Paper - The Origin of the Otic and Optic Primordia in Man
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Bartelmez GW. The origin of the otic and optic primordia in man. (1922) J. Comp. Neural., 34: 201-232.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Origin of the Otic and Optic Primordia in Man
Department of Anatomy, The University of Chicago, and the Laboratory of Embryology, Carnegie Institution of Washington
Ten Figures
Introduction
The early history of the nervous system in man and in the great majority of other mammals is but imperfectly known. In the Whole order there is only a single detailed study of the origin of the cranial ganglia. The data on this phase of human development are for the most part included in descriptions of single specimens; they are few and incomplete and marred by faulty interpretations due to the lack of a human series and neglect of comparative material from other forms.
We shall here confine our attention to the otic and optic primordia, although the identification of them in the various embryos of the series is based in part upon our interpretation of the primary subdivisions of the nervous system. This evidence will be presented in a subsequent paper.
Material
The present observations are based upon the study of complete serial sections of twelve normal embryos ranging between stages of three and sixteen somites. In addition, the data from the published descriptions of four others have been available, so that the series is a reasonably complete one, even though some of the specimens leave much to be desired in the matter of histological detail. We have models of eight of the embryos, and in the case of some of them several models were prepared. The specimens studied in this connection are as follows:
| Designation of Embryo | Number of Somites | Length | Condition | |
|---|---|---|---|---|
| 1 | H279 Univ. of Chicago (3709) | 4 | 2.5 mm in formol Fair, abundant mitoses | |
| 2 | ‘Klb’ Normentafel no. 3 | 5 to 6 | 1.8 mm in alcohol | Excellent |
| 3 | 391 Carnegie Coll. (Dandy 1910) | 8 | 2mm in formol | Fair |
| 4 | H87 Univ. of Chicago Coll. | 8 | Circ. 2 mm in forol | Good |
| 5 | Eternod embryo ‘DuGa’ (1896) | Probably 9 | 2.12 mm from number of sections in the series | Excellent |
| 6 | H637 Univ. of Chicago Coll. | 11 | Distorted. 1.85 mm from number of sections | Excellent histologically |
| 7 | H197 Univ. of California Coll. | 12 | 1.15 mm (much flexed) | Good |
| 8 | H392 Univ. of Chicago Coll. | 11 | 3.6 mm in formol | Fair clumped mitoses |
| 9 | #4 New York Univ. Coll. (Wallin 1913) | 14 | 2.3 mm after fixation | Good histologically |
| 10 | H8 Univ. of Chicago Coll. | 14 | 3.3 mm in alcohol | Poor |
| 11 | ‘Pfst. III’ Normentafel #6 | 14 | Circ. 2.6 mm | Excellent |
| 12 | 470 Carnegie Coll. | Probably 16 | 3.3 mm in 95% alcohol | Fair clumped mitoses |
Acknowledgments
This paper is part of a study of human development during the early part of the period of somite formation, begun in 1915 in the Carnegie Laboratory at Baltimore in conjunction with Dr. H. M. Evans (1917). The complete account is to appear as a joint paper in the Carnegie Institution’s “Contributions to Embryology.” Although Dr. Evans’ own material, his extensive studies on embryos in the European collections and in the Mall collection have been used freely, this part appears under my name as I am assuming complete responsibility for the interpretation of the nervous system. Needless to say, I am under great and varied obligations to Doctor Evans which I gladly acknowledge. The work was begun at the suggestion of the late Dr. F. P. Mall, to whom we owe much. Dr. G. L. Streeter has continued to support it and has helped and advised. Most of the drawings are the work of Mr. J. F. Didusch, Whose understanding help has been invaluable.
We would express our appreciation of the courtesy of Profs. Franz Keibel, A. C. F. Eternod, and H. D. Senior for permission to study the young human embryos in their collections.
To the following physicians we are indebted for the embryos of our own series, for their cooperation and for the care which they took to preserve these delicate specimens: Dr. J. P. Spooner, of Peru, Indiana, for H279; Alpheus B. Streedain, of Chicago, for H87; Dr. Edwin Hirsch, of Chicago, for H637; Dr. Robert T. Legge, of Berkeley, California, for H 197; Dr. Ethel Rice, of Chicago, for H392; Dr. J. F. Burkholder, of Chicago, for H8. The exceptional success we have had in obtaining young embryos has been due in large measure to the support and cooperation of Dr. R. R. Bensley.
The Otic Primordium
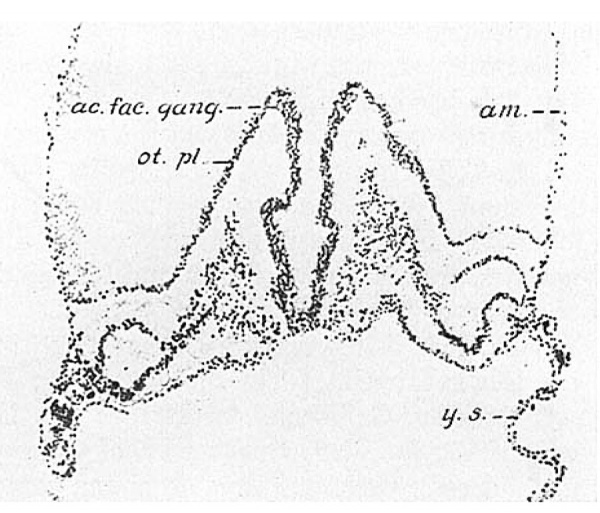
The earliest sensory primordium that can be recognized in man is a thickening of the ectoderm opposite the neural folds of the hindbrain. This is the beginning of the otic plate. Several statements in the literature indicate that the otic plate appears early in human development. In 1908 Keibel identified ‘die Horplatte’ in the Unger embryo (Normentafel 4), which had about nine somites. Wilson (’14, p. 325) suggested that a pair of diffuse thickenings in his two- to three-somite embryo (H3) might be the ‘auditory areas.’ Tracing the otic plate back through our series leaves little doubt but that he was correct in this. Ingalls (’20, p. 67) likewise identified it in his slightly older specimen (Carnegie Collection no. 1878), and with greater certainty, as he had several of our models of older stages for comparison. Figure 1 shows the conditions in the first of our presentseries, a four-somite embryo (H279) with wide open neural folds.The dorsal part of the fold near the beginning of the second subdivision of the hindbrain (cf. p. 207) is enlarged; a swelling protrudes toward the ectoderm, ac. fac. g<mg.; and there is a corresponding ventricular sulcus which does not show clearlyin the photomicrograph. This is Very like the condition described by Schulte and Tilney (’15) in the cat. The enlargement is termed acousticofacial ganglion in accordance with the customary mammalian usage (cf. p. 214). The ganglionic anlage is capped by a single layer of ectoderm cells which appears as if it had slipped over the top of the neural fold. The adjacent ectodermis obviously thickened as the otic plate, at. pl. The section,which is nearly horizontal through this region, shows almost the whole rostrocaudal extent of the otic plate. In most sections it fades off gradually into the surrounding ectoderm so that its limits are difficult to determine.
Fig. 1 A photomicrograph of section 54 of the four somite embryo H27(Univ. of Chicago Coll.). X 100. The plane of section is almost horizontal to the hindbrain. ac. fac. gomg., anlage of the aeousticofacial ganglion. The overlying cap of ectodermal cells is more deeply stained than the ganglion. ct. pl.,otic plate; a,m., amnion; y. 3., yolk sac.
Both the ganglion and the otic plate are present in the six-somite ‘Klb.’ of Keibel, but our tracings are not sufficiently detailed to permit of an accurate description. At the time they were made the presence of the anlagen was not suspected. They can be found in the eight-somite embryo first described by Dandy (’10) (Mall, No. 391). Figure 2a is taken from a section through the middle of the otic plate of this embryo and shows the ganglionic primordium clearly on the right side. This appears as an out-pouching of the neural fold with the characteristic cap of overlying ectoderm.
