Paper - The Peripheral Nervous System in the Human Embryo at the End of the First Month (10 mm)
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Streeter GL. The peripheral nervous system in the human embryo at the end of the first month (10 mm) (1908) Amer. J Anat. 8(1): 285–302.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Peripheral Nervous System in the Human Embryo at the End of the First Month (10 mm)
By
Professor of Anatomy, University of Michigan, Ann Arbor.
(With 3 Plates And 1 Text Figure.)
The period of development under consideration represents the completion of what we may call the primary stage in the growth of the nervous system. The primary neurones forming the peripheral nerves are by the end of the first month well laid down; all their chief peripheral branches and plexuses are indicated, and centrally the nerve roots can be traced to their distribution in the brain and spinal cord, where the nuclei of the motor roots can be outlined and the sensory roots can be recognized as definite bundles extending up and down in the wall of the neural tube. The higher neurone systems, however, are still in a most rudimentary state, and in sections through the brain and cord at this time we see only the primary apparatus differentiated. Such co-ordinating centers as the pons, olive and cerebellum are still undeveloped, and the forebrain is not much more than an undifferentiated thin-Walled tube. It can thus be seen that the period with which we are dealing represents a definite stage in the growth of the nervous system, the stage of the primary brain, and a stage which is of particular importance for a proper conception of the embryology of this system.
A general view of the nervous system as it exists in embryos at the end of the first month is represented on Plate I. It can be seen that the reconstruction shown there corresponds in age almost exactly with the well known His reconstruction of his embryo KO of 10.2 mm. N1. That investigator early recognized the significance of the stage of the primary brain. However, since His, ’88, published his monograph and description of the embryo KO there have been introduced many improvements in the methods of work, and before all others should be mentioned the Born wax plate procedure. It is with such aid and by means of the increased supply of available embryos that it was possible by Lewis, ’02, to elicit further detail and obtain greater completeness in our conception of the formation of the brachial plexus and the development of the nerves of the arm, and Bardeen, ’07, the nerves of the leg, and myself the nerves of the occipital region (Streeter, ’04). With this in mind it seemed desirable to go over the same ground covered by His in his reconstruction of the embryo K0 With the purpose of bringing out the same structures with more accurate detail and in more plastic form, and at the same time to incorporate the results of the Work of the investigators just mentioned.
Some of the features brought out by this study with regard to the cranial nerves were reported at the Chicago meeting of the Association of American Anatomists (Streeter, ’08). It is the purpose of the present paper to include the whole peripheral nervous system of the same embryo.
Material and Methods
The embryo on which this study is based constitutes No. 3 of Professor Huber’s Collection, and was kindly loaned by him for this purpose. After fixation in alcohol the specimen measured 10 mm. greatest length. Its estimated age is 31 days. Unfortunately there was no clinical history obtainable. The embryo is cut in a series of 5 micra sagittal sections, and the tissues are in an excellent state of preservation. The description and drawings are based in part on wax plate models and in part on profile reconstructions made in the usual way by superimposing transparent papers.
Brain and Spinal Cord
The general form of the brain and spinal cord and their relation to the body outlines is shown in Plate I. The spinal cord is largest in the cervical region, and from there slowly tapers down, being somewhat larger again in the lumbar region, and finally abruptly tapers oif at the coccyx. In transverse section the cord possesses a trapezoid outline with rounded corners, the width of the ventral half being a little greater than that of the dorsal half. A large ventricle or central canal extends throughout its whole length. By the lanceolate border of this canal the cord is divided on each side of the median line into an alar and basal plate.
Toward the head the spinal cord enlarges and gradually merges into the rhombencephalon. In this transition the basal plates become thicker, and the alar plates become wider, and at the same time flare apart. As the alar plates spread apart the narrow dorsal seam, that exists between the two in the spinal region, widens out into the broad roof of the fourth ventricle. The most striking feature of the rhombeneephalon in this embryo is its large size as compared with the rest of the brain. Its general form is shown in the drawing. It will be noticed that that part of the alar plate cephalad to the trigeminal nerve, which is to take part in the formation of the cerebellum, shows as yet little signs of differentiation.
