Paper - The fundamental plan of the vertebrate brain
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Kingsbury BF. The fundamental plan of the vertebrate brain. (1922) J. Comp. Neural. 461-490.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Fundamental Plan of the Vertebrate Brain
Benjamin Freeman Kingsbury (1872-1946)
Department of Histology and Embryology, Cornell University, Ithaca, New York
Thirty-Three Figures
Introductory
In a recent paper (Kingsbury, ’20) I pointed out that many reasons both from fact and theory called for a fundamental modification of the primary morphological plan of the brain as given by His (’88, ’89, ’92, ’93) and commonly presented by current text-books as established. The His conception of the brain Was that of a primitively tubular structure (cf. His, ’88, fig. 12). With the development by His of the very useful and fundamental idea of the existence of paired primary motor and sensory zones medially united by lines of suture, and the incorporation of this with the earlier interpretation of the brain as a tube, the logical conclusion was that these zones extended throughout the tube and that the ventral and dorsal sutures of the neural tube, marked by floor plate and roof plate, were at the anterior end of the tube supplemented by a third, terminal, suture (frontale Endnaht). In the discussion that follows these postulated or demonstrated lines of closure will be referred to as sutura neurochordalis (ventralis), sutura dorsalis, and sutura terminalis (anterior), respectively.
The His conception fitted in excellently with the already well established view of brain segments ;[1] since if the brain is composed of three ‘primary segments’ in turn divisible into five or six ‘secondary segments,’ these would only possess a segmental value if they were subdivisions of a fundamentally tubular structure and were serially comparable and metamerically equivalent. His therefore proposed a revision of the subdivisions of the brain — partly it is true for nomenclatural reasons — and his grouping and subdivision of the brain set forth in 1893 is the one generally accepted and the usual basis for text—book presentation of the brain. Both Herrick (’10) and Johnston CO9) have proposed modifications of the His schema of brain segments.
Likewise, the attempt to analyze the neural tube in terms of more primitive segments comparable with the somites of the mesoderm, the so—called ‘neuromeres’ found in the tubular plan of the brain an essential foundation for the interpretation, since clearly these could only possess value as primitive neural segments if they extended transversely to include the entire tube wall. Since to each neuromere there should belong primitively a motor and a sensory nerve the problem of the cranial nerves is also involved, as well as the ‘primitive segmentation of the head.’
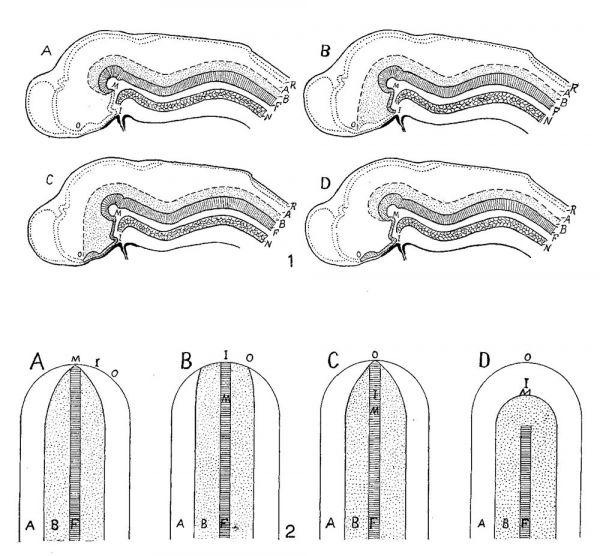
Since the publication of the papers by His dealing with the fundamental plan of the brain there have appeared three definite interpretations departing in important respects from his own. These are, in sequence, those of Johnston (’09), Schulte and Tilney (’15), and my own (’20). In order to bring out clearly the differences in interpretation and the points requiring verification two series of diagrams are presented: 1) of the brain in medial section, 2) of the brain plate (figs. 1 and 2). The latter series is essential in the comparison, inasmuch as it is the cephalic portion of the neural plate which by growth and folding produces the brain. It should, of course, be appreciated that these diagrams are purely schematic and intended merely to present the respective interpretations. The critical landmarks in the comparison of these interpretations are the mammillary recess (M ), the primitive infundibular recess (I), the preoptic recess (optic recess) (0), and the extent of the floor plate (F). These points are designated in both series of diagrams by the significant letter. The floor plate is cross—lined, lamina basalis sparsely dotted, the lamina alaris is clear, while the line of secondary closure conVerting the brain plate into the vesicular brain is outlined by heavy dots.
The His interpretation (B of figs. 1 and 2) requires little explanatory comment. The floor plate (F) and his sutura neuro—chordalis terminated at the primitive infundibular recess (I) which marked the anterior medial limit of the brain plate. Anterior to this the sutura terminalis closed the neural tube in front, meeting the sutura dorsalis (roof plate). The line of demarcation (sulcus limitans) between the primary motor zone (B) and the primary sensory zone (A) terminates at the preoptic recess (0). His’ own diagrams which are, of course, well known were copied in my earlier paper and may be compared.
Johnston (’09), entirely correctly as I think will be adequately shown later, placed the anterior limit of the brain plate at the preoptic recess.[2] C of figures 1 and 2 conveys his interpretation. Apparently he conceived the floor plate as extending throughout the brain plate, i.e., to the preoptic recess (cf. Kingsbury, ’20, p. 122).
Schulte and Tilney (’15), from a study based on twenty-six models of the brain in embryo cats, came to conclusions unique in two respects: a) the brain plate (and the floor plate) were believed to extend no farther forward than the mammillary region (M). The line of secondary closure (of the neuropore, i.e., sutura terminalis) would be correspondingly increased in extent so that not only the optic chiasma, but the entire infundibular region would be thus included. A distinct advance was their recognition that the sulcus limitans could not be traced forward beyond the mammillary recess. 1)) The second unique feature of their interpretation was the inclusion within the limits of the more cephalic portion of the alar plate of neural crest elements. As this does not closely concern the aspects considered in this paper, no discussion will be given, nor is there in A of figures 1 and 2, which present their views, any diagrammatic expression of this side of their interpretation.
My own interpretation is represented by D, figures 1 and 2. There it may be seen that the floor plate extends no farther forward than the fovea isthmi (cf. Kingsbury, ’20), whereas the medial cephalic limit of the brain plate would include the optic chiasma. The unique feature of the interpretation and the one on which in my opinion its value largely rests is that of a primary continuity across the middle plane of ‘nervous materiaI’ — alar and basilar plates - anterior to the fovea isthmi and floor plate, to both of which structures considerable morphological significance is believed to attach. In figure 1, D, the ‘prefoveal’ extent of the brain plate is indicated by heavy stippling.
Thus each of these interpretations, although differing quite fundamentally, made I think a distinct advance toward an understanding of the correct relations. My earlier paper outlines certain of the bearings of the interpretation offered by me. These it will be seen affect not alone or mainly the brain, but possess a broader bearing. The approach to the interpretation was from the embryological side rather than the neurological, and the writer’s interest has centered not so much in affording a satisfactory basis for analyzing the brain as determining the developmental pattern[3] of the body. The early morphogenesis of the brain cannot adequately be considered by itself. The problem of the development of the brain is also that of the head and the primitive plan of the brain plate, and the growth changes that transform it into the vesicular brain must be in full harmony with the facts of development for other cranial structures.
