Rabbit Development
| Embryology - 7 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
As an embryological tool, the rabbit (Taxon- Oryctolagus cuniculus) along with human was a species which show birth defects with thalidomide (teratogenic effects not detected with prior testing on other species).
These animals are herbivores with a very high breeding rate and number of offspring produced. Rabbit ovulation is induced by mating allowing an accurate staging of embryonic age and pregnancy.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Rabbit Development | Rabbit Embryology | Oryctolagus cuniculus development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Taxon
Oryctolagus cuniculus
Taxonomy Id: 9986 Rank: species
Genetic code: Translation table 1 (Standard) Mitochondrial genetic code: Translation table 2 Other names: New Zealand rabbit[includes], rabbits[common name], European rabbit[common name], Japanese white rabbit[common name], domestic rabbit[common name], rabbit[common name], Lepus cuniculus[misnomer]
Lineage( abbreviated ): Eukaryota; Metazoa; Chordata; Craniata; Vertebrata; Mammalia; Eutheria; Lagomorpha; Leporidae; Oryctolagus
Genome
- The Oryctolagus cuniculus haploid genome is estimated to be 3500 Mb.
- The diploid genome is organized in 21 pairs of autosomes and two sex chromosomes.
- Rabbit gene sequences are more similar to human sequences than rodent ones.
- Links: Genome Project Report
Rabbit Reproductive Cycle
|
|
Developmental Timeline
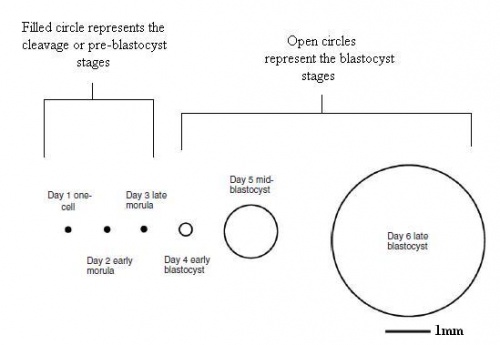
|
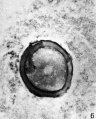
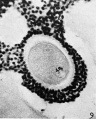
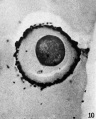
| Early growth of the rabbit morula and blastocyst.[8] |
Early development data from an in vitro development study.[9]
Fertilization - penetration of most ova during the first hour after ovulation.
|
Gastrulation |
Gonad Development
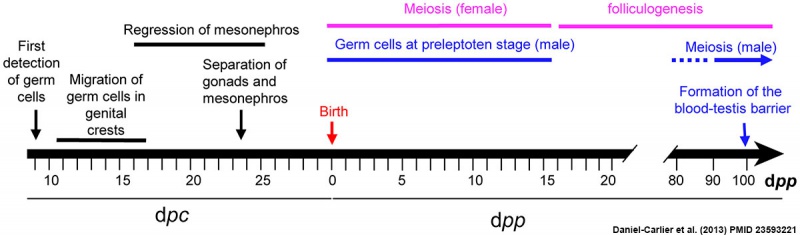
Timeline of rabbit gonad development.[3]
Day post coïtum (dpc)
- 9 - first germ cells are detected in both sexes.
- 14 - gonad macroscopically evident, the mesonephros and gonads are still connected and interactions between tissues are probable.
- 16 - most germ cells already entered the genital ridges (crests).
- 16 to 25 - regression of the mesonephros.
- 23 - gonadal and mesonephric tissues are separated by connective tissue. Thought to prevent the migration of cells and other substances.
Days post partum (dpp)
- birth - XX gonads first signs of meiosis.
- 50 - XY gonads first signs of meiosis.
- 70 - blood-testis barrier is definitively complete.
(text modified from reference[3])
- Links: Genital System Development
Historic Images
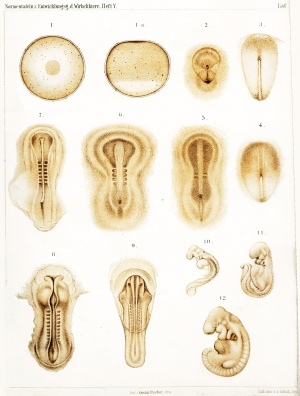
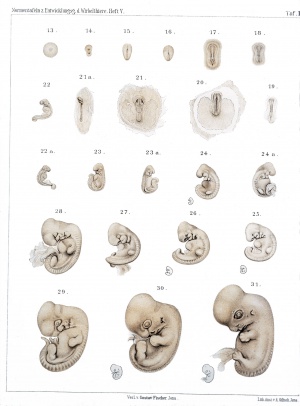
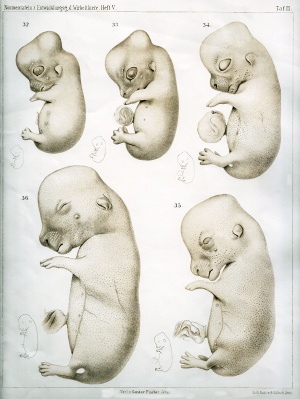
The following plates are from Normal Plates of the Development of Vertebrates Vol. 5. 1905 Rabbit (Lepus cuniculus) by Charles S. Minot and Edwing Taylor.