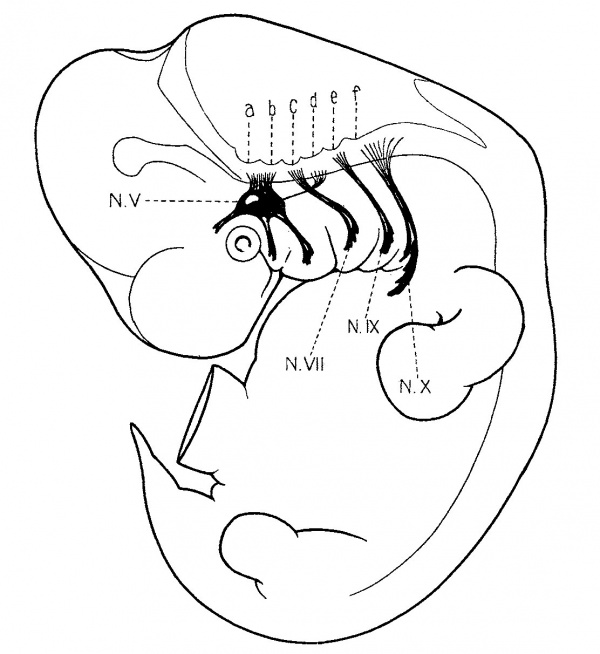
Fig. 1. Composite sagittal view of a 10 mm. human embryo (Huber collection No. 3), showing the rhombencephalon and the o-called rhombic grooves, which exist temporarily as transverse furrows in the floor of the ventricle. They are connected by the fifth, seventh, ninth and tenth nerves with the branchiai arches. The fourth groove (d.) is an exception and has no corresponding visceral nerve. Arising from it can be seen the sixth nerve, extending forward to reach the anlage of the external rectus muscle.
The rhombic grooves present in the floor of the fourth ventricle were described in the paper previously mentioned (Streeter, ’O8), and their relation to the cranial nerves was also referred to. It will only be necessary here to refer to the accompanying fig. 1., in which is shown a composite sagittal View of the embryo. The six rhombic grooves are indicated by the letters a. to f. Extending from the rhombic grooves to the branchial arches may be seen the trigeminal, facial, glossopharyngeal and vagus nerves. The correspondence of the arches, the nerves and the grooves strongly suggests the branchiomeric character of the whole group. Arising from groove d. is the n. abducens extending forward to the region median to the trigeminal. This is included in the drawing, though it is recognized that it may have no determinative connection with the fourth groove with which it stands in relation.
The outlines between the mesencephalon, thalamencephalon and prosencephalon are easily made out, and correspond closely with the His KO embryo (Tab. II, fig. 3). It is to be noted, however, that in this case the olfactory bulb is not as well developed as indicated by the His drawing, the beginning olfactory pouch being represented by only a slight depression in the brain wall just lateral to the lamina terminalis.
The general structure of the neural tube at this stage is shown in a. series of cross sections given in figs. 20-26 in the monograph of His, ’88, mentioned above. They show the division of the wall into three layers, the so-called ependymal, mantle and marginal zones; the first is made up of deeply staining primitive cylindrical cells, the second of clumps of budding neuroblasts, and the third is almost wholly fibrous. In the mantle zone are developed the nuclei of origin of the motor nerve roots, which can be outlined as shown in the reconstructions. The marginal zone consists of a reticulated framework through which the various fibre tracts make their way. At this time the only tracts that stand out prominently are the dorsal funiculi of the spinal cord, consisting of processes from the dorsal roots of the spinal nerves, and in the brain the corresponding tractus solitarius and spinal tract of the trigeminal nerve. In addition to these a portion of the marginal zone can be outlined which contains developing longitudinal fibres, and represents the so-called ventral ground bundle. In the cord it serves to connect the different levels of the mantle layer. It is continued through the hindbrain, and longitudinal fibres grow through it in that region, eventually resulting in the three main longitudinal tracts: the fasciculus longitudinalis medialis, the lemniscus and the tractus pyramidalis. This ground bundle has been modelled out and forms the support to the reconstruction shown in Plate II.
Cerebral Nerves
For purposes of description the nerves of the head will be grouped in accordance with their relation to the four functional systems of Gaskell, the significance of which has been emphasized in the recent writings of Johnston, C. J. Herrick, a.nd others. According to Gaskell the functional systems are determined by the two chief activities of the organism; first, actions in relation to the external world (somatic), and second, internal activities having to do with the processes of nutrition, etc. (visceral). In each case there is the double activity on the part of the nervous system, sensory and motor, making in all four primary functional divisions. Now some of the cranial nerves consist of elements belonging exclusively to one functional division, for example, the n. abducens which consists entirely of somatic motor fibres, while others are complex nerves containing elements of more than one system, such as the n. glossopharyngeus which contains elements from three functional divisions, somatic sensory, visceral sensory and visceral motor. The nerves will therefore be grouped according to the predominance of their functional elements as follows:
| Somatic Sensory | Somatic Motor | Visceral |
|---|---|---|
| Olfactory | Oculomotor | Trigeminal |
| Optic | Trochlear | Facial |
| Acoustic | Abducens | Glossopharyngeal |
| Hypoglossal | Vagus and Accessory |
In general the basal plate of the neural tube is motor and the lateral plate is sensory; thus the somatic motor group arises entirely from the basal plate, while the visceral group arises in large part from the lateral plate and in lesser part from the basal plate. The somatic sensory group is specialized and these nerves have individual processes or lobes of the nervous system devoted to them, i. e., the olfactory bulb, the eye bulb and stalk, and the tuberculum acusticum.
