and colleagues showed through in vitro DNA cleavage experiments that this system could be reduced to two components by fusion of the crRNA and tracrRNA into a single guide RNA (gRNA). Furthermore, they showed that re-targeting of the Cas9/gRNA complex to new sites could be accomplished by altering the sequence of a short portion of the gRNA. Thereafter, a series of publications demonstrated that the CRISPR/Cas9 system could be engineered for efficient genetic modification in mammalian cells. Collectively these studies have propelled the CRISPR/Cas9 technology into the spotlight of the genome-editing field. |
(text extract above from original article[9]
References
- ↑ Tang L, Zeng Y, Du H, Gong M, Peng J, Zhang B, Lei M, Zhao F, Wang W, Li X & Liu J. (2017). CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol. Genet. Genomics , 292, 525-533. PMID: 28251317 DOI.
- ↑ Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y, Sun Y, Bai Y, Songyang Z, Ma W, Zhou C & Huang J. (2015). CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell , 6, 363-372. PMID: 25894090 DOI.
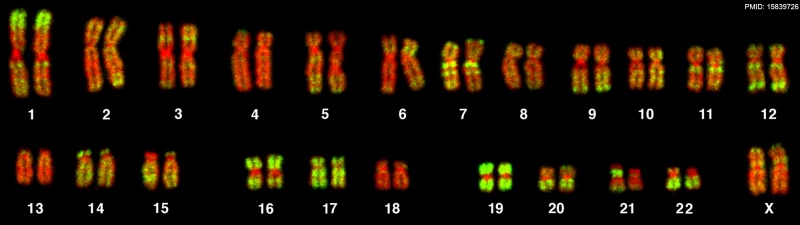
- ↑ Bolzer A, Kreth G, Solovei I, Koehler D, Saracoglu K, Fauth C, Müller S, Eils R, Cremer C, Speicher MR & Cremer T. (2005). Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol. , 3, e157. PMID: 15839726 DOI.
- ↑ Shoubridge EA. (2009). Developmental biology: Asexual healing. Nature , 461, 354-5. PMID: 19759608 DOI.
- ↑ 5.0 5.1 5.2 Schadt EE, Turner S & Kasarskis A. (2010). A window into third-generation sequencing. Hum. Mol. Genet. , 19, R227-40. PMID: 20858600 DOI.
- ↑ Sandve GK, Gundersen S, Johansen M, Glad IK, Gunathasan K, Holden L, Holden M, Liestøl K, Nygård S, Nygaard V, Paulsen J, Rydbeck H, Trengereid K, Clancy T, Drabløs F, Ferkingstad E, Kalas M, Lien T, Rye MB, Frigessi A & Hovig E. (2013). The Genomic HyperBrowser: an analysis web server for genome-scale data. Nucleic Acids Res. , 41, W133-41. PMID: 23632163 DOI.
- ↑ Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA & Charpentier E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science , 337, 816-21. PMID: 22745249 DOI.
- ↑ Lander ES. (2016). The Heroes of CRISPR. Cell , 164, 18-28. PMID: 26771483 DOI.
- ↑ 9.0 9.1 Maeder ML & Gersbach CA. (2016). Genome-editing Technologies for Gene and Cell Therapy. Mol. Ther. , 24, 430-46. PMID: 26755333 DOI.
Online Textbooks
- Genomes (2nd edition) Brown, T.A. New York and London: Garland Science ; c2002 PMID20821850
- Introduction to Genetic Analysis Griffiths, Anthony J.F.; Miller, Jeffrey H.; Suzuki, David T.; Lewontin, Richard C.; Gelbart, William M. New York: W. H. Freeman & Co.; c1999
- Modern Genetic Analysis Griffiths, Anthony J.F.; Gelbart, William M.; Miller, Jeffrey H.; Lewontin, Richard C. New York: W. H. Freeman & Co.; c1999
- Human Molecular Genetics 2 Strachan, Tom and Read, Andrew P. New York and London: Garland Science; c1999
- Genetics for Surgeons Morrison, Patrick J.; Spence, Roy A.J., authors Hatchwell, Eli, series editor London: Remedica; c2000
- Sequence - Evolution - Function: Computational Approaches in Comparative Genomics Koonin, Eugene V; Galperin, Michael Y. Norwell (MA): Kluwer Academic Publishers ; c2003
- The Genetic Landscape of Diabetes [Internet] Dean, Laura; McEntyre, J.R. Bethesda (MD): National Library of Medicine (US), NCBI; 2004 Jun
- Madame Curie Bioscience Database Chapters taken from the Madame Curie Bioscience Database (formerly, Eurekah Bioscience Database) Eurekah.com and Landes Bioscience and Springer Science+Business Media; c2009
Reviews
Salsman J & Dellaire G. (2017). Precision genome editing in the CRISPR era. Biochem. Cell Biol. , 95, 187-201. PMID: 28177771 DOI.
Harrison MM, Jenkins BV, O'Connor-Giles KM & Wildonger J. (2014). A CRISPR view of development. Genes Dev. , 28, 1859-72. PMID: 25184674 DOI.
Götz M & Huttner WB. (2005). The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. , 6, 777-88. PMID: 16314867 DOI.
Articles
Tang L, Zeng Y, Du H, Gong M, Peng J, Zhang B, Lei M, Zhao F, Wang W, Li X & Liu J. (2017). CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol. Genet. Genomics , 292, 525-533. PMID: 28251317 DOI.
Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y, Sun Y, Bai Y, Songyang Z, Ma W, Zhou C & Huang J. (2015). CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell , 6, 363-372. PMID: 25894090 DOI.
Search PubMed
Not easy to generate a good search term for this topic.
Search "Genetic Development" All (151839) Review (31389) Free Full Text (54818)
Search Pubmed: Genetic Development | Genome
Genetic Terms
| Genetic Terms (expand to view) | ||
|---|---|---|
genetic abnormalities | Molecular Development | meiosis | mitosis
| ||
|
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Human Genome Project (HGP) | History of the Human Genome Project | Project Timeline
- NCBI Human Genome Resources
- Online Mendelian Inheritance in Man
- NHGRI Catalog of Published Genome-Wide Association Studies | PDF
- Genome-Wide Associations (GWA) Karyogram
- Idiogram Album David Adler
- Genetic Education Resources for Teachers
- MITOMAP A human mitochondrial genome database.
- Galaxy is an open, web-based platform for data intensive biomedical research. Whether on this free public server or your own instance, you can perform, reproduce, and share complete analyses.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 1) Embryology Molecular Development - Genetics. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Molecular_Development_-_Genetics
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G