The ‘otic sulcus’ is manifest in a model of this region made at 400 diameters. It is a broad shallow pit near the dorsal edge of the fold extending through four of the 10 μm sections. In this case also it belongs to the second hindbrain segment and lies just caudal to the first visceral pouch.[1]
The depression to the left in the figure is not the beginning of the otic pit, but a chance wrinkling of the ectoderm.
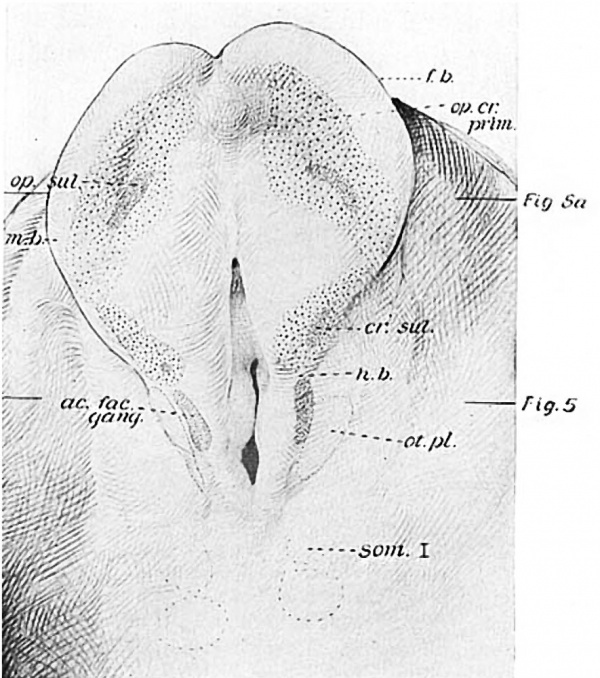
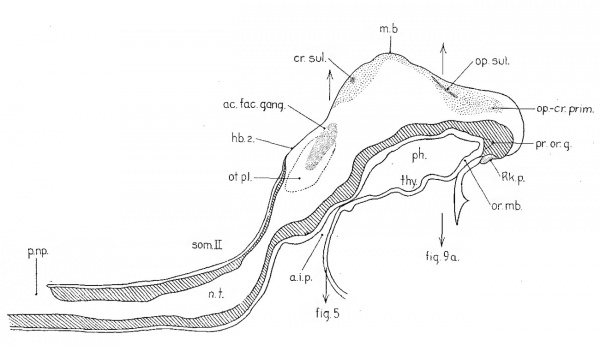
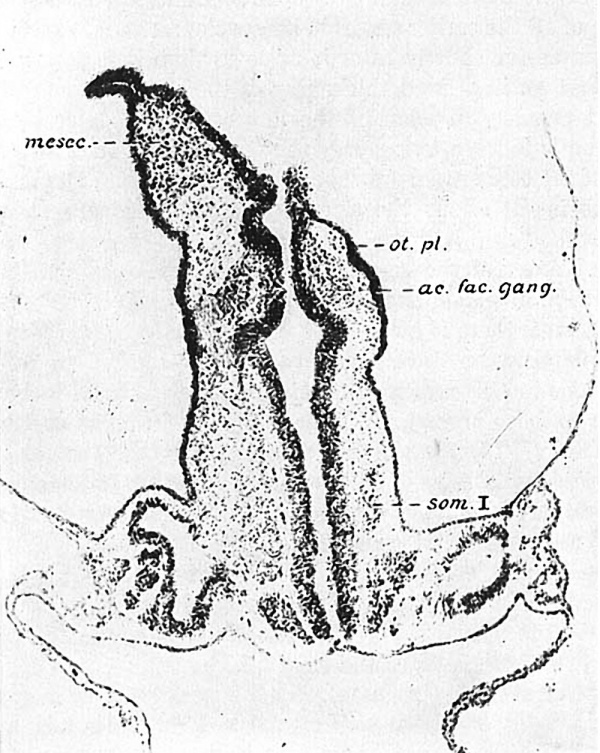
With the illustrations at hand it will be easier to visualize these relations in the other eight—somite embryo, H87. Figure 3represents the dorsal aspect of a model and figure 4 is from a projection reconstruction of the embryo cut in the mid-sagittal plane and viewed from the right. The sensory anlagen are plotted in from a detailed study of the sections and indicated by stippling. In the former figure we see the broad expanse of forebrain, still in the neural-plate stage. The deep neural groove has progressed as far forward as the midbrain, behind which are two large hindbrain subdivisions. From the second of these the acousticofacial ganglion is arising and laterally in the ectodermis the otic plate reaching back as far as the third hindbrain segment. The primary brain segments are better shown in figure 4.
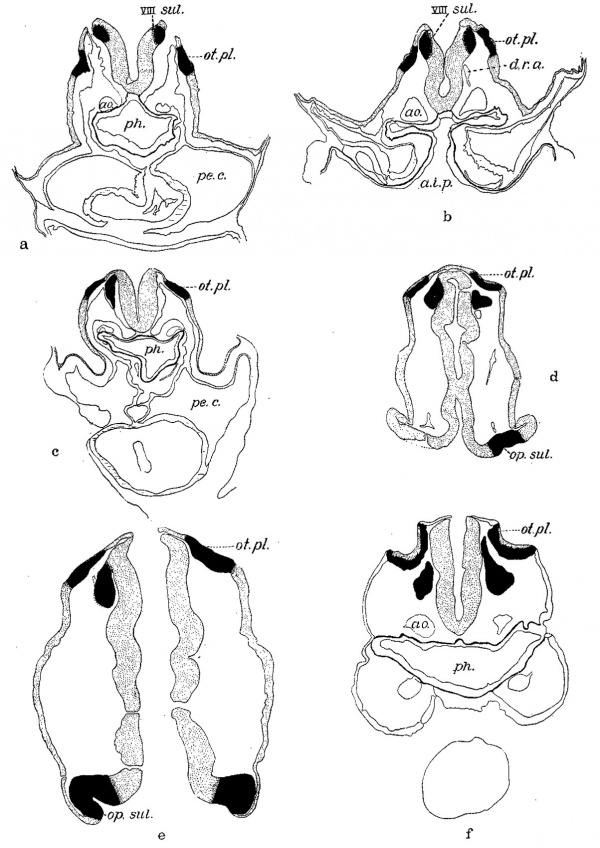
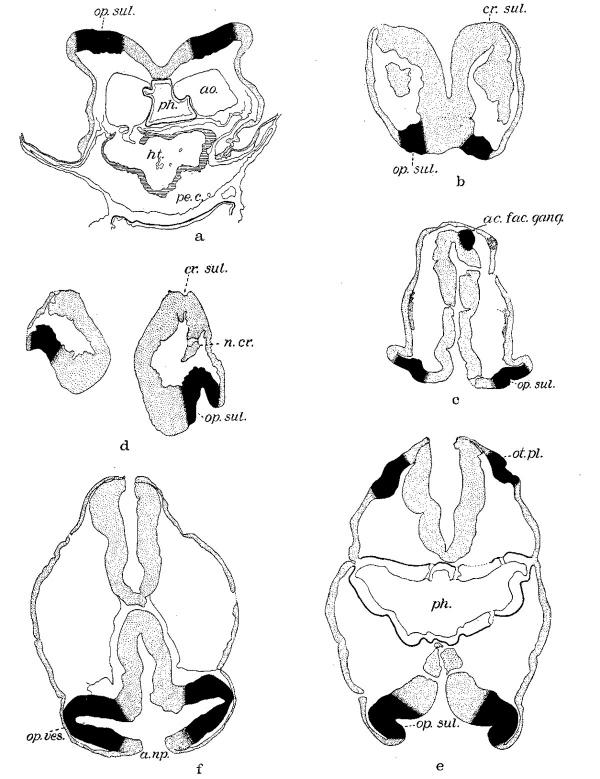
Fig. 2 A series of tracings made from the sections with the Edinger apparatus, X 200, and reduced to 66% diameters in reproduction. The solid color marks the otic plate and the acousticofaeial ganglion and optic anlage; the rest of the nervous system, including the neural crest and the ectoderm, are stippled, the outer boundary of the pharynx is indicated by a heavy line and the myoepicardium marked by horizontal lines. The mesenchyme is not shown except Where it was shrunken and then its outer limits are drawn. a. Mall embryo no. 391—eight somites—section 45. b. H87—eight somites wsection 64 (cf. figs. 3 and 4). c. Eternod ‘Du Ga’—nine somites—section 49. d. N.Y.U. embryo no. 4—fourteen somites—section 32. e. Pfannenstiel III—fourteen somites—section 41. f. Mall embryo no. 470-sixteen somites—s1. 2-1-7. a.i.p., anterior intestinal portal; ao., aorta; d.r.v., dorsal second aortic ramus; op.sul., optic sulcus; pe.c., pericardial cavity; ph., pharynx; VIII sul.; otic sulcus. In this and all other figures of sections the right side of the embryo appears at the observer’s left.