Nerves of Special Sense Organs
These nerves belong to that large group of afferent nerves that connect the integument with the central nervous system, the somatic sensory nerves. The general cutaneous nerves supplying the whole surface of the body compose the bulk of this group, and they are represented in the head by fibres belonging to the trigeminal, ninth and tenth nerves. In addition there are in the head region special somatic sensory nerves. The union of nerve and integument has resulted in these cases in the formation of special sense organs, that is the olfactory organ, the eye and the ear, composed partly of nerve elements and partly of integument. In this sense the olfactory nerves, the retinal ganglion cells and the acoustic nerve, though differing so widely in their adult morphology, may be considered as analogous in development and function.
N n. olfactorii
At the end of the first month the ectodermal olfactory pit is definitely formed and is already in the process of difierentiation, evidenced by the increase of body—protoplasm of its epithelial cells. A corresponding pouch is just beginning to form at the olfactory area at the base of the cerebral hemisphere, the anlage of the olfactory bulb. The fibres which later connect the two, nn. olfactorii, cannot yet be made out.
N. opticus
In case of the optic apparatus the chief contribution on the part of the integument is the lens; and the nerve contribution is the retina. The optic nerve corresponds to the olfactory stalk, and it is formed by the conversion of the optic stalk into a fibre tract connecting the retina with the brain. In the embryo studied the optic stalk consists of a thick-walled hollow tube whose cavity still freely communicates with the general brain cavity. The lower border is indented by the choroidal fissure which gives it a crescentic outline in cross section. The structure of this stalk is like that of the wall of the hemisphere, consisting of a thick ependymal layer covered in by a thin fibrous marginal zone. This marginal zone later thickens at the expense of the ependymal layer and is converted into a framework through which the nerve fibres from the retina make their way to the brain. At the end of the first month we cannot yet refer to this stalk properly as the optic nerve.
N. acusticus
This nerve is in a more advanced stage of development than the optic and olfactory nerves. The epithelial part of this apparatus, the ear vesicle, is already pinched ofi from the skin, as is shown in Plate I, and consists of a closed sac consisting of a double pouch having an upper vestibular portion and a lower cochlear portion; at the junction of the two on its median side is attached the ductus endolymphaticus.
The acoustic ganglion lies closely against the cephalic and median border of the ear vesicle. Two groups of fibres from the ganglion can be made out entering into connection with the cells of the vesicle; the upper group corresponds to the nerves to the superior and lateral ampullae and the utricle, and the lower group the nerves to the saccule and posterior ampulla. The ganglion is somewhat elongated and consists of an upper and lower portion. The upper portion is wholly vestibular, and the lower is partly vestibular and partly cochlear. The cochlear part, or ganglion spiral, is the thickened border of the lower division of the ganglion of which it is a derivative. The acoustic nerve emerges from the proximal end of the ganglion and enters the brain just lateral to the n. intermedius. The nerve at this time consists almost wholly of vestibular fibres. It is shortly after this that a Well defined separate cochlear trunk can be made out connecting the ganglion spirale with the brain. What has been mistaken in younger embryos for a cochlear trunk are the fibres from the lower division of the vestibular ganglion. The acoustic fibres enter the brain wall near its border opposite the third and fourth rhombic grooves, and spread out in the marginal zone just dorsal to the spinal tract of the trigeminal nerve to form the anlage oi’ the tuberculum acusticum.
Somatic Motor Group
The hypoglossal and the three nerves to the extrinsic eye muscles (nn. oculomotorius, trochlearis and abducens) that compose this group are all shown in Plates I and II. As can be seen in Plate II, their nuclei of origin may be considered as a cephalic continuation of the ventral motor column of the spinal cord. This is particularly evident in case of the hypoglossal nerve, whose nucleus and emerging fibres form a continuous line with the ventral roots of the cervical nerves, and their close relation is shown by the tendency of the two to unite in the formation of a plexus. The eye nerves are in the same series with the hypoglossal, and always maintain a similar position near the median line and directly beneath the floor of the ventricle, though they are separated longitudinally at varying intervals. The character of the muclei of origin is the same in all four nerves.
There are no ganglia on these nerves such as are found in the spinal region, though occasionally a ganglion and also at times a dorsal root is associated with the more caudal roots of the hypoglossal. In such cases the ganglion is to be regarded as a precervical one, the exact counterpart of the spinal ganglia. This ganglion when present is the so-called Froriep ganglion.
The n. oculomotorius arises from a group of neuroblasts forming the ventral part of the mantle layer in the mesencephalon. These neuroblasts converge to form small rootlets which pass through the ground bundle and emerge on the ventral surface of the neural tube in the concavity of the cephalic bend. Here they unite into a common trunk, which at first passes directly ventralward and then making a slight angle bends lateralward and finally breaks up median a11d between the first and second divisions of the trigeminal nerve, in the cellular mass which is to form the eye muscles.