|
|
|
Pincus G. and Enzmann EV. The Comparative Behavior of Mammalian Eggs in Vivo and in Vitro. (1935) J Exp Med. 62(5):665-75. PMID 19870440
The following drawings were compiled in the textbooks: Bailey, F.R. and Miller, A.M. (1921). Text-Book of Embryology. New York: William Wood and Co; Foster, M., Balfour, F. M., Sedgwick, A., & Heape, W. (1883). The Elements of Embryology. (2nd ed.). London: Macmillan and Co.
|
Rabbit's ovum between 70-90 hours after impregnation, after E. van Beneden. |
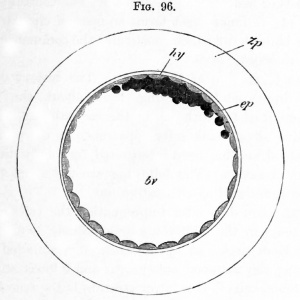
|
Historic drawing of a Transverse sections of embryonic disks of rabbit, (a) Kolliker, (b) Rabl.
|
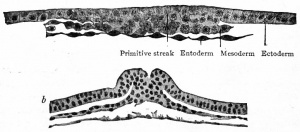
|
Transverse section through primitive groove of rabbit embryo, van Beneden. |
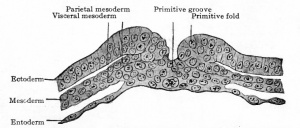
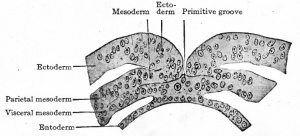
|
Transverse section through primitive groove of rabbit embryo, van Beneden. |
|
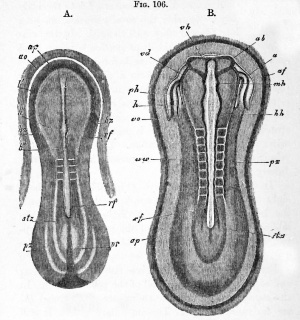
Embryo rabbits of about nine days from the dorsal side, Kolliker. |
|
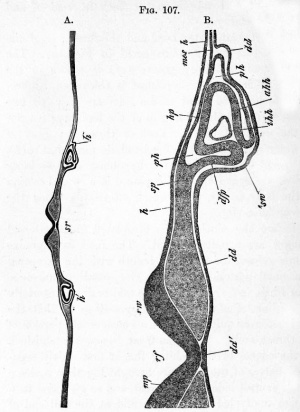
Embryo rabbit of about nine days transverse section through the head, Kolliker. B. is a more highly magnified representation of part of A. |
|
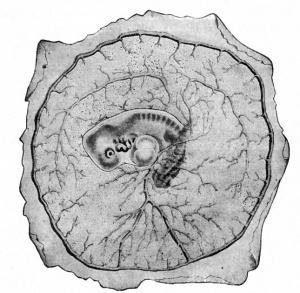
Surface view of area vasculosa of a rabbit embryo of 11 days, van Beneden and Julin. |
|
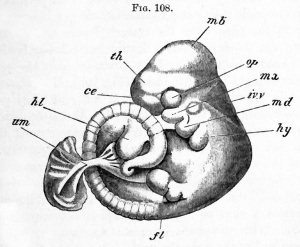
Advanced embryo of a rabbit (about twelve days), by Mr Weldon. |
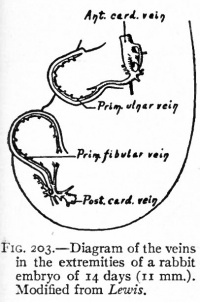
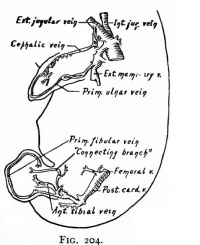
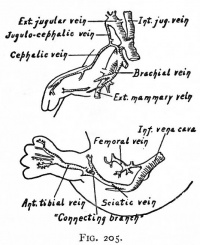
| Limb Vasculature (veins) | ||
|---|---|---|
|
|
|
| Rabbit embryo of 14 days (11 mm), modified from Lewis. | Rabbit embryo of 14 days and 18 hours (14.5 mm), modified from Lewis. | Rabbit embryo of 17 days (21 mm), modified from Lewis. |
Rabbit Placentation
Rabbit implantation and placentation is a centric (or fusion) type, where the blastocyst adheres only to the epithelial cells (apical region) by trophectoderm forming projections.[10]
Neural Development
The data below is summarised from an excellent study of early neural development in the rabbit.[11] The same authors have studied neural development in the pig.