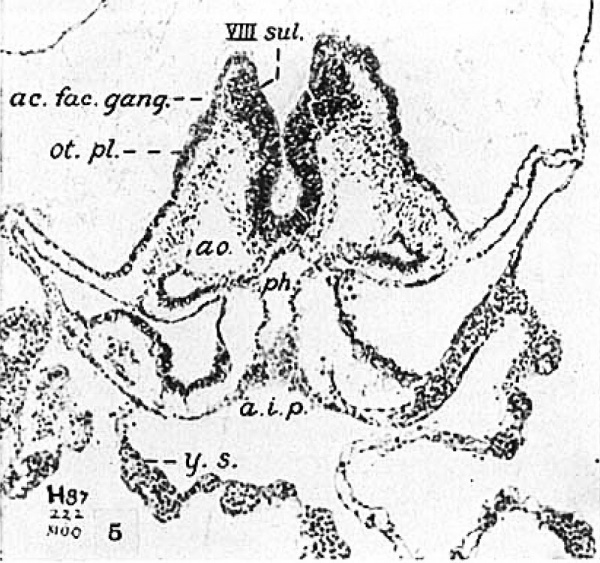
Here We find the summit of the cranial flexure marked by a boss, the midbrain, m.b., limited fore and aft by slight constrictions of the neural folds. Caudal to it the two hindbrain sub-divisions are obvious. Cross section pictures of the otic region are given in figures 5 and 2b which are taken from successive sections, the level of which is indicated in the two general views. The photomicrograph (fig. 5) brings out clearly the apparent evagination of the neural fold at the site of the ganglionic anage, an earlier stage of which We saw in the eight-somite embryo of the Carnegie Collection (Dandy) in figure 2a. No actual
evagination occurs, however, as we shall see: the sulcus is merely an effect of the lateral migration of the ganglionic cells en masse. Caudally they are already more loosely arranged than they
appear in the figures and the epithelial arrangement which characterizes the neural fold is disappearing. The delaminating mass is, however, differentiated from the surrounding mesenchyme by the closer proximity of the cells to one another and by their deeper stain.
Fig. 3 A geometric projection of the head end of a model of H87 (Univ. of Chicago Coll.), drawn by J. F. Didusch and reduced to 100 diameters. Upon it the otic and optic-crest primordia are plotted and represented by stippling. , The densest areas indicate the position of sulci ; as. few. gang., aeousticofacial ganglion; cr. sul., neural-crest sulcus corresponding in this case to the position of the future Vth ganglion; f.b., forebrain; h.b., hindbrain; m.b., midbrain; op.cr.prim., optic-crest primordium; 0t.pl., otic plate; s0m.I, first somite.
Fig. 4 A projection reconstruction of the medial aspect of H87 as if cut in the midsagittal plane. Only nervous system and gut are represented. The cut surfaces are indicated by hatching. The primordia are indicated as in figure 3. a.i.p., anterior intestinal portal; h.b.2, second subdivision of the hindbrain; N .T. , closed region of neural tube; or.memb., oral membrane; ph., pharynx; p.np., posterior neuropore; por.g., preoral gut; Rk.p., Rathke’s pouch; som.II, these letters indicate position and extent of second somite; thy., thyroid evagination. Other abbreviations as in figure 3. X 120.
At the caudal end of the anlage it is possible that some cells are migrating out into the mesenchyme, but the plane of section is oblique, making the microscopic picture difficult to interpret.
In the next embryo, ‘Du Ga’ of Eternod, we find a great increase in the cephalic mesenchyme; the head and especially the first two visceral arches are taking form. The neural folds have closed at the caudal levels of the otic plate, but are still open in the region of the acousticofacial ganglion. Figure 2c represents the relations in a section through the caudal end of the right ganglion which is no longer in direct continuity with the neural fold except along its dorsal edge. From this well—fixed specimen, as well as from H637 which is equally good histologically, and from the description of Veit (’l8), it is obvious that there has been a loosening up of the ganglionic anlage followed by a separation of the entire mass from the neural fold so that it has delaminated before the two folds meet to form a tube.
Fig. 5 A photomicrograph of section 63 from the eight—somite embryo H87‘. X 100. The plane of section passes through the ganglionic anlage on both sides and through the rostral end of the otic plate. ac.fac.g¢mg., acousticofacial ganglion; a.i.p., anterior intestinal portal; ao., aorta; 0t.pl., otic plate; ph., pharynx; y.s., yolk sac; VIII sul., ganglionic sulcus.
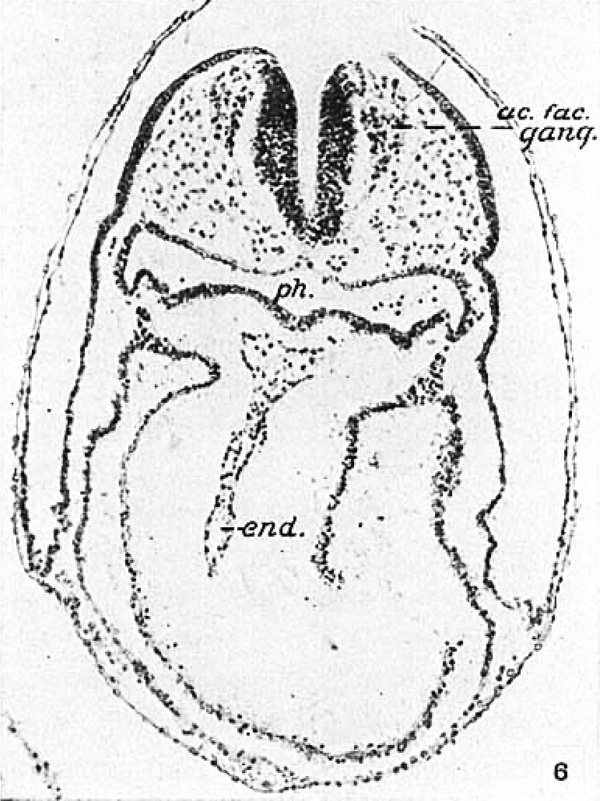
Fig. 6 A photomicrograph of section 50 from the eleven-somite embryo H392 (Univ. of Chicago Coll.). The plane of section passes in front of the otic plate and shows the ganglia separated from the neural folds except at the dorsal edges. The tube is closed immediately caudal to this level. end., cardiac endothelium.
A word should be said here concerning this interpretation of Veit’s findings in his excellently preserved eight-somite embryo. His clear, accurate description and objective figures leave no doubt but that his caudale Ganglienleiste is what we interpret as the acousticofacial ganglion. Having but the one specimen at his disposal, Veit lacked the necessary data for identifying the subdivisions of the neural folds, and consequently his suggestions as to the fate of the Ganglienleisten are purely speculative.