Figs. 1 and 2 Schemas to illustrate the interpretations of Schulte and Tilney (A), His (B), Johnston (C), and Kingsbury (D), of the fundamental plan of the brain and brain plate, respectively, in terms of primary motor and sensory zones. A, alar plate; B, basal plate; F, floor plate; I, primitive infundibulum; M, mammillary recess; N, notochord; 0, preoptic recess; R, roof plate.
Furthermore, neither the brain nor the head can be considered by itself developmentally or for that matter phylogenetically and any adequate interpretation of the developmental plan of the head and brain must be in full harmony with the developmental pattern of the body as a whole. Also I venture to add it should be appreciated that in as much as all vertebrates conform in the fundamental structural plan of the adult body, there must likewise be conformity in the developmental plan. In other words, the differential growth of all vertebrates must of very necessity follow the same pattern. The full significance of this is I think frequently overlooked. It is quite essential that the developmental pattern be established as well as the developmental processes ascertained.
Without considering structural aspects purely neurological wherein the interpretation ‘D’ would clarify difficulties, there are certain general questions of importance in the choice of interpretations that may be answered by an appeal to the facts and which the present paper aims to consider. These are: a) The determination of the cephalic end of the primitive brain plate. Johnston, followed by Kingsbury it will be recalled, included the chiasma; His terminated it at the infundibular recess, Schulte and Tilney at the mammillary recess. The existence of the primitive optic furrow of Johnston. b) The question as to the existence and extent of a sutura terminalis as distinct from a sutura dorsalis. c) The significance of the sutura neurochordalis of His and the primitive extent of the floor plate. In the examination of these specific questions the general significance of conception ‘D’ of figures 1 and 2 will come out in clearer outline.
Material. In the choice of forms in which to test the questions, two were selected for consideration in this paper — the shark (Squalus acanthias) and the chick. Important considerations here were: a) the availability, since it is quite important that a close series of stages at critical epochs be at hand; 12) ease of orientation. Medial sagittal sections through the head are quite essential. Model reconstructions from transverse series alone are far less useful and trustworthy. A considerable number of series cut in the sagittal plane (for the head) at important stages were prepared supplementing the series already available in the collection of the Department of Histology and Embryology, Cornell University, and those of Prof. S. H. Gage Which he kindly placed at my disposal. In addition, series were prepared for and by Mr. H. B. Adelmann for his investigation of the origin of head mesoderm. His studies have materially aided the present study and his paper Will be referred to subsequently and due acknowledgment made. 0) Both of these forms have been previously utilized in important studies of the early development of the brain and, While the literature in itself afforded no complete basis for conclusions, its existence was nevertheless helpful. d) The two forms belong to quite remote classes of vertebrates.
Observations
1. The extent of the brain plate and the primitive optic furrow
Squalus accmthias. In addition to the qualifications above mentioned, the shark possesses the important feature of a sharply defined brain plate. The early development of the brain of the shark has been investigated by a number, particularly von Kupffer (’06), Locy (’95), Neal (’98), and Johnston (’09). Despite this fact, there was not furnished in the literature sufficiently exact information to permit without repeated examination answers to the questions proposed.
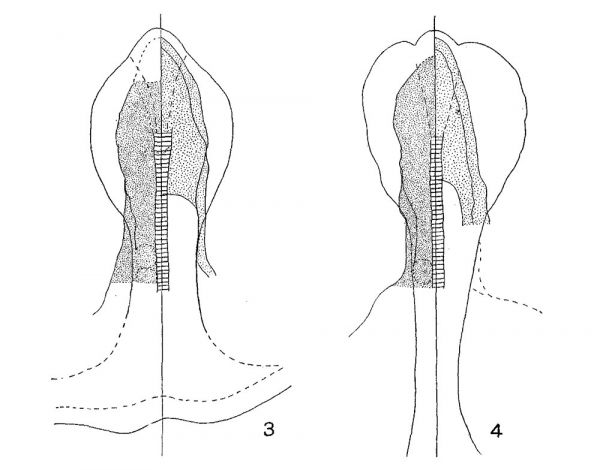
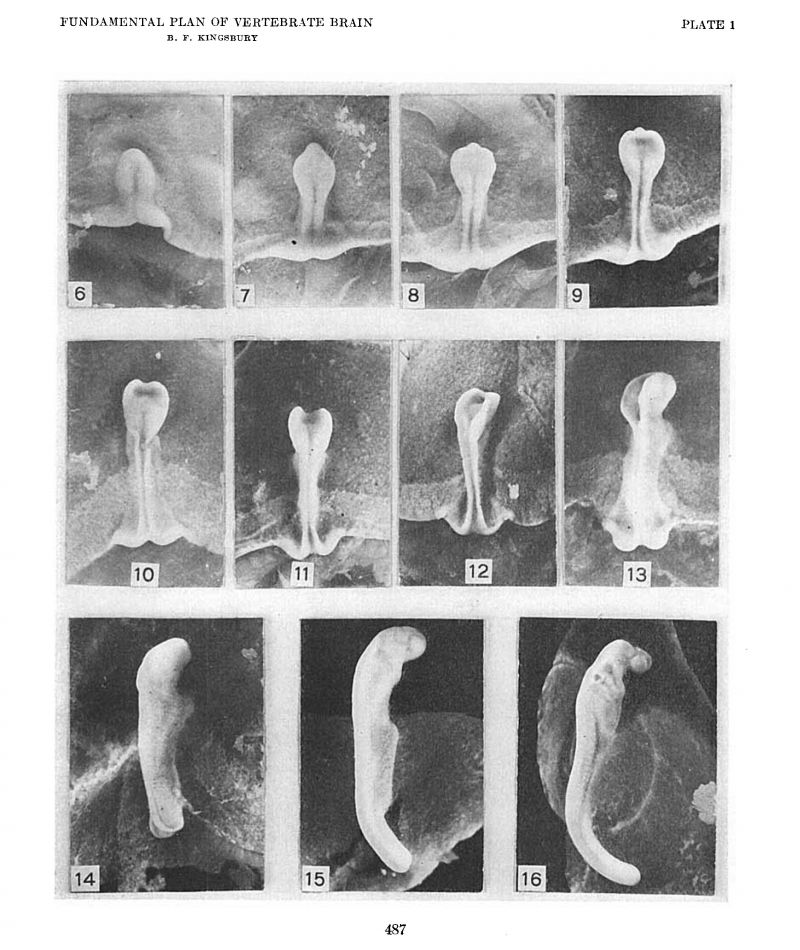
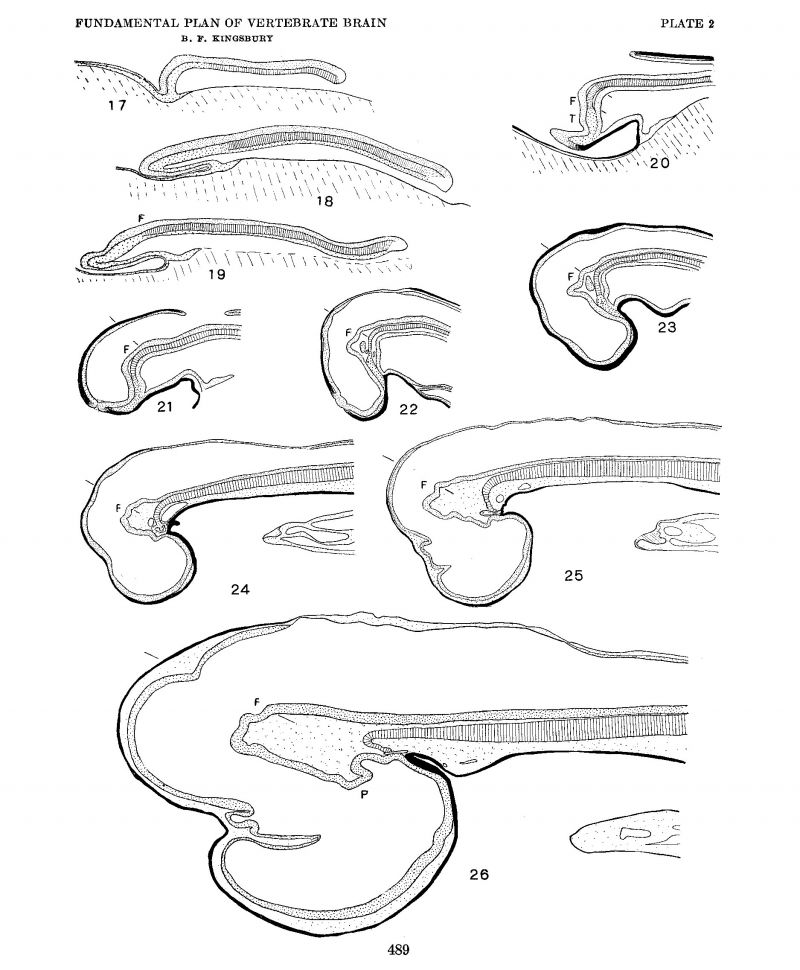
To determine the first point at issue, i.e., the extent of the brain plate, the location of the retinal areas and the existence of a primitive optic furrow (Johnston), surface views of a close series of shark blastoderms were examined, photographed at a magnification of ten diameters, and sectioned, usually in the sagittal plane, and a careful medial plane reconstruction prepared. Certain of these surface views are reproduced as figures 6 to 16. Middle plane reconstructions from the embryos of figures 6, 11, and 13 are given on plate 2 as figures 17, 19 and 20, respectively, while plottings of neural plate, notochord, mesoderm and ento derm from embryos of figures 7 and 9 are given as text-figures 3 and 4.
Johnston (’09), it may be recalled, gave evidence that, a) the retinal foveae (evaginations) were connected across the median plane by a furrow, the same which His might have termed the ‘basilar furrow’ as the internal counterpart of his ‘Basilarleiste.’ This furrow Johnston concluded might persist as a postoptic recess, while the infundibulum was a secondary development. The optic chiasma he found occupied the cephalic border of the brain plate (cf. Kingsbury, ’20, p. 122).