The n. trochlearis arises from a group of neuroblasts similar and lying just caudal to those of the oculomotor. The rootlets derived from them, instead of emerging directly ventralward, curve dorsalward to reach the roof of the isthmus, where they decussate and emerge as a solid trunk which passes down lateral to the neural tube and breaks up among the cells which are to form the superior oblique muscle.
The n. abducens arises from a group of neuroblasts forming the median part of the mantle layer directly beneath the fourth rhombic groove. See fig. 1. The rootlets arising from these neuroblasts pass directly ventralward and after emerging they unite to form the main trunk, which bends forward immediately at an angle of nearly 90°, and passes forward into the region of the terminal rootlets of the oculo— motor, as shown in Plate I. The relative positions of the abducens and the facial nerve will be referred to under the description of the latter nerve.
Visceral Group
The facial, glossopharyngeal and vague form a series of similar nerves which consist almost wholly of visceral fibres. As can be seen on Plates II and III, the visceral motor fibres arise from the nucleus ambiguus, which consists of a column of neuroblasts continuous with the lateral horn cells of the cord. The visceral sensory fibres arise from the peripheral ganglia and enter the alar plate of the neural tube and form a longitudinal strand which in the adult we know as the tractus solitarius. In addition to these visceral fibres there is a small number of somatic sensory fibres, supplying the integumcnt of the adjoining region, which arise and have a course similar to the visceral sensory fibres. In aquatic vertebrates there are also the special somatic sensory fibres of the lateral line system, whose fibres join the rootlets of the facial, glossopharyngeal and vague to reach the brain, and the ganglia from which these fibres are derived become incorporated in the geniculate, petrosal and nodosal ganglia. We still have in the human embryo a trace of these organs in the form of a temporary thickening of the ectoderm directly over the ganglia of these three nerves.
The n. factalis is characterized by a large predominance of visceral motor fibres. They make up the bulk of the adult nerve, and in the higher vertebrates they have been made use of to supply the muscles of expression. These motor fibres arise from a group of neuroblasts situated beneath the third rhombic groove. fibre bundles are assembled and pass directly lateral under the floor of this groove gradually converging to form a solid trunk which emerges from the neural tube just median to the acoustic ganglion. The trunk curves backward, as can be seen in Plate I, and breaks up among the cells of the hyoid arch, from which the facial musculatureiis to be derived. The sensory fibres of this nerve are derived from the geniculate ganglion. From the proximal end of the ganglion the fibres are assembled to form the so-called n. intermedius which enters the alar plate of the neural tube and forms the beginning of the tractus solitarius as shown in Plate III. From the peripheral end of the ganglion the fibres pass down to form the chorda tympani, and finally leave the main trunk of the nerve to enter the mandibular arch, eventually joining the third division of the trigeminal nerve. The great superficial petrosal is another peripheral derivative of this ganglion which makes its appearance a little later. Though the facial and acoustic nerves are closely united in position, and connecting fibres are usually present between the two in the adult, it must still be remembered that this is only due to the fact they lie close together. Further than that they have nothing in common, being nerves which belong to entirely different embryological and functional classes. The term facial-acoustic complex should only be used in the sense of position.
In a paper already referred to (Streeter, ’08,) it was pointed out that the facial (motor root) and abducens nerves occupy positions in the embryo which are relatively reversed in the adult. The facial at first lies directly under the third rhombic groove, while the abducens is more caudal and is under the fourth rhombic groove. As development progresses the nuclei of these two nerves shift their relative positions, the abducens migrating forward. This migration results in the bending of the motor root of the facial out of its original course and produces the genu facialis which is characteristic of the adult.
The n. glossopharyngeus forms a more typical visceral nerve than either the facial or vagus. It possesses a ganglion of the root and ganglion of the trunk, the latter being temporarily connected with the placode over the third arch. As can be seen from the relative size of the ganglia the nerve consists mostly of sensory fibres, connected peripherally with the structures developing from the second (r. tyrnpanicus) and third (r. lingualis) arches. The tympanic branch is not well defined until we come to embryos between twelve and fourteen mm. long. Centrally the rootlets enter the brain wall and, joining with the fibres from the facial, extend caudally (Plate III) as the tractus solitarius. The motor rootlets of this nerve arise from a group of neuroblasts in the nucleus ambiguus series, situated beneath the floor of the fifth rhombic groove. The motor bundles extend directly lateral beneath this groove and pass under the spinal tract of the trigeminal and then emerge from the brain wall and join the main trunk of the nerve.