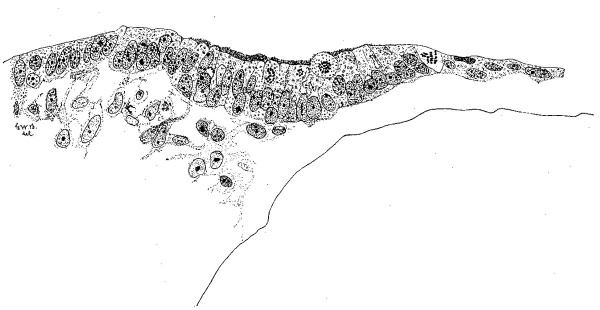
Turning now to the otic plate, no hint of an otic pit is to be found in either our eight- or nine-somite stages, nor did Keibel and Elze (’08) find any in their nine-somite embryo (N T No. 4). In the eight-somite specimen (H87) the otic plate is distinguished by a fighter peripheral zone. An additional feature appears in ‘Du Ga’ (nine somites) where Doctor Evans noted a peripheral brush border on the otic plate when he studied the a series in 1910. In this embryo the plate appears as a boss on the side of the head. When we come to the eleven—somite embryo, H637, we find the invagination just beginning. The histological appearance in this case is shown in figure 7, which represents a median section through this early otic plate. As we pass down from the dorsal ectoderm (from right to left in the figure), it will be seen that the pseudostratified condition of the ectoderm is preserved, but the distal moieties of the cells have elongated while the nuclei remained basal in position. Thus the clear peripheral zone is formed which characterizes the otic plate in all the older members of the series. When aparticular cell begins to divide the nucleus migrates peripheralward and, as the mitotic figure develops, most of the cytoplasm flows up around it. The resemblance of the dividing cells to the germinal cells of the central nervous system is obvious and they occupy the same relative position in the epithelium. The brush border appears over the central cells of the otic plate, and in this section resembles a band of short exceedingly delicate cilia. At other levels it looks more like a granular exudate. This is probably not due to inadequate fixation, as the specimen came from an unruptured tubal pregnancy with a strikingly normal appearing implantation site and was immediately opened and placed in Zenker stock solution. That the embryo was normal is Very probable. There were no immediate symptoms clinically except a lapsed menstrual period. Unfortunately for the embryo, it was cut and folded in the process of opening, but cytologically it is excellent. The plate cells show a Well-developed internal reticular apparatus (‘canalicular apparatus’) which has not been detected in any other cells of the ectoderm at this stage. It appears as a series of clear spaces in the cytoplasm. It will be recalled that Ramon y Cajal (’12) found the internal reticular apparatus to be the first cytoplasmic differentiation which appears in the histogenesis of the neuroblast.
Fig. 7 A section through the middle of the otic plate of the eleven—somite embryo H637 (Univ. of Chicago C011,). Camera drawing with 2-mm. Zeiss apochromate and comp. ocular 2 at 1000 diameters and reduced one-half in reproduction. The wavy line below at the right marks the external boundary of the neural tube. There is no mesenchyme between it and the otic plate.
The otic pit in the next two embryos is no further advanced in development than in that just described. In both it is shallow, in the eleven-somite H392 confined to the rostral end of the plate. The differentiation of the ganglion from the neural fold is complete histologically in H 197 (fig. 8) and figure 6 illustrates a slightly later stage in transverse section. In the fourteen-somite New York University embryo (Wallin) a greater portion of the plate is invaginating, as may be seen in figure 2d on the right (at. pl.). The ganglion appears iI1 this section and in the one shown in figure 9c. It is this which Wallin (’13) interpreted as the trigeminal ganglion, for he failed to recognize the otic plate. In Pfannenstiel III (fourteen somites) the otic pit has deepened and lies somewhat ventral of the center of the plate. The appearance in section may be seen in figures 2e and 9c; in the former the rostral end of the plate and the ganglion are shown on the left, while on the right the plane of section passes behind the ganglion. The section shown in figure 9e lies still further caudad and exhibits the progress made by the otic invagination in this embryo. Ectoderm and neural tube are perfectly distinct in both sections and were continuous across the midline in life, as is obvious from the study of a model constructed at a magnification of 200 diameters. The statement in the Normentafel indicates that Keibel and Elze (’08) also considered the neural tube closed in this region. Low (’08), however, was misled by the artificial breaking of the ectoderm and separation of the neural folds which extends far back into the region of the somites. Such cracks can be avoided, even in freshly preserved embryos, only by the most scrupulous care in dehydration and clearing if the material is to be imbedded in paraffin.
The otic plate of the fourteen-somite embryo H8 presents an intermediate stage between the last specimen and that shown in figure 2f. The whole plate is invaginating, but the pit is not so deep as it is in the sixteen-somite Mall embryo there figured. The marked increase in the thickness of the otic epithelium will be noted in this case. In both embryos the ganglion is almost completely separated from the neural tube. This separation was apparently not so complete in the fifteen-somite embryo of Giglio-Tos (’02).
Fig. 8 A photomicrograph of the twenty-ninth section of the twelve-somite embryo H 197 (University of California Coll.). X 100. The section passes horizontally through the hindbrain region. On the right of the figure the whole rostrocaudal extent of the otic plate appears with the beginning of the otic pit. The relations of the underlying acousticofacial ganglion (ac.fac.gang.) to it and to the neural folds are well shown. It will be seen that the neural fold is thinner Where the ganglion has separated off.
- ↑ Manifestly our interpretation of the nervous system of this embryo does not agree with that of Dandy (’10). Having identified the cranial flexure, the otic plate and its associated ganglion throughout our series, it has been possible to interpret correctly the subdivisions of the neural folds. What he has termed the second brain vesicle is in reality the second division of the hindbrain.
Discussion — The otic primordia
The findings in respect to the otic plate require little comment, Wilson (’14) and Ingalls (’20) found it difficult to determine the limits of the plate in their two-to-three-somite specimens and this is true of all the earlier stages in its development. It is relatively more extensive shortly after it appears than it is later. There is at first an ill—defined thickening of the ectoderm opposite the second primary division of the hindbrain, but later While the ganglion is differentiating only the cells at the dorsal and rostral ends of the ectodermal area continue to elongate. The invagination begins at about the time the neural folds are closing at this level.
The history of the so-called acousticofacial ganglion has not been made out adequately in any mammal. It cannot be done in man until there is a complete series of Well-preserved embryos available between fifteen and twenty-five somite stages. The conditions in this region of carnivore embryos seem to be quite similar to those in man. Weigner (’01) for the ferret and Schulte and Tilney (’15) for the cat consider that the geniculate and acoustic ganglia arise from a common anlage. It remains to be seen whether, in mammals as in many other vertebrates, the otic plate contributes cells to the eighth ganglion. Certainly, such an addition cannot be great. As for the geniculate ganglion, it is certain in the human that part of it arises from the epibranchial placode of the hyoid arch which is constantly present in fourteen-somite stages and later (cf. the findings of Giglio-Tos in his fifteen-somite specimen). This contribution from epibranchial placodes has been well established for all cranial nerves in Ichthyopsida which have a gustatory component, largely through the work of Landacre and his students. In the case of Ameiurus, Landacre (’10) found that the entire ninth ganglion arises from such a placode, and in the adult of this fish the ninth appears to be a pure gustatory nerve. The evidence here is so clear-cut as to Warrant the working hypothesis that in other vertebrates the gustatory ganglion cells of the seventh, ninth, and tenth cranial nerves arise from epibranchial placodes. The other components are probably derived from the neural crest, and there is abundant evidence in our series for such a proliferation at the levels corresponding to these nerves.
It should be said in conclusion that the acousticofacial ganglion in man is manifestly derived from the wall of the neural tube before its closure. The ganglion cells separate off, leaving the dorsal part of the fold thinner here than elsewhere as is well shown in figure 8. Even the few sections here figured leave no doubt of this. The evidence clearly excludes the possibility that the ganglion is formed from cells intermediate between neural and somatic ectoderm because the cells lying dorsal as well as medial to the ganglionic anlage enter into the formation of the definitive neural tube.
It is obvious from what has been said that the origin of the more rostral cranial ganglia in man must be studied largely in neural—fold stages. Much of the confusion in the literature is due to the failure to recognize this, to ignorance concerning the functional components of the cranial nerves, and to the fact that distinct components may have different origins.
The Optic Primordia
The earliest anlage of the optic vesicle is so intimately associated with the cranial neural crest that the two must be discussed together. We will confine our attention to the extent and relations of the latter, as the details of this proliferation will be described elsewhere. The transformations which lead to the formation of the primary optic vesicle will be considered after we have sketched the development of the ‘optic-crest primordium as a whole.
