Paper - A Study of the Structural Unit of the Liver
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Mall FP. A study of the structural unit of the liver. (1906) Amer. J Anat. 5:227-308.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Study of the Structural Unit of the Liver
From the Anatomical Laboratory of the Johns Hopkins University.
With 74 Figures and 7 Tables.
Introduction
In studying the structural development of an organ it is necessary to consider the systems within it as a whole and to determine their relations to one another. Analytical methods, which must precede synthetical methods, have shown that organs are built up of like parts, or structural units, which are analagous to the leaves of a tree. However, in the growth of an organ the units are not thrown off annually, but are gradually shifted and transformed into new units. It follows that in a study of the kind proposed it is always necessary to consider the unit in relation to the organ as a whole throughout its development, and to do this we must constantly resort to reconstruction. Of course this cannot be done with much success, in the ordinary sense of the term, but for the present purpose, the tables may be considered as reconstruction. Geologists, geographers, archaeologists and anatomists each have their own methods of reconstruction, and I have utilized them all, more or less, in the present study.
I undertook the study of the structural development of the liver on account of its well-known and sharply-defined lobule. It was thought at the beginning that this lobule was the simplest of all the structural units and therefore the most suitable for a study of this kind. It soon became evident that the lobule was not the structural unit, and that both lobules and units were extremely difficult to follow in their development, for they are constantly blended with adjacent lobules, or units as the case may be. Furthermore, lobules or units once formed do not remain, but sprout, fracture and rearrange themselves, thus making the various pictures obtained complex and difficult to interpret.
The work has been carried on during a number of years, after being laid aside in order to take up the same question in other organs. These secondary studies, —usually made by others connected with me,— have aided my work for the liver materially, which I new venture to present in a more or less connected form.
Historical Note
In 1664 Wepfer described lobules in the liver of the pig, and two years later they were again described by Malpighi who gave them their name. Malpighi states that the livers of all vertebrates are conglomerate glands, being composed of lobules which in turn contain acini. For a long time after this the capital problem in the anatomy of the liver was the study of the structure of the lobules and their relation to one another.
In 1733 Ferrein described these lobules as being composed of two substances, brown and yellow (substantia fusca and substantia flava) which formed respectively its medullary and cortical portions. In general this description was accepted by anatomists, sometimes, however, with a reversal of the arrangement of the colors in the medullary and cortical portions of the lobule.‘ In 1832 E. H. Weber showed that the two colors of the lobule are due to an unequal distribution of blood in it, and a year later Kiernan, in his classic paper,[1] denied altogether that the lobule was composed of two substances as described by Ferrein. We owe to Kiernan our present conception of the lobules, he described their shape and relation to one another, the amount and character of tissue between them, and what is more, their relation to the vascular system; he also introduced our present nomenclature. The defining line around a lobule was broken up into spaces and fissures, spatia interlobularia when three lobules came together, and fissurae interlobulares between each two adjoining lobules. The spaces and fissures, which were not always easy to demonstrate, were no doubt included by Ferrein in the cortical portion of the lobule. It is seen that Kiernan’s interlobular spaces and fissures form a network between the lobules, and for this reason Theile calls them the substantia reticularis, and the lobule proper the substantia granosa. It was also shown that the order of the reticular and granular substances are reversed in hepatic congestion; in it the brown reticulum encircles yellow granules, pseudogranules, as Theile calls them. The yellow “ pseudolobules ” are tough and more consistent than the true brown lobules.
Before the time of Kiernan the usual confusion of terms naturally arose. For instance, Autenrieth accepted Ferrein’s cortical and medullary portions of the lobule, only he reversed the order of their colors. Evidently he was describing “ pseudolobules.” Merkel, who also no doubt studied hyperaemic livers, did the same. Krause took the “ happy mean ” course and described pseudolobules, i. e., the yellow interlobular connective tissue, with hepatic veins in their center. Cruveilhier made the same blunder. Numerous other terms were used in a variety of ways, as, for instance, acinus which meant anything froina cell to an entire lobule, according to different authors.
The lobule, as described by Kiernan, received its strongest support in its having on its periphery the terminal twigs of the portal vein, hepatic artery, bile duct, and an increased quantity of connective tissue, which in the pig forms a distinct capsule. Had it not been for an occasional animal with a lobule so well outlined and a great authority like J. Muller, it is probable there would still be much confusion in spite of the “ happy means ” and the innumerable terms. The study of the structure of the liver illustrates beautifully the value of great minds in the study of any subject. We see during a period of two centuries that the generalizations of Malpighi, Ferrein, and J. Muller are consistent and practically correct in spite of the great amount of confusion and opposition brought from many quarters. Taking all of the facts into consideration, analysis by means of injection experiments, finally gave us a structural unit of the liver which has withstood all opposition.