The n. vagus like the facial represents a branchial nerve the motor fibres of which have in man undergone special development, for the purpose of supplying a group of muscles derived from its branchial arch or arches. The large motor trunk of the facial nerve, as we have seen, is distributed to the muscle cells of the hyoid arch and, as these cells group themselves into the muscles of expression and spread forward over the face, the facial branches are drawn along with them. In a similar way the more caudal rootlets of the vagus become predominantly motor and form a distinct bundle which we know as the spinal accessory nerve, and this bundle is distributed to a group of muscle cells originally be~ longing to the more caudal branchial arches, and in man are destined. to form muscles for the arm girdle, the mm. sternocleidomastoideus and trapezius. As these muscles spread out into their eventual position the nerve is drawn down across the neck with them. Coincident with the increased importance of this musculature as we ascend the vertebrate scale we meet with increased development of the spinal accessory, and it obtains additional rootlets of origin by spreading down into the region of the spinal cord. As can be seen in Plate I, the spinal accessory may reach as far down as the fourth cervical segment. The nucleus of origin of the spinal accessory and other motor rootlets of the vagus constitutes the nucleus ambigous of the medulla oblongata and the lateral horn of the spinal cord, the two being directly continuous. This is best shown in Plate II.
The neuroblasts of the basal plate of the neural tube form two columns, a larger median one and a smaller lateral one; the median column furnishes somatic motor fibres to the ventral spinal roots and hypoglossal, and at intervals further forward the motor nerves of the eye; the lateral column furnishes visceral motor fibres to the dorsal spinal roots and to the vagus (spinal accessory), glossopharyngeal, facial and trigeminal nerves. In the 10 mm. embryo these neuroblast columns are longitudinally continuous from the spinal cord into the vagus region, and there is present a continuous series of median (ventral) and lateral rootlets. As other structures develop the columns become interrupted and particularly the lateral column; thus in the nucleus ambiguus of the adult we have a broken series of discrete nuclei.
The motor neuroblasts of the vagus point dorso-lateralward and form rootlets which emerge just ventral to the entrance of the sensory roots. After emergence they turn forward and form a common trunk which in the spinal region lies between the dorsal roots and the side of the spinal cord. The more caudal rootlets are devoid of sensory fibres; but as we go forward we meet with ganglionated rootlets. In the vagus as in the glossopharyngeal there are the ganglia of the roots and the ganglion of the trunk (ganglion nodosum). The ganglia of the roots represent a series diminishing in the caudal direction, as shown in Plate I. In the adult the more caudal ganglia usually disappear except for traces of scattered ganglion cells found occasionally on the rootlets of the spinal accessory division. These more caudal vagus ganglia are not to be mistaken for the Froriep ganglion, which represents a persistent precervical ganglion. In the one case we have a series diminishing from the head toward the tail, and in the other it is in the opposite direction. Owing to the tendency to regression on the part of more caudal of the vagus root ganglia the vagus complex, as was shown in a former paper (Streeter, ’04), becomes differentiated into a fore part or vagus division which is predominantly sensory, and a back part or accessory division which is almost wholly motor. In the 10 mm. embryo there is no division between the two parts.
On entering the wall of the neural tube the sensory fibres immediately unite in a longitudinal tract continuous with similar fibres from the facial and glossopharyngeal thus completing the formation of the tractus solitarius. In Plate III is shown a cross section of the neural tube in the vagus region, and on it is indicated the position of the tractus solitarius. The marginal or reticular zone of the alar plate in this region is mostly made up of the longitudinal fibres of this fasciculus, and directly ventral lies the similar group of fibres belonging to the trigeminal nerve. The relation of the two is best shown in Plate II.
Peripherally the fibres from the ganglia of the roots together with the remaining motor fibres that are not included in the accessory bundle are collected into a common trunk and pass down caudally to be lost in the ganglion nodosum. After they emerge at the distal end of this ganglion they bend medialward and lose themselves on the wall of the oesophagus.
The n. trigeminus possesses the largest ganglion of the Whole embryo. From this ganglion the three large peripheral divisions extend ventralward. The ophthalmic division passes forward and subdivides into the frontal and nasociliary nerves, the latter forming a long slender well defined branch passing just dorsal to the eye stalk. The maxillary and mandibular divisions pass downward and break up in their terminal branches among the cells of the maxillary process and mandibular arch respectively. Centrally the ganglion is connected with the brain by a thick short trunk which enters the wall at the pontal bend and opposite the first and second rhombic grooves. Within the wall the fibres immediately form a flattened longitudinal tract, as shown in Plate II, part of which extends caudally as the spinal tract, and part extends forward and upward to enter the cerebellar ridge. These fibres must be considered as containing both somatic and visceral elements, between which no difference could be made out ernbryologically.
In its motor elements the trigeminal nerve departs somewhat from the type represented in the other three nerves of this visceral group. In the others the nucleus of origin is in the basal plate, and the nerve rootlets exhibit a characteristic curved course to reach the point of emergence; while in the trigeminal the nucleus is more lateral and lies directly against the entering sensory fibres, so that the fibres of the motor root pass directly ventralward to fuse with the mandibular division.
It is possible that the motor nucleus of the trigeminal is to be considered as an hypertrophied example of one of the dorsal motor nuclei found in the adult ninth and tenth nerves, both median and lateral to the tractus solitarius and which have not yet been recognized in the embryo.