The rostrally expanded neural folds in the younger members of the series show slight isolated thickenings which are difficult to interpret, if indeed they have any significance. When we come to the eight-somite embryo No. 391 (Dandy), we can identify definite primordia. Here, as we have seen, the otic plate and acousticofacial ganglion have appeared. Rostral to them we find the lateral third of each fold is thickened, the neural epithelium protrudes slightly toward the underlying mesenchyme and
Fig. 9 A series to show the early development of the optic primordia corresponding to that of the otic anlagen. (See figure 2.) Here the optic primordium and otic anlagen are indicated by the solid color. a. H87—eight somites—section 28. b. Eternod ‘Du Ga’—nine somites—section 12. c. Wallin embryo- fourteen somites—section 24. d. H392—e1even somites—section 9. e. Pfannenstiel III—fourteen somites—section 29. f. Mall no. 470—sixteen somites—slide 1-4-5.
in some sections there is a corresponding sulcus dorsally, i.e., on the future ventricular surface. On the left side three such thickenings can be recognized, one near the rostral end of the folds, one in the region of the cranial flexure, that is, in the mid-brain, and a third in the first hindbrain segment (of. p. 218 infra). A few sections through the latter level show a further differentiation in that the thickened neural fold has no lower (external) boundary. It seems to merge with the mesenchyme beneath. As the later stages show, this is the very beginning of the neural-crest proliferation; in other words, there is an active migration of cells from the neural folds. The histologic picture in these particular sections is not above reproach, but under low powers of the microscope it presents the same appearance as do the perfectly preserved embryos of this period. These specimens make it clear also that we are not dealing in this case with an artefact. It is significant that the proliferation begins in the region where the semilunar ganglion can subsequently be identified.
In H87 (eight somites) it has been possible to study these primordia carefully, as the ‘plane of section is particularly favorable. The forebrain here is in the form of a broad neural plate (fig. 3) which is in marked contrast with the domed folds of the four-somite H279, the Pfannenstiel-Kromer embryo (‘Klb’) and the eight-somite Dandy embryo. On the other hand, it resembles several embryos in this respect, e.g., the three-somite Carnegie No. 1878 (Ingalls), Wi1son’s ‘H 98’ (nine somites), I-I637 (eleven somites), the -twelve-somite H 197, and the fourteen-somite N. Y. U. specimen (Wallin).
From the levels which show the pouch of Rathke [7] (fig. 4, Ric. go.) there are lateral thickenings of the folds extending caudally into the hindbrain, as is indicated by the stippling in figures 3 and 4 (op. cr. prim. and c1'.). This longitudinal ridge may be divided into two parts, a larger broader area which converges rostrally toward its mate of the other side and a more caudal one which is narrower and more laterally situated. The constriction between them is in the region of the midbrain. This primordium gives rise not only to the optic vesicle, but to neural crest as well; that is, to ganglion cells and head mesenchyme. Hence the somewhat labored term ‘optic-crest primordium.’ It is a continuous zone on the left side of the H87; on the right it is difficult to be certain of it in two adjacent sections through the midbrain, a distance of 16 ,u. Brachet (’06, p. 244), in his amphibian studies, has pointed out the interesting and perhaps significant fact that the cranial neural crest and retina arise from homologous regions of the neural folds, and the continuity of the primordium in this series adds interest to our speculations on the subject.
As the figures show, the large rostral part of the primordium has a shallow sulcus on the upper, i.e., ventricular, surface which marks the position of the future optic evagin.ation and is, in fact, the optic sulcus. As we approach the midbrain the primordium becomes narrower and involves the lateral part of the neural fold, and there is no sulcus (fig. 3). On the left at least the primordium continues into the hindbrain where cells are migrating out from it. Here, then, we have a typical neural crest. Some of these cells remain relatively closely packed and enter into the formation of the semilunar ganglion. Others become head mesenchyme.
It is worthy of note, in View of the recent work of Landacre (’21),that promptly after the proliferation begins the mandibular and hyoid arches begin to take form (of. the Ziegler model of Eternod’s embryo ‘Du Ga’).
The appearance of a section through the rostral area of the primordium is shown in figure 9a, where both optic su.lci appear. The primordium is indicated by the solid color. _It is improbable that all of this eventually enters into the optic vesicle, as we shall see below.
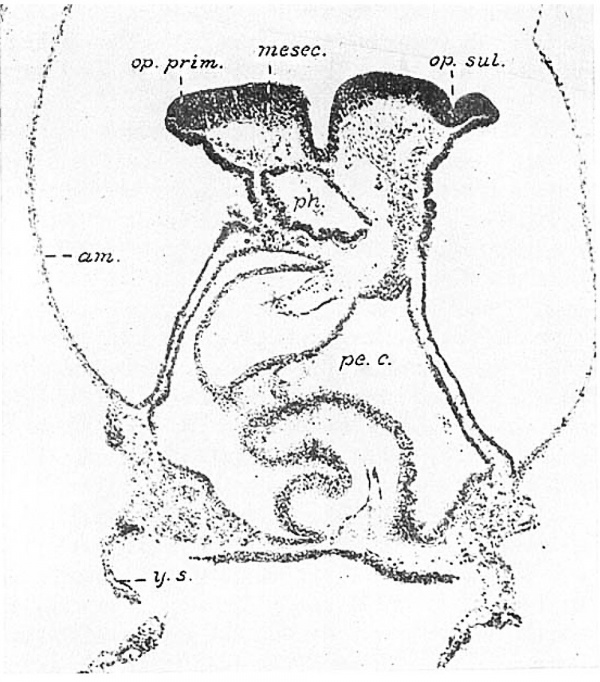
It will be best to turn now to the eighth member of the series, the twelve-somite embryo H 197, because the plane of section in the forebrain is very like that of H87, whereas in the three intermediate stages it is less favorable. There is a great dorsal fiexure in H 197 like that in the Wilson embryo H98 (’14) and consequently it was possible to obtain sections perpendicular rather than tangential to the surface of the forebrain folds. The optic-crest primordium shows several changes. Its rostral division is Wider, the optic sulcus has deepened so that the optic anlage is sharply delimited on all sides from the rest of the area. Mesial and rostral to it cells are migrating out from the primordium as mesectoderm. Caudally the primordium continues without a break into the hindbrain ‘where, as figure 8 (mesec.) shows, the mesectoderm is clearly differentiated from the rest of the mesenchyme by its deeper stain. The optic sulcus appears on the right side of figure 10; on the left the section passes through the rostral end of the optic primordium (op. pm'm.). The more medial portion of the thickened primordium in this figure shows the indefinite lower boundary which is characteristic of a mesectodermal proliferation. It would seem, then, that the optic anlage arises only from the lateral part of the area indicated by stippling in figure 3, at levels corresponding to the caudal part of the forebrain, i.e., the future diencephalon.
Fig. 10 A photomicrograph of the seventeenth section of the twelve-somite embryo H 197 (Univ. of California). X 100. On the right the section passes near the middle of the optic anlage (op.sul.); on the left through its rostral end (op.prim.). Medial to the optic evagination the thickened neural fold is» pro- liferating mesectoderm (mesecz). The section passes horizontally through the heart. pe.c., pericardial cavity; am., amnion; ph., pharynx; y.s., yolk sac.
H 197 is the only embryo we have seen which clearly shows mesectoderm formation in the forebrain, nor has it been observed in any other mammal. It can hardly be an abnormal activity in this particular case, for as a whole the embryo fits perfectly into the series. More than that, there are hints of such a process in another undoubtedly normal specimen, as We shall see. It is in better condition histologically than most young human embryos, as the photomicrographs (figs; 8 and 10) demonstrate.