Lobules of the liver are certainly not well marked in most animals and it is seen by the foregoing that the lobules were as often found encircling the portal twig as around the hepatic twig. A glance at fig. 1 will show why either arrangement is correct. With the facts before him, it is not remarkable‘ that E. H. Weber denied the anatomical existence of the lobule, 11. e., a lobule that can always be seen and is always the same. However, J. Miiller, with the livers of the pig and of the polar bear as examples set the question at rest for a time.
The “psuedo lobule ” of Theile, with the strong connective tissue of the portal space as a center, is tougher than the true lobule which has only a delicate reticulum to hold it together. Theile has shown that it is easy to isolate the “pseudo lobule” of the dog’s or the rabbit’s liver, while it is impossible to isolate the true lobule. In fact, if livers of these animals are crushed and washed in a stream of water, the whole system of lobules is isolated, clustered around the branches of the portal vein, forming a specimen which may be likened to a bunch of grapes.
Sabourin has described the liver as being composed of biliary lobules with the terminal bile ducts as their centers.[2] He has accepted the pseudolobule of Theile as the true unit of the liver, which in Phoca[3] is outlined by a capsule as the hepatic lobule is in the pig. The biliary or portal lobule has been used as a basis by Berdal[4] in his histology and has been advocated by myself for a long time.[5] Recently it has been discussed as the secreting lobule by J. B. MacCal1um,[6] and- has been defended from an embryological standpoint by F. T. Lewis.[7]
Fig. 1. Diagram of a transverse section of a group of lobules with lines indicating the course of the capillaries. The lobules as usually understood are marked with circles. P, a portal unit; n, nodal point; p‘ a portal unit outlined as in partial congestion.
In all other glands we make the duct the center of the structural unit. From this center often the artery and the framework radiate. In the liver everything radiates from the so-called interlobular space,-arterial and portal blood vessels, bile duct, lymphatics, nerves and connective tissue; the liver develops from this point; physiologically everything centers there. So, viewed from any standpoint, it is the center of the structural unit.
Throughout my description I shall use the term portal structural unit, portal unit, structural unit, or unit, for the clump of tissue which surrounds each terminal branch of the portal vein. In order to avoid confusion I shall use the term lobule in its old sense,—a.s the hepatic lobule,for after much discussion carried on during two centuries, it has become well established. The old idea, the idea of Wepfer, Malpighi, Ferrein, Kiernan, and J. Muller should be marked with the word lobule ; the new idea, associated with the unity of structure, should be called the unit.
Vascular Proportion
Roux has stated in one of the theses in his Habilitationsschrift[8] that the lobular subdivisions of the liver are due, in their arrangement and form, to the vascular system. This same idea is again brought forth several years later in the Introduction to his Archiv.[9] In this he says, on page 17, “ Die Gliederung der gewohnlichen, baumartig veriistelten Driisen in Lappcheu erscheint durch die gestaltenden Wirkungen der Epithelien, also der specifischen Teile, bedingt und ist, so weit dies richtig ist, Selbstdifferenzierung der Driisensubst-anz. Bei der Leber dagegen, einer Netzdriise, erscheint die normale Grosse und Gestalt der Lappchen und auch die lobulare Gliederung selber durch die Blutgefasse bedingt, und zwar einmal durch die geeignete Kapillarlttnge wie zweitens durch die Eigenschaft der letzten Veriistelungen der Vena portae, beim Wachsthum des Kapillarnetzes dichotomische Verzweigungen in letzterem auszubilden. Die acinose Gliederung des Leberparenchyms stellt somit eine Von dem Blutgefasssystem abhiingige Diflferenzierung der Driisensubstanz dar.” A much more extended discussion of the growth and proportion of the vascular system is given by Thoma in his numerous papers, but that which relates to the capillaries in particular is to be found in his brilliant study of the blood-vessels of the area vasculosa of the chick.[10] It is not possible to discuss in detail the many observations and arguments in this model research without extending this paper far beyond the space of this Journal. However, an excellent summary of Thoma’s work is given in his Pathology, from which I will quote several pages from the English translation.[11] Thoma’s work is summed up in three laws or histomechanical principles (page 265), as follows:
(1) " The increase in the size of the lumen of the vessel, or what is the same thing, the increase in the surface of the vessel wall, depends upon the rate of the blood-current. The surface of a vessel wall ceases to grow when the blood-current acquires a definite rate. The vessel increases in size when this rate is exceeded, becomes smaller when the blood-stream is slowed, and disappears when it is finally arrested.
“ This law which brings the growth of the surface of the vessel wall into dependence upon the rate of the flow of the blood is, I consider, the first and most important histo-mechanical principle which determines the state of the lumen of the vessel under physiological and pathological conditions. It will be further proved, however, in many places in the general, as well as in the special parts of this book.