A merely cursory inspection of figures 9 to 13 of plate 1 and the corresponding median plane reconstructions of plate 2 (figs. 18 to 22) suffices to confirm the conclusions of Johnston on the first point, since it is evident that his first transverse furrow on the brain plate is continuous with the retinal foveae and later with the optic Vesicles, and therefore well deserves the name of ‘primitive optic furrow.’ It would seem to me, however, that it also constitutes the primitive infundibulum[4] and is in fact at one and the same time primitive optic furrow and primitive infundibular furrow. While it is probable that in the unequal growth of the region it becomes, as Johnston affirms, the relatively insignificant postoptic recess, I have not noted any indications that such is the case, doubtless because sufficiently late stages have not been examined. Although it differentiates relatively late, the same comparison of figures indicated quite clearly that the chiasma is included within the extent of the brain plate. In the later stages medial sections (figs. 23 to 26) alone are not conclusive, but, interpreted from the relations of the optic stalk in the more lateral sections, they sustain J ohnston’s conclusions in this regard also.
On the other hand, no confirmation is afforded for the Views of Schulte and Tilney. According to these authors, in the eat there exists in the neural tube a landmark of importance the ‘tubercle of the floor.’ This marks the anterior end of the neural plate, thus forming the ventral lip of the neuropore and also the anterior end of the floor plate. Ventrally the tubercle of the floor is “in relation with the blind extremity of the foregut (while) the stomo— daeum approaches but hardly reaches it in front” (p. 339). In later stages “it forms a transverse ridge intervening between the mamrnillary and infundibular regions.” The optic vesicles at an early stage form the cephalic extremity of the neuraxis and, between these there exists an ‘optic sulcus’ crossing the midplane anterior to the tubercle and partially interrupted (caudally) by it. The optic vesicles during growth, as they interpret, decrease in size contributing the infundibular region caudally and the anlages of the thalamencephalon and telencephalon anteriorly (cephalically). The line of closure of the neuropore they thus consider very extensive, including lamina terminalis, optic chiasma, and infundibulum as well.
As stated in my earlier paper, the critical point is the interpretation of the ‘tubercle of the floor,’ and it was there commented that the figures offered by them in illustration “neither conclusively show that it corresponds to the anterior end of the neural plate nor that it marks the anterior boundary of the mammillary recess.” As to the first point above it may be urged that their figure 1 of plate 27 (eat of four somites in which the neural folds have not yet fused) shows, on the contrary, that there intervenes between the ‘tubercle of the floor’ and the anterior end of the brain plate the primitive optic furrow (their ‘optic sulcus’) and a terminal ridge in which, in the light of J ohnston’s work and my own observations, I should be inclined to see a chiasmatic ridge. Their figure (1 of plate 27) might well be compared with figure 10 (9 and 11) in this paper of the neural plate of the shark. flatten out the neural plate as it is shown in their model in the cat and the equivalence would be quite striking.
The shark, however, possesses no well—defined ‘tubercle of the floor.’ The slight elevation, which from their description of the relations the tubercle of the floor bears to foregut and stomodaeum would correspond to it, is marked in figure 20 by the letter T. It also agrees with the tubercle of the floor in being caudal to the primitive infundibular recess. This slight elevation in the shark is clearly but the mechanical expression of the underlying ‘preaxial mesoderm.’ In the neural plate stages, figures 7, 8, and 9, there is indicated, somewhat faintly it is true, a wedge-shaped area[5] tapering caudally from a rounded ‘apical lobe’ which from its photographic reproduction might easily be interpreted as a typical ‘tubercle of the floor’ extending quite to the anterior edge of the neural plate. The comparison of the Schulte and Tilney figure 1, plate 27, with figure 10 of this paper, already called for in connection with the optic sulcus, might be again appealed to as establishing the equivalence of this wedge-shaped area with the tubercle of Schulte and Tilney. However, examination of transections shows that in such stages as those of figures 9, 10, and 11 there is no or but slight elevation of the neural plate and no marked thickening of the neural plate which corresponds to the area reproduced by the photograph. Plottings were made of mesoderm, entoderm, notochord, and neural plate showing their position and extent in the embryos of figures 7 and 9, and these are reproduced here as figures 3 and 4, respectively. From their examination it is quite evident that the appearance seen in the surface examination of shark neural plates and which the camera reproduces is mainly an expression of the greater thickness of the underlying preaxial tissues where entoderm, mesoderm and notochord are indistinguishably united —a region with whose significance Adelmann (’22) has more particularly to do. The progressive transformation of this region as seen in the.median plane from these or comparable stages may be seen in the comparison of figures 17 to 21 (plate 2).