The optic anlage of Eternod’s nine-somite embryo ‘Du Ga’ (fig. 9b) is no farther along than in the eight-somitc H87. In the eleven-somite H637 it is but little younger than in H 197 and the neural fold medial to it is greatly thickened. There are two spots where cells seem to be preparing to leave the primordium, and this evidence from an absolutely normal specimen affords the best evidence for regarding the extensive proliferation in H 197 as normal. Unfortunately, the mechanical injuries to the head in H 637 make modeling practically impossible. In the other embryo of this group, H392 (eleven somites), the optic sulcus is decidedly deeper than in H 197, as may be seen by comparing figures 9d and 10. Here, as in ‘Du Ga,’ the progress of the cranial flexure has bent the rostral end of the neural folds ventrally so that in the transverse series the first sections pass tangentially through the midbrain. Because of the oblique sections of the neural folds, it is impossible to get convincing pictures of neural—crest formation.
So far as the more caudal part of the optic-crest primordium is concerned, it is clear that in H637 (eleven somites) the optic anlage is continuous with the neural—crest proliferation of the mid- and hindbrain. In the beautiful 5 μm sections of this series one can observe every stage in the slipping out of the epithelial cells from the neural fold and Veit’s (’18) excellent description of his ‘craniale Ganglienleiste’ can be verified in detail. In ‘Du Ga’ and H392 and the N. Y. U. No. 4 (fourteen somites) there is a continuous crest anlage in the midbrain and the pre-otic hindbrain. In the first it reaches almost to the acoustico-facial ganglion, while in I-I392 (eleven somites) it cannot be recognized below the trigeminal level.
Early history of optic vesicle
We may turn now to the mass movements in the rostral division of the anlage which produce the optic vesicle. The general features of the process may be gathered from a survey of the sections reproduced in figure 9. The first is taken from H87 (eight somites). On the left we have a slightly more caudal level than on the right as the head end curved somewhat to the left. The level of the section is indicated on figures 3 and 4. The thickening of the neural fold, viz., the optic anlage, is indicated by the solid color. The optic sulcus and the bulging of the anlage into, the mesenchyme stand out‘ clearly. From the lateral portion of it the optic vesicle will arise, as We have determined by the study of the corresponding region in the older embryos. This is an earlier stage of the optic primordium than has been described for any mammal.
figure 9b is from the twelfth section of Eternod’s nine—somite embryo ‘Du Ga.’ The rostral limb of the cranial flexure appears below; above is the caudal mesencephalic limb. Only the optic anlage is indicated by the solid color; it shows the characteristic A thickening and the optic sulcus. Above in the figure is the mesectodermal proliferation with its corresponding ventricular sulcus. The optic anlagen in this specimen begin four sections (40p) behind the rostral end of the neural folds and can be recognized in five or six sections.
The next stage we have is in the fourteen—somite embryo of the New York University collection. In this case the forebrain appears to have lagged behind the rest of the embryo in development. Unlike the preceding specimen (fig. 9b) or the following one (fig. 9d), this has the anlagen cut transversely (fig. 90). Active evagination has begun and on one side there is a deep pit at the center of the primordium, which is, however, confined to the single 5;; section here drawn. Figure 10 (op. sul.) shows the deepening optic sulcus as it appears in the twelve-somite embryo H 197. The optic portion is differentiated from the rest of the anlage as has been said and appears as a long narrow field measuring 60 X 200; with the sulcus running through the middle. The optic anlage of the eleven-somite H637 is practically identical in appearance with this one.
Figure 9d was made from the ninth section of the eleven-somite embryo H392. The space separating the two neural folds here is the neural groove, the bottom of which is one—tenth of amillimeter caudalward in the series. The sulcus is cut obliquely, but is actually deeper than in any case we have considered. It will be noted that the optic evaginations are still directed ventrally. They correspond closely in position and extent to those of ‘Du Ga,’ in fact these two embryos are very similar in most respects.
Our next stage is found in the fourteen—somite embryo ‘Pfst. III’ (Keibel u. Elze, ’08, Taf. 6), which is well known from the work of Low (’08) and others. This has the earliest optic anlage that has been described hitherto in man. It begins about 130;; behind the rostral tip of the nervous system and is present in twenty—one of the 10;; sections. Throughout this region the neural folds are still open. A section through the middle of the anlagen may be seen in figure 9e and a more caudal level appears in figure 2e. The striking change here is that the optic evaginations are now directed laterally toward the overlying ectoderm as a result of the rapid approximation of the neural folds. Ac- cording to Low, there is an area of contact between the young optic vesicle and the overlying ectoderm. Keibel und Elze describe the Vesicle as ‘close’ to the ectoderm, Bach und Seefelder (’14) intimate that there is no actual contact nor do Doctor Evans’ tracings show any. Certainly, there is no mesoderm between the two epithelia and in the immediately following stages the lateral side of the vesicle comes into close contact with the future lens epithelium. This is true of two embryos in the Carnegie Collection, No. 12 (fourteen somites) and N0. 470 (sixteen somites). ‘In the latter, as figure 9f shows, we have a fully formed optic vesicle resembling that of the His embryo ‘EB’ (’04) and of the twins described by Watts (’15). It is younger in the fourteen-somite embryo H8 in which the lumen is narrower and there is no ectodermal contact, although the anterior neuropore is as small as in the sixteen-somite No. 470.
Discussion - The Optic Vesicle
If we compare e and f of figure 9, it is clear that the optic anlage has begun to balloon out, by an interstitial growth as well as by a thinning of the wall. The optic sulcus has become V-shaped so that the anlage is a trough about as long at the base as it is deep. This holds not only for the fourteen—somite H8 and the sixteen-somite #470 (Carnegie coll.), but also for the embryos of Watts and that described by Bremer (’O6). It is not until the twenty—three—somite stages that the vesicle has assumed a more or less spherical shape. It is diflicult as yet to say how much of the area indicated by the solid color in figure 9e enters into the formation of the definitive optic vesicle. The narrow band of neural epithelium which separates vesicle and head ectoderm appears to grow very rapidly as the optic sulcus widens and deepens, and this growth certainly plays a part in the approximation of the neural folds. After their closure this lateralmost part of the original neural plate constitutes all there is of brain wall" separating the two Vesicles dorsally. It is of course possible that all of the dorsal and lateral diencephalic wall which we find between the optic stalks in later stages is derived from it. On the other hand, it seems more probable that, as the vesicle gradually pinches off, some of the original evagination is incorporated into the brain wall both dorsal as well as rostral and caudal to the developing optic stalk. Perhaps it would be better to say that a portion of the lateral brain wall is at first dragged out with the optic evagination. This would hold particularly for the zone between vesicle and head ectoderm. Schulte and Tilney (’15) have presented strong evidence that this is so for the cat. On the basis of a complete series of models, they have described an absolute decrease in the size of the optic vesicle during the process of its separation from the brain and the formation of the stalk. It is not unlikely that the same conditions obtain in man. With the rapid enlargement of the optic ventricle the Wall of the vesicle becomes thinner, Whereas the diencephalic Wall dorsal to it remains as thick as before.
These conditions emphasize another significant fact. The optic primordium is laterally placed, but not in direct continuity with the future skin ectoderm. The future roof plate and part of the alar plate intervene. This strongly supports the theory that the vertebrate eye originated Within the central nervous system. Other evidence for this has been convincingly presented by Parker (’08). If the optic vesicle and its derivatives Were lateral ectoderm incorporated into the neural plate, it would be necessary to assume that the hiatus left by the separation of the vesicle Was filled by an ingrowth of neural epithelium from either side. Such ingrowth should be manifest first as a notch in the side of the anterior neuropore or later as a suture. There is no evidence of such conditions in the pertinent stages We have examined, and We may confidently say that mammalian ontogeny offers no support for the theory of the peripheral origin of the eye.