- 6 - 8 somite stage - the flat neural plate transforms into a V-shaped neural groove (beginning at rhombo-cervical level)
- 8 and 9 somite stage - multiple closure sites occur simultaneously at three levels
- incipient pros-mesencephalic transition
- incipient mes-rhombencephalic transition
- level of the first pairs of somites
results in four transient neuropores
anterior neuropore
- 9-11 somite stages - anterior and rhombencephalic neuropores close
- mesencephalic neuropore is very briefly present
posterior neuropore
- largest and remains open longest
- 9-10 somite stages - tapered (cranial) portion closes fast within
- wide (caudal) portion closes up to a narrow slit
- further closure slows
- 22 somite stage - full closure occurs
compared with chick and mouse - sequence of multiple site closure resembles that of the mouse embryo, but other important aspects of neurulation resemble those of the chick embryo. In contrast to mouse and chick, no time lag between closure at the three closure sites in the rabbit was seen
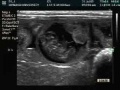
Ultrasound
|
Ultrasound of a 16 day rabbit embryo. |
Uterus
The rabbit's uterus has six longitudinal folds, symmetrically disposed ; the line of insertion of the mesentery (mesometrium or broad ligament) corresponds to the space between two folds, which alone participate in the formation of the placenta; accordingly we may designate them as the placental folds.[12]
Rabbit Immune Development
Rabbits generate their antibody repertoire in three stages.[13]
- Neonatal repertoire is generated by B lymphopoiesis in fetal liver and bone marrow (limited by preferential V(H) gene segment usage).
- Between 4 and 8 weeks after birth gut-associated lymphoid tissue (GALT) a complex primary antibody repertoire.
- The primary antibody repertoire is subsequently modified during antigen-dependent immune responses (the secondary repertoire).
Rabbits uniquely develop a primary antibody repertoire through somatic diversification of Ig genes (dependent on intestinal microbial flora).
The esacculus rotundus is located at the ileocaecal junction as an enlargement of the large intestine and contains lymphoid tissue.
Postnatal Rabbit Growth
Postnatal growth data from 2 to 34 weeks of age at biweekly intervals for New Zealand white rabbit.[14]
- 17 male and 12 female rabbits, with the data tabulated separately.
- Skeletal growth was complete at 28 weeks, with the 34 week values mature adult lengths.
Mean body weight
- 2 weeks of age was 6% that at 34 weeks
- 16 weeks was 72% of the weight at 34 weeks
- weight continued to increase in the adult.
Mean body length
- 2 weeks was 40% that at 34 weeks
- 16 weeks was 91% of mature adult
Mean femoral length
- 2 weeks was 38% of adult
- 16 weeks was 95% of adult
Mean tibial length
- 2 weeks was 38% of adult
- 16 weeks was 94% of adult
References
- ↑ Kobayashi T, Castillo-Venzor A, Penfold CA, Morgan M, Mizuno N, Tang WWC, Osada Y, Hirao M, Yoshida F, Sato H, Nakauchi H, Hirabayashi M & Surani MA. (2021). Tracing the emergence of primordial germ cells from bilaminar disc rabbit embryos and pluripotent stem cells. Cell Rep , 37, 109812. PMID: 34644585 DOI.
- ↑ Proks P, Stehlik L, Nyvltova I, Necas A, Vignoli M & Jekl V. (2018). Vertebral formula and congenital abnormalities of the vertebral column in rabbits. Vet. J. , 236, 80-88. PMID: 29871755 DOI.