The ‘apical lobe’ is intimately related to the ‘pre—oral’ extension of the foregut, and it is the great expansion of the more lateral region of the neural plate in this region that sets it off as an apical lobe.
Figs. 3 and 4 Plottings from series cut in the transverse plane of Squalus acanthias embryos, 2.2 mm. and 2.6 mm. length, 6 and 8 somites, respectively. These are the embryos of figures 7 and 9, plate 1. In each, the notochord is indicated by cross-barring; the stipple areas on the right and left mark the extent of the entoderm and mesoderm, respectively; the two lines on the right bound the lateral wall of the archenteric cavity; the outline of the median ‘wedge—shaped area’ is shown in broken line, and closely corresponds to the territory of the prechordal plate at these stages.
Whether the tubercle of the floor in the cat is a mechanical expression of a moulding of the neural plate upon the “blind extension of the foregut” cannot be determined from the figures of Schulte and Tilney, but it seems hardly probable. A mechanical crowding in the forward growth seems more likely to be responsible for its presence.
Chick. While in the chick embryo the brain plate is less clearly defined in the early blastoderm and the retinal foveae do not appear until after the neural folds have formed, fundamental agreement with the relations existing in the shark nevertheless exists.
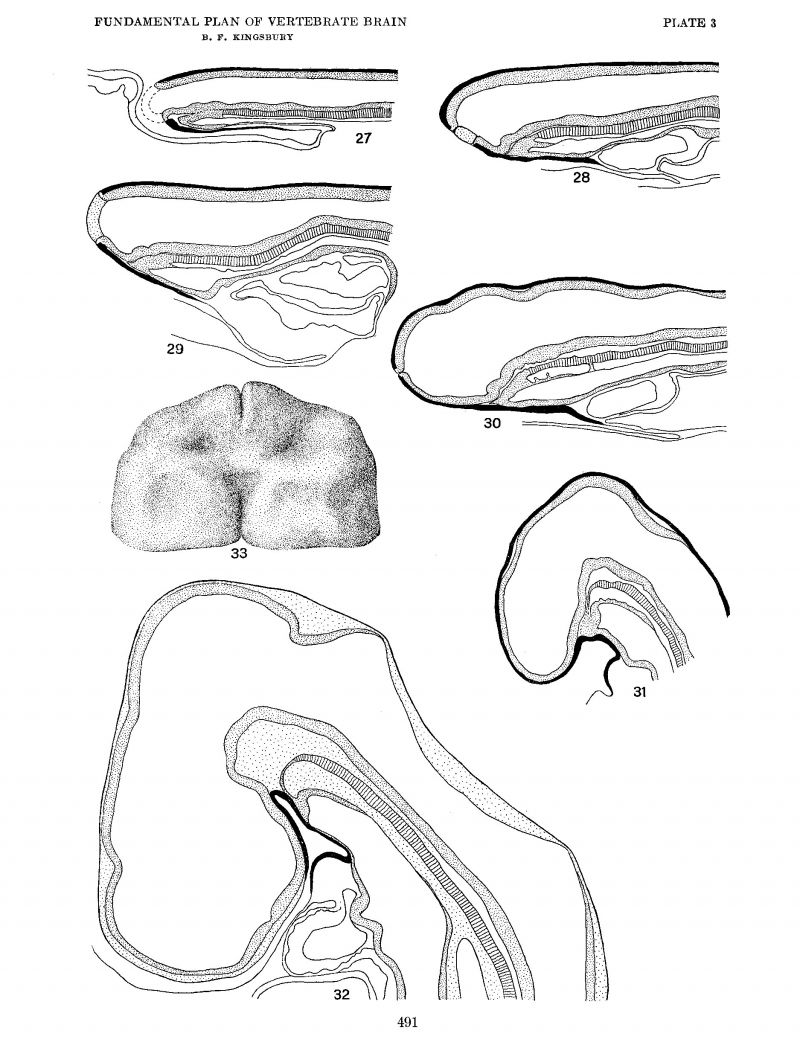
Despite the large amount of work done on the development of the chick, adequate descriptions of the early relations and transformations of the neural plate are lacking, and it was necessary to undertake a detailed study of a sequence of stages. Eighteen median plane reconstructions from sagittal sections were made by Mr. Adelmann and myself, of which six are here shown in accurate outline, but diagrammatically reproduced, as figures 12 to 32. Of these, the reconstructions of figures 27, 29, 30, and 32 were also utilized by Mr. Adelmann in his paper, who added four additional ones.
Johnston (’09) does not describe the condition in the chick in detail, but it has been found that in this form as well as in the shark the primitive infundibular furrow is also a primitive optic furrow. The earliest stage in which the furrow appears is one of six to seven somites (fig. 27). Here We see two slight furrows With equally slight prominences overlying a region where notochord mesoderm and entoderm are blended and intimately in contact (at least) with the neural plate. An earlier stage (four somites) illustrates the above—mention.ed important characteristics of this important region without the presence of these furrows or slight depressions. There is an obvious pushing forward and downward of this region in the growth expansion. The more anterior of these furrows becomes the primitive infundibular furrow, as may be seen by comparing figures 27, 28, 29, and 30. In the interval of development which they represent the optic vesicles have appeared, and by tracing the infundibular furrow to either side it is evident that it leads directly into those lateral expansions of the tube, fully substantiating Johnston’s interpretation. The community of relation of optic vesicle (furrow) and primitive infundibulum which exists in the interior of the tube has an external counterpart, as is Well shown in the His models of the developing chick brain where optic vesicle and infundibular fold are continuous with one another. As the brain expands the primitive infundibulum becomes extended cephalo-caudally and loses its direct and primary communication with the optic vesicles (figs. 30, 31, 32).
A comparison of these figures (figs. 27 to 30) will also substantiate the important point that the primitive infundibulum (primitive optic furrow) is within the territory of the brain plate. The preoptic recess appears later (fig. 32). From the relations, which were well ascertained by Johnston (’09), it is apparent that the optic chiasma, although it appears only subsequently, marks the anterior boundary of the optic furrow and the anterior limit of the brain plate medially.
From the examination of the relations in the chick, as in the case of the shark, the interpretations of Schulte and Tilney lack substantiation. In the chick, as in the mammal (e.g., cat, Schulte and Tilney), the early formation of the neural folds disguise relations that are clearer in the shark due to the greater expansion of the brain plate (of. figs. 9, 10, 11) before the neural folds rise up. Were one to ‘open out’ the neural folds in, say, the four- to six-somite chick as in the four-somite eat of Schulte and Tilney, the morphological identity would be quite striking.
The prominences seen in the median plane reconstructions of the chick (figs. 27 to 30) correspond closely to Schulte and Tilney’s tubercle of the floor. Whether the second (slight) furrow corresponds with the future mammillary recess was not determined. I am inclined to regard these prominences—as indeed the furrows—as but expressions of the growth mechanics of the region and caused mainly by a crowding forward.