The anatomical evidence here, as in vertebrates generally, indicates that the optic primordia are lateral in position from the outset. The experimental evidence which has been Well summarized by Mall (’19) is conflicting. The experiments of Stockard on Amblystoma (’14) are the most complete. In order to substantiate his theory of cyclopia, this investigator set out to prove that the earliest optic anlagen are median in position. In most of the embryos which survived the removal of the middle third of the rostral end of the neural plate the optic Vesicles were subsequently lacking, whereas they were usually present when lateral moieties were extirpated. Stockard was convinced that the evidence demonstrated the existence of a median origin of the two subsequently lateral vesicles. ' It is possible that he did not consider all the factors involved and that the results may be explained differently. It may be that his median extirpations had a much more general effect than he assumed and were in fact comparable to the general inhibition experiments with anaesthetics. The growing tip of the nervous system was removed, and this, in terms of Child’s gradient hypothesis, is the dominant region - of highest metabolism.- According to the severity of the injury, the development of one or both eyes was more or less inhibited. The extirpations of lateral areas would be more convincing if there had. been any attempt to map out the morphologically differentiated optic areas and remove them. Even then the regulatory restitution of an entire optic vesicle from a fragment of the primordium intrudes itself. It might prove possible to make small definitely localized injuries and trace them through their subsequent migrations, as Patterson (’10) did in his gastrulation experiments, and thus obtain more conclusive evidence.
Comparative Data
From the comparative point of view, there are several interesting aspects of these observations. Man is the only vertebrate species on record in which the otic primordium appears before the optic. The otic plate can be recognized at an extraordinarily early period-earlier, in fact than in any other form for which We have accurate data. Conversely, the optic anlage is differentiated relatively later than in most other mammals, if we take into consideration the fact that the earliest stage described‘ in the literature corresponds to our fourth stage (fig. 9d). The optic sulcus can be identified in man at a slightly earlier stage than that in which the otic plate appears in other mammals. The following résumé includes only the available mammalian literature.
Artiodactyls
Bonnet’s (’01) account of early sheep embryos gives only enough to make it clear that the optic primordium precedes the otic in this form. Neither was identified in his twelve-somite specimen (p. 10), but a fourteen-somite embryo (fig. 13) had well-developed optic vesicles, which indicates that the first anlage will be found at least as early as it is in the human. ‘Das Ohrgriibchen’ is first mentioned in a nineteen—somite stage.
The Normentafeln of Sakurai (’06) for the deer are more complete. The optic pit is first indicated in table 15 (eleven-somite embryo), While the otic plate appears at fourteen somites (table 17).
Keibel’s first stage of the optic Vesicle in the pig is a well-defined pit in the forebrain of a nine—somite embryo (’97, table 30). There is also the first hint of the otic plate here, but it must be remembered that the optic anlage doubtless appears earlier than this stage. The ten-somite series from which the Ziegler model was made has early optic vesicles rather than simple ‘foveolae.’ There is a marked evagination with a relatively wide lumen as yet directed ventrally, not laterally.
Carnivores
Weigner (’01) found the ‘first signs’ of an optic Vesicle in ferret embryos 1.2 to 1.5 mm. in length in which the neural tube had not yet completely closed and the otic pits were already present. His data are not sufficiently exact to make it clear which primordium is actually the first to appear. In his careful study of a three—somite ferret embryo, Yeats (’11) refers to an ‘optic prosomere,’ ‘and it Would seem from his figures that there is an optic sulcus here. No mention is made of otic primordia.
There are two excellent papers on the early sensory anlagen in the cat. The earlier Work of Martin (’90) can be best considered in the light of the more complete and thorough studies of Schulte and Tilney (’15). Here (p. 322) it is probable that the optic anlage was indicated in the three—somite embryo. The four—somite specimen had optic sulci which resemble those of man (fig. 9d) rather than the optic ‘foveolae’ of the pig. In both cases the neural folds were still open throughout their extent. In the older one they identified trigeminal and acousticofacial ganglia, but the otic plate is not mentioned. It is possible that the latter appears early in the cat, although it certainly does not precede the optic anlage as in man.
The neural crest deserves further study in the carnivores. Weigner found no evidence for mesectoderm formation in the ferret and Schulte and Tilney affirm that all cells which leave the neural folds enter into the cranial ganglia. Martin (p. 342) found ‘neural crest’ beginning ‘dicht hinter’ the optic vesicle and extending through the midbrain giving rise to the sensory components of the third, fourth, and part of the fifth cranial nerves. If the microscopic picture in these forms is really not complicated by a mesectodermal proliferation, they are particularly favorable material for the study of the origin of the cranial ganglia and especially of such problems as the origin and fate of the muscle sense cells of the oculomotor nerves. On the other hand, it is also possible that the particular embryonic stages during which the mesectoderm migrates out have not yet been studied. It would seem that in the cat the nervous system is differentiating more rapidly than the axial mesoderm and that a close series, so far as the number of somites is concerned, may have distinct gaps in it. In man the period of pro-otic mesectoderm formation is limited to stages between eight and twelve somites, and it may therefore be that this phenomenon occurs between five— and seven-somite stages in the cat—a period which was not represented in the Columbia University series.
For the dog we have the descriptions of Bischoff C45) and Bonnet (’O1). Figure 36 of the former shows an embryo of about ten somites with obvious optic vesicles. The 1atter’s figure 39 is taken from a section of an eight-to—nine-somite specimen and seems to pass through the edge of the optic primordium. Bonnet’s ten-somite embryo has both optic vesicles and otic pits. Here, then, as in the cat the two primordia arise at about the same time, the optic, however, taking precedence.
Rodents
When one considers the large collections of rodent embryos in many embryological laboratories, it is surprising that so little work has been done on the early development of the nervous system in these forms. The only complete study is the Normentafel of Minot and Taylor (’05) for the rabbit. In the first embryo of their series they noted the broad expansion of the neural folds rostrally and suggested that this might be the first indication of the optic anlage. This specimen had five somites. Since the eight—somite embryo (no. 4) had well-developed vesicles, it is probable that there were optic anlagen in the cephalic plate of the former. The thickening for the otic plate as well as the ‘acoustico—facial ganglion’ and the trigeminal ganglion were recognized in the nine—somite specimen of table 5.
Keibel, in 1889, figured the optic primordia of a guinea-pig embryo 16 days, 7 hours, old in sagittal section (fig. 44 and 45) and Foriep (’05, p. 157) has reproduced a photograph of a transverse section from a 3—mm. embryo. In both instances there is a deep broad pit situated laterally in the neural fold. Bischoff’s monograph on the guinea-pig furnishes no data on this subject.
Insectivores
For the mole we have concrete data in the classic monograph of Heape (’87). He recognized the optic grooves in an embryo of three somites (stage E) where they are as Well developed as in our eleven-sornite H392 (fig. 9d). Heape calls attention to the very early appearance and typical form of the optic primordia in this form where the eye is subsequently degenerate. The otic plate is referred to in stage J when the optic Vesicles are well developed and the neural folds are closed as far forward as the cranial flexure. His figure 25 is from a section of a fourteen-somite embryo in stage H and shows a slightly thickened otic plate.
Primates
Our knowledge concerning the pertinent stages of primates other than man is due. entirely to Selenka and Huprecht. Selenka (’91, ’O0) obtained three embryos from the period We are considering. His figure of the Hylobates embryo ‘Ab’ (’00, fig. 24) represents a dorsal View of a three—somite specimen, of which the rostral end of the neural plate resembles that of H87 (eight somites). It shows two Well-marked converging grooves which may Well be the optic sulci. The thirteen-to-fourteen- somite embryo of Semnopithecus (‘Wa,’ Selenka, ’03, figs. 11 and 12) has large optic vesicles, but no otic plate is indicated. There is a deep otic pit in the twenty—three somite Cercocebus embryo, ‘Cc,’ however.
From the Normentafeln of Tarsius (I-Iuprecht and Keibel, ’O7) it would seem that the eight-somite embryo (figs. 8 and 9) had optic anlagen somewhat further advanced than those of our eight-somite embryo H87 (fig. ‘9a). The youngest stage in which the otic plate could be recognized is that of twelve somites (table 5), where the neural folds were closed in the region of the optic vesicles. Here, then, as in most other forms, the optic primordia precede the otic in development.
The scanty and inaccurate data of this survey emphasize the need of detailed studies of early mammalian embryos both on their own account and for the light they will ‘throw on human development.
Conclusions
- The earliest sensory anlage in man is the otic plate which can be recognized in an embryo of two to three somites as a diffuse thickening of ectoderm in‘ the hindbrain region. A four-somite embryo shows the beginning of the associated acousticofacial ganglion, though its fate is not yet completely known.