- For a description of the motor nuclei connected with the tractus solitarius see E. L. Mellus, ‘02.
In that case we must conclude that either the nucleus ambiguus and ventral motor root are not present in this nerve, or that they are represented by the mesencephalic motor root. In analyzing these nerves it is to be remembered that in man the typical visceral cranial nerve has three central terminations:
- Sensory root (tractus solitarius).
- Curved ventral motor root (nucleus ambiguus).
- Straight dorsal motor root (nucleus vagi dorsalis).
These three elements may be represented in the different nerves in different proportions. The ninth nerve approaches the mean and all elements are fairly represented. In the vagus the curved ventral motor roots are increased in proportion in the caudal portions and form the spinal accessory. In the facial the sensory root (n. intermedius) is diminutive, while the curved ventral root becomes the main trunk of the nerve. In the trigeminal it is the straight dorsal motor root that forms the principal motor supply, while the curved ventral motor root is either not present or is represented by its mesencephalic root.
Spinal Nerves
At the end of the first month each segmental nerve of the trunk possesses a sharply outlined spinal ganglion, whose constituent cells are in the early stages of differentiation. On examination it can be seen that many of these cells consist of a prominent nucleus surrounded by a. thin rim of ill defined body protoplasm. p In other cells the body protoplasm has increased in the form of a process at one or both ends. Cells of this kind are clustered so that their processes_ unite to form fibrous strands. These strands in turn fuse into larger bundles and lead toward the two poles of the ganglion. At the proximal pole they become grouped into the dorsal roots, which enter the spinal cord in an uninterrupted longitudinal series. In the cord they unite and extend up and down in the marginal zone in the form of a flattened band of fibres which later constitutes one of the dorsal funiculi of the cord.
The fibres from the distal pole of the ganglion unite in a common bundle which is almost immediately joined by the fibres of the ventral root, the two together forming the main trunk of the nerve. The ventral roots consist of a continuous series of rootlets emerging from the ventrolateral border of the neural tube, and derived from the neuroblasts of the mantel layer of its basal plate. These neuroblasts form a longitudinal column which, as shown in Plate II, is continuous with and of the same character as the nucleus of the hypoglossal nerve.
At the same time that the dorsal and ventral roots unite to form the main trunk they give off lateral fibres to form the dorsal branch, the so—called posterior primary division, which turns back dorsalward and covers in the distal part of the ganglion, and breaks up among the cells which are to form the long muscles of the back.
The remainder of the nerve trunk is continued forward as the ventral branch or anterior primary division. From its median side there is given off the ramus communicans, which extends medianward to the region of the aorta and ends in the sympathetic ganglion cord. The rami communicantes and the sympathetic cord are not shown in Plate I. The main trunk terminates in two branches, the anterior and lateral terminal branches, which correspond to the anterior and lateral cutaneous branches of the adult, and which in the thoracic and abdominal regions end among the cells giving rise to the musculature of the front and lateral body wall.
Throughout the spinal region there is a tendency for the nerve trunks to unite at the level of the lateral terminal branches and form intersegmental loops. This loop — or plexus — formation may involve either the lateral or the anterior terminal branches, or both. We thus have produced the cervical, brachial and lumbosacral plexuses.
In the cervical region the anterior and lateral terminal branches form two separate plexuses; the former produces the deep cervical plexus and the latter the superficial cervical plexus. The superficial cervical plexus consists of the union of the lateral terminal branches into loops from which are given off the cutaneous branches to the auricular, cervical and occipital regions. The deep plexus results in the formation of the ansa hypoglossi and the phrenic nerve. The former is produced by the fusion of the second and third cervical nerves into the descendens cervicis, which unites in a loop with the hypoglossal, together with which the first cervical has been incorporated above. From this loop are given off the short branches which end among the cells that are to form the hyoid musculature. The main trunk of the hypoglossal bends sharply medianward to end in the tongue anlage. The deep cervical plexus was studied in an older embryo (14 mm. Mall embryo No. 144) and described in a former paper (Streeter, ’04). The ansa hypoglossi is shown in fig. 11 and is essentially the same as in the present case, differing only in that the communication between the first cervical and the hypoglossal could not be traced. In Plate II of that paper the r. descendens is labelled wrong; the leader should extend to a point above where it is joined by the descendens cervicis, which can be seen as a slender nerve ‘made up of branches from the second and third cervical nerves.
The phrenic nerve is formed by anterior terminal branches from the fourth and fifth cervical nerves. A contribution on the part of the sixth could not be made out. A view of the nerve can be seen through the arm in Plate I. Owing to the position of the diaphragm at this time the course of the nerve is almost directly ventral, passing over the lung anlage which is not represented in the drawing. Later, as pointed out by His and Mall (Mall, ’01), the points of origin and insertion of the nerve draw gradually apart, due, on the one hand to the descent of the diaphragm and the lengthening of the thoracic cavity, and on the other hand to the subsequent elevation of the cervical nerves which accompanies the development of the structures of the neck. It is thus that there results the long caudal course of this nerve that is characteristic of the adult.