2. The sutura terminalis (anterior)
In the His conception of the brain as a tube a separate anterior closing sutura, sutura frontalis (terminalis, anterior), ‘frontale Endnaht,’ would have a distinct morphologic significance. If, however, it is recognized that the brain is developed from a plate folded together while it grows, such a theoretical significance would not attach to this portion of the secondary line of closure. As is well known, the dorsal suture is first formed somewhere in the neighborhood of the tectum mesencephali, although there is clearly considerable variation in this respect. The closure of the neural tube progressing cephalad (as well as caudad) leaves a slit—like opening of the neural tube anteriorly (chick of eight to nine somites; cf. figs. 27 to 29). Its ventral extremity lies at the site of the future preoptic recess. Due to the expansion of the dorsal portion of the neural tube in this region, it is from the beginning in part ventral in position. figure 33 is from a model of the anterior portion of the head in a chick of eight to nine somites as seen from the Ventral side. Its ventral extremity likewise marks the anterior medial boundary of the neural plate. The suture closes rapidly and apparently last at or near its dorsal extremity, although again there appears to be some variation in different forms and individually. To this entire opening from the earliest stage the term ‘neuropore’ is frequently applied, while others would limit the term to the point of final closure. Possibly it would be well to distinguish these as primitive neuropore and definitive neuropore, respectively.
Inasmuch as the bordering edge of the neural plate in this region marks the lamina terminalis, it would seem that a separate designation would be of value for descriptive purposes. It should, however, be appreciated that essentially it is but the most anterior portion of the sutura dorsalis given a peculiar significance through the growth expansion of the adjacent portion of the brain (and head).
It may be remarked that Lillie (’19) correctly describes in the chick the anterior relations of the sutura terminalis (pp. 105, 147). The interpretations of Schulte and Tilney (’15) are as obviously Wrong. Johnston (’11) has quite adequately discussed this region (p. 39) and with what he there states my observations agree.
3. The sutura neurochordalis (ventralis)
The His conception of the origin of the body through the apposition or concrescence of a germ ring is of course well known. The idea was an old one with him and appears as early as 1874, prompted by his work on the development of the salmon and strengthened by examination of the shark (’7 7). Apparently the entire vertebrate body was thought of as formed by concrescence in the midplane of right and left moieties, although by both word and figure he seems to have recognized a primitive continuity of material at the anterior end of the body axis. Inasmuch as the primitive line of junction established the neural plate externally and the chordal plate internally, His designated it as the ‘neurochordale Naht.’ This two-fold effect of concrescence His recognized as early as 1874 and comments on in 1877, but the designation was not given until he turned his attention to the structural plan of the central nervous system. GoronoWitsch (’93) was apparently the first to give it technical form as the ‘sutura neurochordalis (ventralis),’ although he failed to grasp the significance of the term.
Hertwig (’92) gave a new significance to the neurochordal suture through his recognition that it marked the line of closure of the blastopore.
The interpretations of His and of Hertwig and their bearing have been, I think, adequately discussed in my earlier paper (Kingsbury, ’20). It remains to examine more closely than was then attempted the value and significance of the term ‘neurochordal suture’ as applied to the brain.
The neurochordal suture, according to His, becomes in the neural tube marked out by the floor plate and this—in accordance with his conception of the origin of the body—extended to the anterior end of the neural plate. The distinction of neurochordal suture and floor plate should of course be appreciated. The neurochordal suture is a structurally negative term; an ideal plane of junction. The floor plate is a structural differentiation. The term may be used either in a purely descriptive way or as a structure of ontogenetic significance. Reference to my earlier paper (p. 115) is indicated in this connection.
The problem of the neurochordal suture may perhaps be stated in question form as follows: Does the floor plate mark in the neural plate (tube) the line of closure, either actual or potential, of the blastopore? Does the notochordal plate likewise coincide with the line of closure of the blastopore? If so, these two structures should be when first laid down coextensive. Since I have shown that a differentiated floor plate extends no farther forward than the fovea isthmi, the primary extent of the notochord would thus fall far short of the point marked out as its anterior end by His, namely, the point where sutura terminalis, sutura neurochordalis, ectoderm, and entoderm (i.e., pharyngeal membrane) adjoin (cf. the His fig. 1, 1892, 2 paper, reproduced in my earlier paper as figure 4).
The question as to whether or not it is permissible to recognize a primitive suture, actual or potential, uniting the right and left halves in the epichordal portion of the body is a purely embryological one and it is not proposed to enter into its discussion here. More pertinent at this time is the determination as to whether or not floor plate and notochordal plate are primarily coextensive, and hence whether, granting the propriety of the term suture, it may likewise be designated as neurochordal suture. This determination is somewhat difficult for two main reasons: 1) At the early developmental stage in which notochord and neural plate are in juxtaposition the floor plate is still undifferentiated. Likewise in most forms at least the anterior end of the notochord is not at first clearly demarcated from the prechordal plate (preaxial mesoderm). 2) The neural plate (tube) and notochord grow markedly and at different rates, the former obviously more rapidly, so that the neural tube becomes bent away and separated from the notochord so that by the time the fovea isthmi is evident and the floor plate distinguishable through its differential change, the points for comparison are quite remote from each other. These difficulties do not, however, render valueless a comparison of neural plate and notochordal plate and recourse may again be had to the median plane relations in progressively older stages.
Shark
Sixteen median plane reconstructions were made of shark embryos from 1.5 to 23 mm. length, of which ten are reproduced as plate 2. The magnification is the same for all so as to permit more readily Visualization of the growth changes. figures 17, 19, and 20 are from the embryos shown in figures 6, 11, and 13, respectively. Figures 18, 21, and 22 are of stages intermediate to those of 9 and 10, 13 and 14, and 15 and 16, respectively. figures 23 to 26 are of progressively older stages, all of them, however, older than that represented by figure 16.
While in figure 17 the notochord "is included in the entoderm and its prospective cephalic end is indeterminate Without a comparison with the mesoderm and with later stages, in the succeeding stages figured it is separated off save at its cephalic and caudal ends Where prechordal plate and marginal zone (blastoporic lip) are united to it. Both of these zones mark regions Where notochord, paraxial mesoderm and entoderm are confluent. In the case of the marginal zone, of course, the neural plate must be added to these. The prechordal plate is a region of great significance in understanding the morphology of the head. Without stopping to consider the neural plate—notochordal plate relations more closely at this point, it is obvious at once from a survey of these figures that not only does the anterior end of the notochord fail to reach the anterior end of the neural plate, but falls far short of the primitive infundibular fold, being separated from it by the greater extent of the prechordal plate (cf. figs. 17 to 20) in which it ends.
Turning to a more exact comparison of notochord and neural plate, in order to determine if possible the point in the neural plate with which the anterior end of the notochord primitively corresponds, it should be noted that only in the last figure of the series (fig. 26) is the fovea isthmi and the cephalic end of a differentiated floor plate distinguishable, where it has been designated by the letter F. The interval of the medial stretch of floor between this point and the tuberculum posterius of Von Kupffer is in the shark embryo of this stage extensive. P marks the latter landmark. No significant alterations in form development take place between this stage and the 40-mm. embryo figured in my earlier paper (figs. 1 and 8), with which it may be compared. Each preceding earlier stage has been compared and the equivalent point similarly designated, back to and including that of figure 19. In this comparison the descriptions and figures of Neal (’98), Von Kupffer C06), and Scammon (’11) have been considered and compared, particularly the fine plates of the former. The medial plane sections in each of these series have been compared with the parasagittal sections and the general plane of the external ‘fissura rhombomesencephalica’ indicated by lines. It appears clear that the expansion and pushing forward of the neural plate carries the point in question forward (and downward) (figs. 18, 19, 20). Subsequently the (cephalo-caudal) expansion of the primitive infundibulum and the increase in extent of the mesencephalic floor shifts it relatively backward (figs. 20, 21, 22, 23) to again advance it with the growth of the rhombencephalon (figs. 24, 25, 26). In comparing figures 20 to 24, the relations of the second head somite material may be noted. In figure 20 the two short lines mark its cephalocaudal extent as it lies paraxially. In figure 21 the neural plate has begun to separate from closer contact with the notochord in this region. In this space a dense mesenchymal growth from the second head ‘somite has appeared in figures 22 and 23. From this somite much at least of the mesenchyme occupying the plica encephali ventralis is derived.