“ A second histo-mechanical principle may be added to this, viz., the growth in thickness of the vessel wall is dependent upon its tension. Further the tension of the wall is dependent upon the diameter of the lumen of the vessel and upon the blood-pressure.
“ The proof of this law is to be sought, in the first place, in the varying strength of the wall of the larger and smaller arteries, veins, and capillaries. In certain diseases of the vessels (arteriosclerosis, aneurism) there are apparent exceptions which will be discussed in their proper place.
“ The third histo-mechanical principle has not hitherto been so completely demonstrated as the first two. It will, therefore, be put forward merely as an hypothesis, which runs as follows: increase of blood-pressure in the capillary areas leads to new formation of capillaries‘.
“The three histo-mechanical principles were, in the first place, employed to explain the developmental processes in the area vasculosa of the chick. In this flat extended area a capillary network is found at an early date in which no arterial and venous channels can be differentiated (fig. 2). A few channels are, however, selected by the blood-stream in consequence of the general direction which is given to it by the position of the ends of the primitive aorta on the one side, and of the venous ostia of the heart on the other. These channels (fig. 2, a, b, 0) contain the more rapidly flowing streams. They, therefore, dilate and become converted into arteries and veins. (fig. 3).
“ Other channels, in which the rate of the flow of the blood has a certain medium force, remain as capillaries, and lastly, some channels which offer great resistance to the stream, and are thus very slowly traversed, atrophy, or disappear altogether. The rapid growth of the selected channels diminishes the resistance to the blood-stream, so long as the capillary area remains unaltered. The blood-pressure in the capillary area accordingly rises and leads to new formation of capillaries. New communications are thus formed between the terminal ramifications of the arteries and veins ; the capillary area is thus relieved, and its blood-pressure falls. Arteries and veins have now become wider and longer, and the capillary area has increased in extent. A larger quantity of blood will flow into it, therefore, and this will involve a corresponding increase in the total resistance to the stream within the enlarged capillary areas. The chain of processes described may therefore be repeated until any one link in the chain becomes incapable of further increase.
Fig. 2. Capillary channels of the area visculosa after forty-eight hours’ incubation. S, peripheral end of the primitive aorta; a, b, c, selected blood channel, X 30. After Thoma.
Fig. 3. Blood-vessels from the area vasculosa. after fifty-seven hours’ incubation. The same part as in fig. 2. S, peripheral end of the ‘primitive aorta; a, b, c, the selected channels of entrance to capillary network; V, V, V, venous exit of latter; d, d, d, the beginning of the second capillary network. X 25. After Thoma.
“If we consider that this chain of processes is constantly repeated within short spaces of time, and that at each time only a slight alteration of the previously existing relations is produced, we may form a fairly accurate conception of the mode of growth of the vascular system.
The details of this will not be considered here. If, however, we apply the above principles to any organ whatever which has a longer existence than the area vasculosa, we must admit that the histo-mechanical principles justify us in assuming that, after the organ has ceased to grow, the rate and presence of the blood in all its capillaries are approximately
Fig. 4. Part of the area vesicular of a. chick incubated seventy-four hours. The dorsal aspect is presented. V, V, veins. The arteries are dark. X 21. After Thoma.
“In this organ, according to the first histo-mechanical principle, all blood channels in which the rate of flow exceeds a certain maximum, must increase in lumen and become converted into arteries and veins. Vice versa, all vascular channels will disappear in which the rate of the bloodstream falls below a certain maximum. If, however, the lumen of the vessel bears a fixed relation to the rate of the blood-current, the interval between the maximum and minimum cannot be great. From this it appears that, after growth is completed, the rate of flow must be fairly uniform in all the capillaries of an organ.
“ The conversion of capillaries into arteries diminishes the resistance of the blood-stream, and leads to an increase of pressure in the capillaries. If, then, according to the third histo-mechanical principle, new capillaries are formed at all places in the capillary area in which the pressure of the blood exceeds a certain limit, these capillaries, again, reduce the pressure by forming new connections between the arteries and veins. The third histo-mechanical principle, therefore, implies that, during the growth of the organ, new capillaries are being formed everywhere, and that, after complete growth, the blood-pressure in all capillary areas of the same organ is fairly uniform.
“ The width of the lumen of the capillary channel at the close of the period of growth must be almost the same in all areas of the same organ, since it depends on the rate of flow, and this rate is uniform in all capillaries of the same organ.
“ These conclusions are in perfect harmony with the actual state of matters. It appears, however, that in the different organs there are great differences in the width of the lumen and in the number of their capillaries, in the rate of flow, and in the quantity of the blood flowing through a given area of the vessel in a given time.