The brachial plexus is shown in Plate I by representing the arm as transparent. In the region of the fifth cervical to the first thoracic nerves there is an exuberant growth of both the anterior and lateral terminal branches, resulting in a solid flattened mass of fibres, which in turn is split by the skeletal anlage into two laminae, from which the various nerves arise. Arising from the anterior or ventral lamina one can recognize the nn. musculocutaneous, medianus and ulnaris, and from the posterior or dorsal lamina the nn. axillaris and radialis. These nerves pass down into the arm and break up in the muscle masses, which they are to supply.
The lumbosacral plexus as compared with the brachial plexus is somewhat retarded in its development. It is formed by the fusion of the trunks of the five lumbar and upper three or four sacral nerves. These nerves and their ganglia, as with the cervical nerves, are enlarged as though stimulated to extra growth by the presence of the limb bud. The nerves unite into a flattened mass of fibres which enters lateralward into the base of the leg bud, the division into anterior and lateral terminal branches being lost in the formation of the plexus. The further course of the fibres is determined by the framework of the leg. Owing to the cell masses of the bony pelvis and the femur the fibres become grouped into four bundles arranged in two pairs, each consisting of a median and lateral trunk. Of the upper pair the median trunk corresponds to the obturator nerve, and the lateral the femoral nerve.
The lower pair corresponds to the sciatic nerve, and its median bundle constitutes the future tibial nerve, and the lateral the common peroneal. From these larger nerves smaller branches split off and become isolated as discrete nerves, and enter the muscle masses between which the main trunks lie.
The Sympathetic System
The ventral migration of the cells derived from the neural crest, that are destined to become sympathetic ganglion cells, is completed by the end of the first'month. The elements derived from the successive segments have by that time fused together, on each side of the body, to form a longitudinal column of proliferating cells, situated lateral to the aorta and directly in front of the developing bodies of the vertebra
This column extends as a rather sharply outlined continuous cellular strand from the occipital region to the level of the lower sacral vertebrae. The differentiation of the individual cells is already under way and nerve fibre formation can be recognized throughout its length, being most pronounced in the thoracic and lower cervical region. This process however has not advanced far enough to produce a breaking apart of the column into segmental nodes, as is characteristic of the adult ganglionic chain.
Dorsally the column remains in connection with the nerve roots and spinal ganglia by means of the rami communicantes, which consist of sharply outlined fibrous bundles, varying from one to three bundles to each segment. The rami communicantes are largest and best developed in the thoracic and lumbar regions. In the cervical region they are least well developed, and owing to the slanting course of the upper cervical nerves they could not be satisfactorily traced in sagittal sections for the upper three segments.
Ventrally in the region of the sixth to the eleventh thoracic segment a definite fibro-cellular plexus extends forward to form the splanchnic nerves and coeliac plexus. The splanchnic nerves extend almost directly forward. As is the case with the phrenic nerve their caudal course is brought about later by the subsequent caudal displacement of the viscera relative to the bodies of the vertebrae. At this time the diaphragm and caudal surface of the heart lie opposite the first and second thoracic vertebrae, and the stomach is opposite the fifth to tenth thoracic vertebrae so that the coeliac region is directly ventral to the origin of the splanchnic nerves from the ganglionated cord.
The cardiac plexus and the cranial sympathetic ganglia owing to the more complicated architecture of those regions could not be outlined with any accuracy. The cephalic end of the ganglionated cord can be traced median to the hypoglossal nerve to the region situated between the ganglion nodosum and the wall of the pharynx. Its extension along the internal carotid artery and commuication with the ganglia of the head could not be made out.
Literature
Bardeen CR. Development and variation of the nerves and the musculature of the inferior extremity and of the neighboring regions of the trunk in man. (1906) Amer. J Anat. 6:259–390.
His, W., 1888. Zur Geschichte des Gehirns sowie der centralen und peripherischen Nervenbahnen beim menschlichen Embryo. Abhand. math.phys. C1. Kgl. Sachs. Gesell. d. Wiss., Bd. XIV.
Lewis WH. The development of the arm in man. (1902) Amer. J Anat. 1(2): 145-184.
Mall FP. On the development of the human diaphragm. (1901) Johns Hopkins Hospital Bulletin 12: 158-171.
MELLUS, E. L., 1902. On a hitherto undescribed nucleus lateral to the fasciculus solitarius. Amer. Jour. Anat., Vol. II.
Streeter GL. Peripheral development of the cranial and spinal nerves in the occipital region of the human embryo. (1904) Amer. J Anat. 4: 84-116.
STREETER, G. L., 1904. The development of the cranial and spinal nerves in the occipital region of the human embryo. Amer. Jour. Anat., Vol. IV.