This comparison of a close series of stages indicates that the point of the prospective fovea isthmi in the neural plate corresponds closely at least to the anterior end of the notochordal plate. It does not seem possible more closely to establish the correlation, due to the differences in differentiation and growth earlier mentioned, without resort to an extensive and supplementary series of models of the region, or experimentally. The point could hardly be tested experimentally in shark or chick. In the Amphibia, however, this would seem quite possible, where, it might be incidentally remarked, I have found (in Amblystoma punctatum) conformity to the developmental pattern of the head outlined here for shark and chick. The comparison instituted entirely suffices, however, to establish clearly the incorrectness of the His diagram (His, ’92, fig. 1; cf. Kingsbury, ’20, fig. 4), in which the notochord extends to the infundibular fold and to the anterior end of the neural plate. The His conception of the brain plate, therefore, which the diagram illustrates fails of substantiation. The His term, ‘neurochordal suture,’ however, deserves, I think, retention, but with full recognition that its use implies the acceptance of a line of blastoporal closure, actual or potential, with which it coincides, and which in the brain and spinal cord is marked by the floor plate whose anterior limit I have placed at the fovea isthmi.
Inasmuch as Johnston (’09) and Schulte and Tilney (’15) did not consider the growth of the brain plate in its relation to the notochord and head mesoderm, the question of the neurochordal suture and its extent is not directly involved in the adjudication of their interpretations. Doubtless in prolonging a floor plate forward to the anterior end of the neural plate they but expressed an acceptance of the His analysis of the neural tube.
It may be pointed out that the primitive furrow of the neural plate which appears in figures 6 to 11 and whose cephalic end is carried forward and down (figs. 11 and 12) and is lost to view with the development of the neural folds corresponds closely to the extent of the notochord. figures 3 and 4, which are plottings to show the extent and relations of notochord mesoderm and entoderm in the embryos of figures 7 and 9, respectively, may be compared. This furrow I believe unquestionably corresponds to the neurochordal suture.
Chick
While there exists in the chick as in the shark the same fundamental plan of relations of neural plate, notochord, prechordal plate and entoderm, with the characteristic morphological transformations of forward growth and down—bending, it has not been possible clearly to determine the point of the fovea isthmi in the neural plate. It is obvious, however (figs. 27 to 32), that the notochord ends ‘far from the anterior end of the neural plate separated from the infundibulum, as in the shark, by the extent of the prechordal plate, and at a level such as would, from the subsequent growth transformations in the brain, correspond closely with the level of the prospective fovea isthmi and the anterior end of the floor plate. The fovea isthmi and floor plate could not be detected, however, earlier than five days’ incubation, and no serious attempt was made to bridge the interval by a detailed study of intervening stages. The essential similarity in the pattern in chick and shark may be seen by a comparison of the figures of plates 2 and 3. The important relations of notochord and prechordal plate are more adequately discussed by Adelmann (’22)
Conclusions
In the foregoing pages the four interpretations outlined in the introductory paragraphs of this paper have been examined from the three points of View proposed on the question of fact, and the conclusion is safe, I think, that the His interpretation and that of Schulte and Tilney fail to satisfy the requirements. Johnston’s observations have been confirmed in the essential feature that the neural plate terminates with the chiasmatic ridge and that the primitive infundibular furrow is likewise a primitive optic furrow. My own interpretation has added what I regard as an important point — the recognition of a primitive continuity of nervous parietes in the brain anterior to (cephalad of) the notochordal axis, together with a more correct evaluation and limitation of the sutura neurochordalis of His. It is felt that there has been given an interpretation also consistent with the actual facts of brain growth and withthe pattern of vertebrate ontogeny which was less apparent if the approach was from the neurological side as in the case of the researches of Johnston and Schulte and Tilney. As has been insisted earlier in this paper, the developmental origin of the brain plate and the morphogenesis of the brain are primarily embryological questions inseparable from the patterzn of morphogenesis of the head and of the body as a whole.
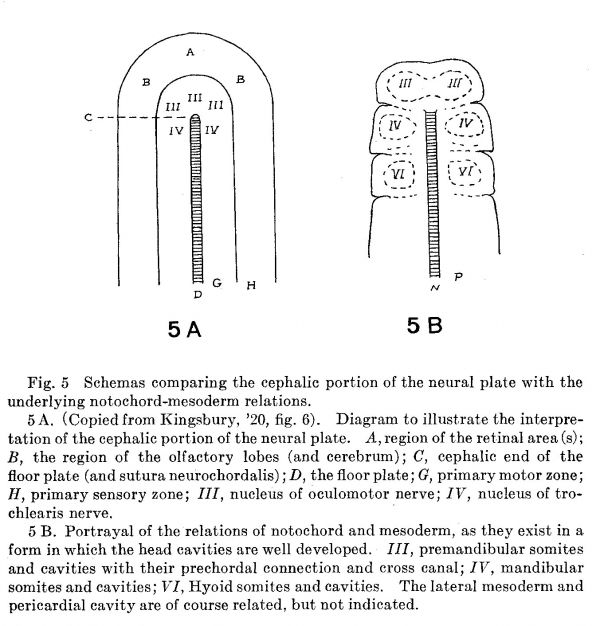
It is not my intention to consider in any detail the embryologic aspects of the problem in this place; it is, however, advantageous to briefly mention some contributory evidence from the embryological side. Stated succinctly, it is clear that the vertebrate brain arises from the dorsal blastoporic lip which by growth effecting essentially a potential closure of the blastopore, produces an arrangement of material in the neural plate illustrated in figure 2, D, in which the neurochordal suture marks such a line of ‘closure,’ while the floor plate is a differentiation along this line expressing the primitively bilateral character of the growth of nervous material. Beneath the neural plate the mesoderm and notochord exhibit a similar arrangement. For a more adequate comparison on this important point figure 6 of my previous paper is here reproduced as figure 5 A while figure 5 B reproduces the plan of arrangement of notochord, preaxial and paraxial mesoderm essentially as it would exist in any form (e.g., shark, turtle, certain birds) in which the ‘head somites’ are Well developed. To make the comparison more forceful, the cranial nerve number marks the somite from which its territory of innervation is derived.
Fig. 5 Schemas comparing the cephalic portion of the neural plate with the underlying notochord—mesoderm relations.
5 A. (Copied from Kingsbury, ’20, fig. 6). Diagram to illustrate the interpretation of the cephalic portion of the neural plate. A, region of the retinal area (s); B, the region of the olfactory lobes (and cerebrum); C, cephalic end of the floor plate (and sutura neurochordalis) ,' D, the floor plate; G, primary motor zone; H, primary sensory zone; III, nucleus of oculomotor nerve; IV, nucleus of trochlearis nerve. '
5 B. Portrayal of the relations of notochord and mesoderm, as they exist in a form in which the head cavities are well developed. III, premandibular somites and cavities with their prechordal connection and cross canal; IV, mandibular somites and cavities; VI, Hyoid somites and cavities. The lateral mesoderm and pericardial cavity are of course related, but not indicated.