- The ganglion arises near, but not exactly at the dorsal edge of the open neural fold and the outermost part of the apparent evagination delaminates from the fold before the process of tube formation is completed. It is clearly derived from the Wall of the definitive neural tube.
- The otic epithelium differentiates by an elongation of the distal ends of the cells and the appearance of a brush border. Between ten- and twelve-somite stages invagination begins and there is a deep otic pit at sixteen somites.
- The otic plate has been identified earlier in man than in any other vertebrate of which We have accurate data, and in this form only does it precede the optic anlage.
- Isolated thickenings (growth centers) of the cranial neural folds appear at a stage of seven to eight somites, which promptly fuse to form a continuous ridge, the ‘optic-crest primordium.’ An associated ventricular sulcus in the forebrain levels of the ridge indicates the position of the optic anlage. This is the earliest stage of this anlage which has been recognized in a mammal.
- The non-optic part of this primordium proliferates mesectoderm and a large part of the trigeminal ganglion.
- The optic anlage appears laterally in the neural fold, but between it and the ectoderm thereis an intervening zone which gives rise to part of the alar_ and roof plates of the future diencephalon. The optic vesicle is therefore derived entirely from the central nervous system.
- At a stage of sixteen somites the optic vesicle is in contact with the overlying ectoderm.
Literature Cited
BAILEY, P. 1916 Morphology of the roof plate of the forebrain and the lateral choroid plexuses in the human embryo. Jour. Cdmp. Neur., v. 26, pp. 79-120. .
BACH, L., UND SEEFELDER, R. 1914 Atlas zur Entwicklungsgeschichte des menschlichen Auges. Leipzig und Berlin.
Brscnorr, T. L. W. 1842 Entwicklungsgeschichte des Kaninchen-Eies. Braunschweig. 1845 Entwicklungsgeschichte des Hundeeies. Braunschweig.
BONNET, R. 1889 Beitrage zur Embryologie der Wiederkiuer, gewonnen am Schafei. II. Ar. f. Anat. u. Physiol., Anat. Abt., S. 1-106. 1901 Beitrage zur Embryologie des Huudes. II. Anat. Hefte, Bd. 16, S. 231-332. .
BRACKET, A. 1906 Recherches sur Pontogénesé de la téte chez les amphibiens. ’ Arch. (1. Biol., T. 23, pp. 165-257.
Bremer JL. Description of a 4-mm human embryo. (1906) Amer. J Anat. 5: 459-480.
CAJAL, S. R. 1912 Algunas variaciones fisiologicas y patologicas del aparato reticular de Golgi. Trab. Lab. Inves. Biol., vol. 12, pp. 127-228.
Dandy WE. A human embryo with seven pairs of somites measuring about 2 mm in length. (1910) Amer. J Anat. 10: 85-109.
ETERNOD, C. A. F. 1896 Sur un oeuf humain de 16.3 mm. avec embryon de 2.11 mm. Actes Soc. helv. de sc. nat., pp. 164-169. 1899 I1 y a un canal notochordal dans l’embry0n humain. Anat. Anz., Bd. 16, S. 131-143.
Evans HM. and Bartelmez GW. A human embryo of seven to eight somites. (1917) Anat. Rec. 11: 355.
FORIEP, A. 1905 Die Entwicklung des Auges. Hertwigs Handbuch der Ent-Wkges., Bd. II, H. 2, S. 139-240. ‘
GIGLIO-Tos, E. 1902 Sui primordi dello sviluppodel nervo acustico-faciale nell’u0mo. Anat. Anz., Bd. 21, S. 209-225.
HIS, W. 1893 Ueber das frontale Ende des Gehirnrohres. Ar. f. Anat. u. Physiol., Anat. Abt., S. 157-171. 1904 Die Entwickelung des menschlichen Gehirns Wéihrend der ersten Monate. Leipzig.
Ingalls NW. A human embryo at the beginning of segmentation, with special reference to the vascular system. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. 274, 11: 61-90.
Johnston JB. The morphology of the forebrain vesicle in vertebrates. (1909) J. Comp. Neurol. and Psychol. 19: 457-539.
KEIBEL, F. 1889 Zur Entwickelungsgeschichte der Chorda bei Saugern. Ar. f. Anat. u. Physiol., Anat. Abt., S. 329-388. 1896 Studien zur Entwickelungsgeschichte des Schvveines. II. Morph. Arbt., Bd. 5, s. 17-169. 1897 Normentafel z. Entwickelungsgeschichte des Schweines. Jena.
KEIBEL, F., UND ELZE, C. 1908 Normentafel zur Entwickelungsgeschiehte des Menschen. Jena.
KRAUSE, R. 1905 Entwickelungsgesehichte des Gehéirorgans. Hertwigs Handbuch der Entwkges., Bd. II, H. 2, S. 82-138.
LANDACRE, F. L. 1910 The origin of the cranial ganglia in Ameiurus. Jour. Comp. Neur., vol. 20, pp. 309-412. 1921 The fate of the neural crest in the head of Urodeles. Jour. Comp. Neur., vol. 33, pp. 1-44.
LENHossr‘:K, M. 1891 Die Entwickelung der Ganglienanlagen bei dem menschlichen Embryo. Ar. f. Anat. u. Phys., Anat. Abt., S. 1-25.
Low A. Description of a human embryo of 13-14 mesodermic somites. (1908) J Anat Physiol. 42(3): 237-51. PMID 17232769 | PMC1289161
MALL, F. P. 1917 Cyclopia in the human embryo. Carnegie Contrib. to Emb., vol. 6, pp.‘5—33.
MARTIN, P. 1890 Die erste Entwickelung der Kopfnerven der Katze. Oestr. Monats. Tierheilk., Bd. 15. '
Minot CS. and Taylor E. Normal Plates of the Development of the Rabbit Embryo (Lepus cuniculus). Vol. 5 in series by Keibel F. Normal plates of the development of vertebrates (Normentafeln zur Entwicklungsgeschichte der Wirbelthiere) Fisher, Jena., Germany.
PARKER, G. H. 1908 The origin of the lateral eyes of vertebrates. Am. Naturalist, vol. 42, pp. 601-609.
PATTERSON, J. T. 1909 Gastrulation in the pigeon’s egg. Jour. Morph., vol. 20, pp. 65-124.
SAKURAI, T. 1906 N ormentafel zur Entwickelungsgeschichte des Rehes (Cervus caprolus). Keibels Normentafel no. 6. Jena.
Schulte HVW. and Tilney F. The development of the neuraxis in the domestic cat to the stage of twenty-one somites. (1915) Ann. N. Y. Acad. Sc. 24: 319-346.
SELENKA, E. 1892 Studien fiber die Entwicklungsgeschichte der Tiere. Wiesbaden. 1900 Menschenaffen. Wiesbaden. Zweite Lieferung. 1903 Ibid., Fiinfte Lieferung.
STOCKARD, C. R. 1914 An experimental study on the position of the optic anlage in Amblystoma punctatum, etc. Am. Jour. Anat., V01. 15, pp. 253-289.
VEIT, O. 1918 Kopfganglienleiste bei einem Embryo Von acht Somitenpaaren. Anat. Hefte, Bd. 56.
Wallin IE. A human embryo of thirteen somites. (1913) Amer. J Anat. 15(3): 319-331.
Watt JC. Description of two young twin embryos with 17-19 paired somites. (1915) Contrib. Embryol., Carnegie Inst. Wash. 2: 15-54.
WEIGNER, K. 1901 Bemerkungen zur Entwicklung des Ganglion acustico-faciale und des Ganglion semilunare. Anat. Anz., Bd. 19, S. 145-155.
Wilson JT. Observations upon young human embryos. (1914) J Anat Physiol., 48(3): 315-51 PMID 17233002 PMC1288949
YEATS, THOS. 1911 Studies in the embryology of the ferret. Pt. I. Jour. Anat. Phys., vol. 45, pp. 319-335.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The Origin of the Otic and Optic Primordia in Man. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Origin_of_the_Otic_and_Optic_Primordia_in_Man
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G