- ↑ 3.0 3.1 3.2 Daniel-Carlier N, Harscoët E, Thépot D, Auguste A, Pailhoux E & Jolivet G. (2013). Gonad differentiation in the rabbit: evidence of species-specific features. PLoS ONE , 8, e60451. PMID: 23593221 DOI.
- ↑ Kawamura Y, Matsumoto K & Sato K. (2012). Stability of the reproductive variables and fetal malformations from control animals and animals treated with thalidomide in Kbl:JW rabbits over two decades. Congenit Anom (Kyoto) , 52, 191-202. PMID: 23181494 DOI.
- ↑ Marshall VA & Carney EW. (2012). Rabbit whole embryo culture. Methods Mol. Biol. , 889, 239-52. PMID: 22669668 DOI.
- ↑ Roellig K, Goeritz F & Hildebrandt TB. (2010). Ultrasonographic characterisation of prenatal development in European brown hares (Lepus europaeus PALLAS, 1778): an evolutionary approach. Reprod. Fertil. Dev. , 22, 448-58. PMID: 20047730 DOI.
- ↑ Kobolak J, Kiss K, Polgar Z, Mamo S, Rogel-Gaillard C, Tancos Z, Bock I, Baji AG, Tar K, Pirity MK & Dinnyes A. (2009). Promoter analysis of the rabbit POU5F1 gene and its expression in preimplantation stage embryos. BMC Mol. Biol. , 10, 88. PMID: 19732419 DOI.
- ↑ Warner SM, Conlon FV & Kane MT. (2003). Inositol transport in preimplantation rabbit embryos: effects of embryo stage, sodium, osmolality and metabolic inhibitors. Reproduction , 125, 479-93. PMID: 12683919
- ↑ Sultana F, Hatori M, Shimozawa N, Ebisawa T & Sankai T. (2009). Continuous observation of rabbit preimplantation embryos in vitro by using a culture device connected to a microscope. J. Am. Assoc. Lab. Anim. Sci. , 48, 52-6. PMID: 19245751
- ↑ Lee KY & DeMayo FJ. (2004). Animal models of implantation. Reproduction , 128, 679-95. PMID: 15579585 DOI.
- ↑ Peeters MC, Viebahn C, Hekking JW & van Straaten HW. (1998). Neurulation in the rabbit embryo. Anat. Embryol. , 197, 167-75. PMID: 9543335
- ↑ Minot CS. Uterus And Embryo - I. Rabbit II. Man. (1889) J Morphol. 2:
- ↑ Lanning D, Zhu X, Zhai SK & Knight KL. (2000). Development of the antibody repertoire in rabbit: gut-associated lymphoid tissue, microbes, and selection. Immunol. Rev. , 175, 214-28. PMID: 10933605
- ↑ Masoud I, Shapiro F, Kent R & Moses A. (1986). A longitudinal study of the growth of the New Zealand white rabbit: cumulative and biweekly incremental growth rates for body length, body weight, femoral length, and tibial length. J. Orthop. Res. , 4, 221-31. PMID: 3712130 DOI.
Reviews
Fischer B, Chavatte-Palmer P, Viebahn C, Navarrete Santos A & Duranthon V. (2012). Rabbit as a reproductive model for human health. Reproduction , 144, 1-10. PMID: 22580370 DOI.
Püschel B, Bitzer E, Blum M & Viebahn C. (2010). Mounting, embedding, and sectioning of early rabbit embryos. Cold Spring Harb Protoc , 2010, pdb.prot5356. PMID: 20150115 DOI.
Püschel B, Daniel N, Bitzer E, Blum M, Renard JP & Viebahn C. (2010). The rabbit (Oryctolagus cuniculus): a model for mammalian reproduction and early embryology. Cold Spring Harb Protoc , 2010, pdb.emo139. PMID: 20150104 DOI.
Articles
Hassoun R, Schwartz P, Rath D, Viebahn C & Männer J. (2010). Germ layer differentiation during early hindgut and cloaca formation in rabbit and pig embryos. J. Anat. , 217, 665-78. PMID: 20874819 DOI.
Idkowiak J, Weisheit G & Viebahn C. (2004). Polarity in the rabbit embryo. Semin. Cell Dev. Biol. , 15, 607-17. PMID: 15271306 DOI.
Search PubMed
Search Pubmed: rabbit development | rabbit embryo | Oryctolagus cuniculus development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Food and Agriculture Organization of the United Nations The Rabbit - Husbandry, Health and Production (1997)
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 | ||
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 7) Embryology Rabbit Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Rabbit_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G