The notochord terminates anteriorly in a zone of proliferation where mesoderm and entoderm are for some time confluent. The material in which the anterior end of the notochord terminates, the prechordal plate, is actually or potentially preaxial mesoderm and furnishes the material from which differentiate the muscles innervated by the oculomotor (IlIrd cranial) nerve. The paper of Adelmann, several times referred to, critically examines the growth transformations in this region and furnishes us with an adequate conception of the significance of the prechordal plate. VVhile the prechordal plate is primitively preaxial, its growth is largely bilateral rather than in the median plane, a feature important in understanding the morphology of the head. Thus, if the series of medial planes in Squalus (figs. 17 to 26) are compared, it is seen that the preaxial mesoderm which separates off from the entoderm and gains a cavity (connecting the cavities of the premandibular somites), becomes eventually drawn out to the sides and disappears without contributing directly to preaxial median structures, unless indeed the most anterior portion connecting the ‘anterior’ head cavities may persist (cf. Adelmann, ’22).
The bilateral growth appears more precocious in the higher vertebrates, particularly mammals, disguising the significance of primitive relations and rendering an appreciation of the morphogenetic pattern dlfi'lC11ll3. With the disappearance of medial mesoderm the notochord may through growth attain secondary relations quite different, as fusion with the hypophysis (cf. Rand, ’17, et al.). In the chick, likewise, the attenuation of the medial preaxial mesoderm may give rise to faulty interpretations if one were to omit comparison with birds, such as the duck, in which the differentiation is more marked (cf. Adelmann). The slight development of the medial preaxial mesoderm in the chick as compared with the shark may be seen on comparison of the two sets of median planes, plates 2 and 3. It should be appreciated, I think, that we are dealing with a mode of differential growth possessing marked plasticity, hence interpretation in terms of a rigid morphology is not adequate.
The bilateral growth characteristic of the mesoderm is clearly also a marked feature of the growth of the neural plate and is indicated in the comparison of figures 6 to 10 particularly. Also, it is precociously so in chick and mammals, and this is unquestionably a factor in the difliculty with which the primitive relations are recognized.
The interpretation of the neural plate here presented furnishes also an adequate basis for the understanding of the morphology of the head as a whole. Certain features of cranial morphology that may be better comprehended on the basis of the present interpretation were indicated in my earlier paper, and since it is the intention to review the matter in a subsequent publication, it will not be further discussed here. As far as the brain tube is concerned, the outstanding feature, in addition to the marked bilateral growth already referred to and the well-known foldings of the tube as a whole due to growth, is the great expansion of the dorsal portion, effecting a rotation, as it were, roughly around the region of preaxial mesoderm as a center or axis. This brings, thus, dorsal regions ventral, ventral aspects more dorsal, more caudal material cephalad—thus reversing the primitive sequence, with the more rostral expansion primitively dorsal. Certain of these rotations are more extreme in the embryo than in later stages.
Little need be added to what was previously said upon the effect of the interpretation upon the relations of alar plate, basal plate, and sulcus limitans in the prechordal portion of the neural tube. The boundary between them as primary sensory and motor zones must necessarily be here indefinite and perhaps indeterminate. The boundary clearly lies, I think, in the region of the mammillary recess. The question, however, possesses no embryological bearing and I gladly leave it to the consideration of neurologic workers.
Only as a comment at this time, it may be said that the mode of growth of prechordal neural plate and preaxial and paraxial mesoderm commented on above furnishes us with a suggestive basis for understanding the morphogenesis of the hypophysis.
It is not the purpose of this paper to consider the question, in how far this conception of the brain plate may assist in the understanding of the purely neurologic problem of the structural pattern of the brain, but there is much that is suggestive. The floor of the midbrain, the ganglion interpedunculare, the corpus mammillare particularly, invite examination, while many fundamental structural relations of the vertebrate brain might be mentioned whose review from ‘the standpoint here presented would be indicated. Inasmuch as other aspects of the broad problem of developmental pattern claim the writer’s attention, he does not plan, for the present at least, to test further the neurological possibilities of the interpretation, even though a strong curiosity in the matter prompts thereto. It is hoped, however, that the concept may afford a sound basis for neurological investigation.
In closing, I wish to acknowledge the kindness of Prof. S. H. Gage who placed at my disposal the chick and shark embryos[6] of the collection in his charge, and the helpful cooperation of Mr. H. B. Adelmann at points where my line of investigation crossed with his.
Footnotes
- ↑ The history of the interpretation of the brain as a tubular organ composed of successive segments is practically coextensive with the knowledge of its development and comparative anatomy. While still earlier observers, apparently note the brain vesicles, to von Baer (’37) may be ascribed the recognition of three primary and five secondary brain vesicles with the well-known designations of the German vernacular — Vorderhirn, Zwischenhirn, Mittelhirn, etc. Huxley (’71) I believe introduced technical designations for these. Balfour, Wilder (B. G.), His, and von Kupffer each modified the scheme of segmentation in accordance with his own conceptions.
- ↑ Mrs. S. P. Gage (’05) earlier came to the same conclusion (p. 426): “The natural corollary follows that the optic chiasma crosses the original margin or dorso-mesal line.”
- ↑ For a number of years (cf. Kingsbury, ’13) I have recognized the importance of distinguishing Process and Pattern as two fundamental aspects of development as of life generally. Recently Child (’21) has likewise recognized the validity of the distinction, this time drawn between Pattern and Material, however.
- ↑ Johnston’s contention that the definitive infundibulm (i.e., depression leading to and into the cavity of the pars nervosa of the hypophysis) is a secondary orlater development is of course entirely correct. Embryologists have, however, so generally applied to this primitive recess the term infundibulum that perhaps for a time at least the designation may be retained, but distinguished as the ‘primitive infundibulum’ or ‘primitive infundibular recess.’ It should be appreciated that it is but an expression of the mechanics of growth of brain and head in their early morphogenesis. The term ‘hypothalamus’ seems to the writer to apply more exactly to the topography of the wall and thus leave a need for an additional and supplementary term. The question of the nomenclature of this portion of the diencephalic floor is discussed by Tilney (’15).
- ↑ This anterior medial wedge—shaped ‘area’ was noted and figured by both Locy and Neal. The former says regarding it (p.551): “This has already been spoken of in Part I as a tongue-like process extending from the median anterior tip backwards to two-thirds the length of the cephalic plate. It continues to be a prominent feature of the cephalic plate for some time. I can offer no suggestion as to its significance, outside the obvious suspicion that it may represent a proboscis of some kind, or that it may be related to the large notochord of this region.”
- ↑ Many of these series bear evidence of the attention Mrs. S. P. Gage had devoted to the problem of the anterior end of the neural plate. That she had definitely rejected as not satisfactory the His plan of arrangement of the zones is apparent from her paper of 1905. Doubtless had her health been spared she would have arrived at some alternative explanation, such as that here presented.
Bibliography
Adelmann HB. The significance of the prechordal plate: an interpretative study. (1922) Amer. J Anat. 31(1): 54-101.
BAER, C. V. Von 1837 UeberEntwickelungsgeschichte der Tiere. Beobachtung und Reflexion. II.