Streeter GL. The nuclei of origin of the cranial nerves in the 10 mm human embryo. Anat. Rec, 2: 111-115.
Plates
Plate I
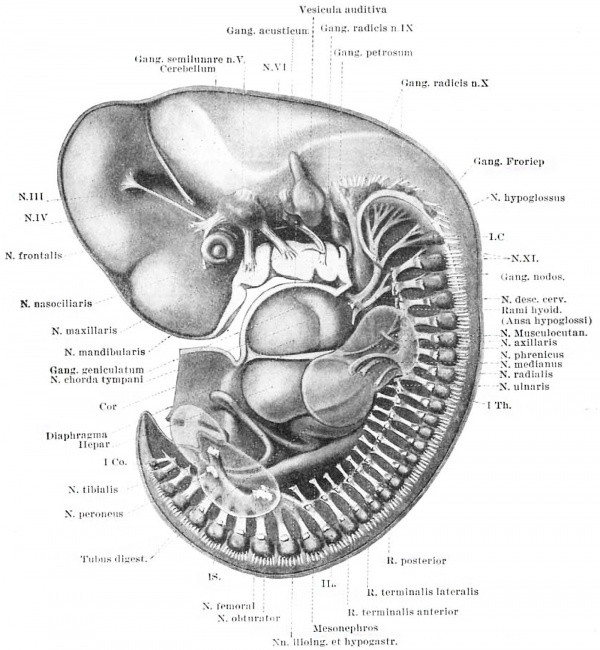
Lateral View of a wax plate reconstruction of a 10 mm. human embryo (No. 3, Huber collection), showing the origin and distribution of the peripheral nerves. The ganglionic masses are represented by darker and the fibre handles by lighter shading. For purposes of orientation the diaphragm and some of the viscera are shown. The arm and leg are represented as transparent masses into the substance of which the nerve branches may be followed. The original model is enlarged 40 diameters and the drawing is about 12 diameters.
Plate II
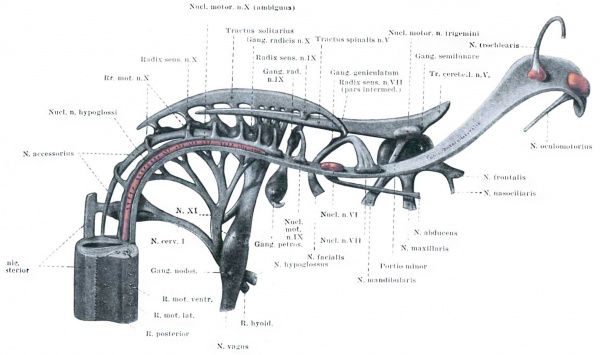
Median view of a model of the cranial nerves in the same embryo shown in Plate 1. A portion of the spinal cord is represented and above that everything is cut away, excepting the sensory bundles and motor nuclei of the different nerves, together with that portion of the marginal zone which is to form the funiculus anterolateralis. The somatic motor nuclei are colored red, and it can be seen that they form a column that is practically continuous with the cells of the ventral horn of the spinal cord. Enlarged about 30 diameters.
Plate III
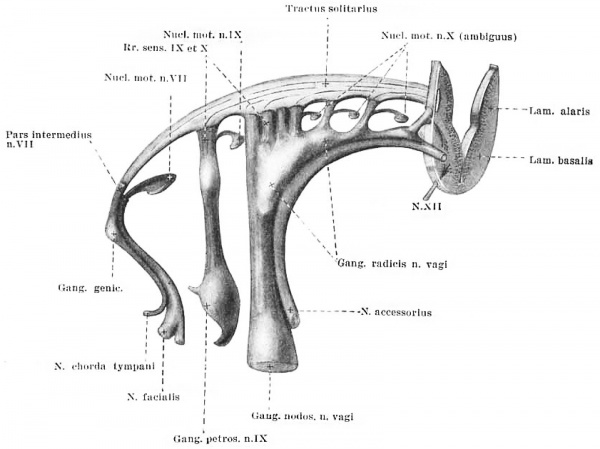
Reconstruction of the facial, glossopharyngeal and vagus nerve group in the same embryo shown in Plate I, showing the series of motor nuclei of origin and the tractus solitarius. It can be seen how the latter is formed by the sensory roots on entering the marginal zone of the neural tube, analogous to the dorsal funiculi formed by the dorsal roots of the spinal cord. A section of the neural tube is included in the reconstruction to show its relation to these different structures. Ganglionic masses are represented by lighter shading than the fibre bundles, and the point at which the rootlets enter the neural tube is indicated by dark rings. Enlargement about 35 diameters.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The Peripheral Nervous System in the Human Embryo at the End of the First Month (10 mm). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Peripheral_Nervous_System_in_the_Human_Embryo_at_the_End_of_the_First_Month_(10_mm)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G