Notice - Mark Hill
Currently this page is only a template and will be updated (this notice removed when completed).
Introduction
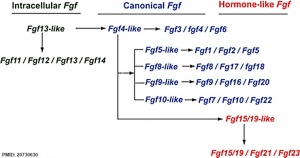
:Fgf gene family evolution
Fibroblast Growth Factors (FGF) were originally identified by their ability to stimulate fibroblast cell proliferation but have a role in a growing number of different tissues development and differentiation and continue to have a role in the adult.
The first two identified factors were originally given the nomenclature of acidic or basic. We now know there to be at least 22 different human FGFs (Fgf1–Fgf23). These protein growth factors are bound by 4 different cell membrane receptors (FGFR1-4). FGFRs belong to the tyrosine kinase receptor family.
The mammalian Fgf family can be divided into the intracellular Fgf11/12/13/14 subfamily (iFGFs), the endocrine hormone-like Fgf15/21/23 subfamily (hFGFs), and the paracrine canonical Fgf subfamilies, including Fgf1/2/5, Fgf3/4/6, Fgf7/10/22, Fgf8/17/18, and Fgf9/16/20.
Protein Properties
Human FGF
- ~150–300 amino acids
- have a conserved ~120-residue core with ~30–60% identity
Some Recent Findings
- FGF-signaling gradient maintains symmetrical proliferative divisions of midbrain neuronal progenitors[1] "For the correct development of the central nervous system, the balance between self-renewing and differentiating divisions of the neuronal progenitors must be tightly regulated. To maintain their self-renewing identity, the progenitors need to retain both apical and basal interfaces. However, the identities of fate-determining signals which cells receive via these connections, and the exact mechanism of their action, are poorly understood. The conditional inactivation of Fibroblast growth factor (FGF) receptors 1 and 2 in the embryonic mouse midbrain-hindbrain area results in premature neuronal differentiation. Here, we aim to elucidate the connection between FGF-signaling and neuronal progenitor maintenance. Our results reveal that the loss of FGF-signaling leads to downregulation of Hes1 and upregulation of Ngn2, Dll1, and p57 in the ventricular zone (VZ) cells, and that this increased neurogenesis occurs cell-autonomously. Yet the cell-cycle progression, apico-basal-polarity, cell-cell connections, and the positioning of mitotic spindle in the mutant VZ appear unaltered. Interestingly, FGF8-protein is highly concentrated in the basal lamina. Thus, FGFs may act through basal processes of neuronal progenitors to maintain their progenitor status. Indeed, midbrain neuronal progenitors deprived in vitro of FGFs switched from symmetrical proliferative towards symmetrical neurogenic divisions. We suggest that FGF-signaling in the midbrain VZ is cell-autonomously required for the maintenance of symmetrical proliferative divisions via Hes1-mediated repression of neurogenic genes."
- Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules[2] "It is widely accepted that tissue differentiation and morphogenesis in multicellular organisms are regulated by tightly controlled concentration gradients of morphogens. How exactly these gradients are formed, however, remains unclear. Here we show that Fgf8 morphogen gradients in living zebrafish embryos are established and maintained by two essential factors: fast, free diffusion of single molecules away from the source through extracellular space, and a sink function of the receiving cells, regulated by receptor-mediated endocytosis. Evidence is provided by directly examining single molecules of Fgf8 in living tissue by fluorescence correlation spectroscopy, quantifying their local mobility and concentration with high precision. By changing the degree of uptake of Fgf8 into its target cells, we are able to alter the shape of the Fgf8 gradient. Our results demonstrate that a freely diffusing morphogen can set up concentration gradients in a complex multicellular tissue by a simple source-sink mechanism."
|
References
- ↑ <pubmed>21074523</pubmed>
- ↑ <pubmed>19741606</pubmed>
Reviews
Articles
<pubmed>20582225</pubmed>
Search Pubmed
Search Bookshelf Fibroblast Growth Factor
Search Pubmed Now: Fibroblast Growth Factor