BALFOUR, F. M. 1881 A treatise on comparative embryology, vol. 2, London.
CHILD, C. M. 1921 The origin and development of the nervous system from a physiological viewpoint. Univ. of Chicago press.
Gage SP. A three weeks' human embryo, with especial reference to the brain and nephric system. (1905) Amer. J Anat. 4: 409-443.
GORONOWITSCH, N. 1893 Untersuchungen fiber die Entwicklung der sog. ‘Ganglienleisten’ im Kopfe der Vogelembryonen. Morphol. Jahrbueh, Bd. 20, s. 187-259.
HERRICK, C. J. 1910 The morphology of the forebrain in Amphibia and Reptilia. J our. Comp. Neur., vol. 20, pp. 413-547.
HERTWIG, O. 1892 Urmund und Spina bifida. Eine vergleichend morphologische, teratologische Studie an missgebildeten Froscheiern. Arch. f. mikr. Anat., Bd. 29, S. 353-503.
HIS, WILHELM 1874 Unsere Korperform und das physiologische Problem ihrer Entstehung. Leipzig.
- 1877 Ueber die Bildung der Haifischembryonen. Zeitschr. f. Anat. u. Physiol., Bd. 2, S. 108-124.
- 1888 Zur Geschichte des Gehirns; sowie der centralen und peripherischen N ervenbahnen beim menschlichen Embryo. Abh. d. math.-phys. K1. (1. Konigl. Sachsischen Gesellschaft d. Wiss., Bd. 14, N0. 8, S. 341-392.
- 1889 Die Formentwickelung des menschlichen Vorderhirns vom Ende des ersten bis zum Beginn des dritten Monats. Abh. d. math—phys. Kl. d. Konigl. Sachsischen Gessellschaft d. Wiss., Bd. 15, No. 8, S. 674-736.
- 1892 Zur allgemeine Morphologie des Gehirns. Arch. f. Anat. u. Physiol. Anat. Abt., 1892, S. 346-383.
- 1893 Ueber das frontale Ende des Gehirnrohres. Arch. f. Anat. u. Physiol., Anat. Abt., 1893, S. 157-171.
- 1893 Vorschlage zur Einteilung des Gehirnes. Arch. f. Anat. u. Physiol. Anat. Abt., 1893, pp. 172-179.
HUXLEY, T. H. 1871 Manual of the comparative anatomy of vertebrate animals.
Johnston JB. The morphology of the forebrain vesicle in vertebrates. (1909) J. Comp. Neurol. and Psychol. 19: 457-539.
JOHNSTON, J. B. 1911 The telencephalon of selachians. Jour. Comp. Neur., vol. 21, pp. 1-114.
KINGSBURY, B. F. 1913 The fitness of organisms from an embryologist’s viewpoint. Science, N. S., vol. 38, pp. 174-179.
Kingsbury BF. The extent of the floor-plate of His and its significance. (1920) J Comp. Neurol. 32(1): 113–135.
KUPFFER, K. VON 1906 Die Morphogenie des Centralnervensystems. Handbuch d. vergl. Entw. ges., edited by O. Hertwig. Bd. 2, Abt. 3, 8. 1-272.
Lillie FR. The development of the chick. (1919) Henry Holt And Company New York, New York.
LOCY, W. A. 1895 Contribution to the structure and development of the vertebrate head. Jour. Morph., vol. 11, pp. 497-594.
NEAL, H. V. 1898 The segmentation of the nervous system in Squalus acanthias. A contribution to the morphology of the vertebrate head. Bull. Mus. Comp. Zool. Harvard Coll., vol. 31, pp. 147-294.
Rand R. On the relation of the head chorda to the pharyngeal epithelium in the pig embryo: a contribution to the development of the bursa pharyngea and the tonsilla pharyngea. (1917) Anat. Rec. 13: 465-491.
SCAMMON, R. E. 1911 Normaltafeln zur Entwicklungsgeschichte der Wirbe1tiere. Edited by F. Keibel. XII. Normal plates of the development of Squalus acanthias. Jena.
Schulte HVW. and Tilney F. The development of the neuraxis in the domestic cat to the stage of twenty-one somites. (1915) Ann. N. Y. Acad. Sc. 24: 319-346.
Tilney F. The morphology of the diencephalic floor. A contribution to the study of craniate homology. (1915) J. Comp. Neur. 25: 213-282.
WILDER, B. G. 1885 Encephalic nomenclature. N.Y. Med. Journ., Mar. 21 and 28, 1885, pp. 325 and 354.
- 1897 What is the morphologic status of the olfactory region of the brain? Proc. Amer. Anat. Assoc., Dec., 1897, pp. 94-99.
Plates
Plate 1
Photographs, all at a magnification of ten diameters, except figure 16 which is X 8. Embryos of Squalus acanthias dorsal aspect.
6. Series 42, no somites. length 1.5 mm.
7. Series 45, 6 to 7 somites, length 2.2 mm.
8. Series not sectioned, length 2.4 mm.
9. Series 48, ca. 8 somites, length 2.16 mm.
10. Series not sectioned, length 2.18 mm.
11. Series 52, 11 to 12 somites, length 2.9 mm.
12. Series 54, 13 to 14 somites, length 3.5 mm.
13. Series 55, 15 somites length, 4.0 mm.
14. Series 59, ca. 17 somites, length 4.8 mm.
15. Series 69, 29 somites, length 5.0+ mm.
16. Series 75, 41 somites, length ca. 7.0 mm.
Plate 2
Median plane reconstructions from sagittal sections of Squalus acanthias. The notochord is indicated by cross—barring, the neural plate stippled, entoderm and preaxial mesoderm, stippled or black, ectoderm generally in black. All figures at the same magnification, X 25.
17. Series no. 42, no somites, length 1.5 mm.
18. Series no. 50, 8 somites, length 2.7 mm.
19. Series no. 52, 11-12 somites, length 2.9 mm.
20. Series no. 55, 15 somites, length 4.0 min.
21. Series no. 56, 16 sornites, length ca. 3.0 mm.
22. Series no. 51, length 4.5 mm.
23. Series no. 70, 28-30 sornites, length ca. 5.0 mm.
24. Series Gage 3, length 9-10 mm.
25. Series no. 46, length 9-10 mm.
26. Series no. 38, length 23 mm.
F, actual or prospective location of the fovea isthmi and the anterior end of the floor plate.
P, Tuberculum posterius (v. Kupffer).
I, Tubercle of the floor (see text).
Plate 3
Median plane reconstructions from sagittal sections of the chick embryo, head region only. The notochord is indicated by cross—barring, the neural plate stippled, entoderm and preaxial mesoderm (prechordal plate) stippled; the ectoderm shown in black.
anterior portion, X 67. To illustrate the ventral end of the ‘sutura terminalis marking the anterior end Of the brain—plate.
All figures at the same magnification, X 50.
27.Series 139, 6—7 somites.
28. Series 106, 10 somites.
29. Series 119, 14 somites.
30. Series 109, 16 somites.
31. Series 127, 22 somites.
32. Series Gage 54s, 30 + somites.
33. Ventral View of a model of the head of a chick, eight to nine somites, Back of it is the ‘hypophyseal area and a shallow Seessel’s pocket continuous caudally with a dorsal pharyngeal groove.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The fundamental plan of the vertebrate brain. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_fundamental_plan_of_the_vertebrate_brain
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G