“ If these facts be compared with the results which were obtained above, according to which the first vascular spaces, the rudimentary capillaries, were formed by the secretory activity of the cells forming their wall, We are compelled to assume that the metabolic processes and other special characteristics of the various organs also exercise a determining influence on the peculiarities which distinguish their capillaries. It must be imagined that the individual characters of the organ, and its size in relation to other organs, decide firstly the number of capillaries in the whole organ and in a single part of the organ; further, the special relations existing between the rate of flow and the lumen of the capillary channel; and lastly, the height of the blood-pressure which will lead to the formation of new capillaries. If, for example, the growth of the capillaries is arrested in one organ at a rate of flow a, corresponding to a lumen b, in a second organ the growth of the capillary lumen might perhaps be arrested at a rate of flow A corresponding to a lumen B. Thus, in the one organ, capillary new formation would occur when the pressure of the capillary blood exceeds the limit c, while, in the other organs, this limit might be higher at the blood-pressure O.
“ The number of capillaries, their lumen, and the rate of flow of the blood—stream passing through them, determine, as has been observed, the total quantity of the blood which flows through the entire organ. We thus arrive at the remarkable result that it is the organ iself which deternii/nes the quantity, the rate of flow, and the pressure of the blood flowing through it; and that this is effected by means of fixed relations which are expressed generally in the three histo-mechanical principles. The conditions which produce the uniformity of pressure and rate of the blood—current in all capillary areas of the same organ are included in these principles.
“ According to the generally accepted view of the problem of the circulation, which was formerly quite sufficient to serve as a basisfor the account of its general disturbances, the pressure, the rate, and the amount of the blood-flow appeared to be directly dependent upon the action of the heart. According to the view given here, on the other hand, it is the metabolic processes in the organs, which determine first for the individual organs, then for the whole of the organs—that is, for the circulation as a whole—the amount ‘of blood propelled within a given time, its pressure and its rate of flow. In this case, the working-power of the heart appears as the equivalent of the sum of the histo-mechanical demands made by the organs.” It will be seen that Thoma concludes, and I think properly, that capillaries of like component parts of an organ are of equal size and length, and that the rapidity of the circulation through them is also equal. This idea I have also tried to develop in various papers upon the structural unit of organs. It appears that each organ is broken up into units which are of equal value from anatomical and physiological standpoints. What takes place in one unit takes place in all of the rest. A good example is to be found in the intestine where the structural unit is a villus surrounded at its base with a circle of intestinal glands (crypts). In the center of the group is the main artery which passes directly to the apex of the villus and ending there divides abruptly into an umbrella of capillaries which lie at the periphery of the villus. These capillaries are about of one diameter and length, and no matter what course is taken by the blood the distance and resistance in passing from the artery to the vein is always the same.
" Ludwig pointed out that the capillaries of an organ 1' Mall, Abhandl. d. K. S. Gesell. d. W1ss., XIV, 1887.
were always equally favored by the circulation, and that many descriptions and illustrations of the blood-vessel, as, for example, of the villus and the glomerules, could not possibly be correct. If in reality the bloodvessels of these structures were arranged as is frequently pictured, the blood would have to take the capillaries in the course of the least resistance, while in those of the greatest resistance it would stagnate or come to a standstill. Thoma’s first law explains how an equal distribution which favors no part of an organ is brought about. In development the vessels in which the blood stagnates degenerate, and in those in which the rapidity is too great the lumen is enlarged. There seems to be a tendency to maintain a “normal” flow of blood through the capillary. After capillaries are well dilated they become arteries and veins, and the thickness of their walls is new dependent upon their tension, according to Thoma’s second law. These two laws are constantly at work, and regulate accurately the diameter and thickness of the walls of the arteries and veins.
Before considering Thoma’s third histo-mechanical principle, it is necessary to discuss his numerous measurements as well as to give data which I have accumulated. The whole question hinges upon the cause of the new formation of capillaries for which Thoma has not found a law, but has merely put forward an hypothesis.
Thoma made many measurements of arteries and their branches and tabulated Bencke’s measurements of the aorta with its branches. These measurements show that the area of all of the branches of the aorta equals about the area of the ascending aorta, being a little less before the thirtieth year of age and a little greater thereafter. Thoma gives a few measurements of small arteries in which the area of the immediate branches equals about that of the main stem. These measurements, however, are not constant in live animals, for if the observations are continued, the caliber of the branches increases out of proportion, and ultimately their area exceeds that of the main stem." This change Thoma ascribes to a change in the vascular tone. In other parts of the same work,“ as well as elsewhere, he appears to be somewhat uncertain regarding the equality of the area of a vessel and the area of all of its branches. Also in a later publication the arguments seem to accumulate against this view." Thoma states, however, that the exceptional cases are found in growing arteries, the umbilical, for instance, which is to be expected, for the peripheral bed is enlarging. After the vessels cease to grow the area of the vascular bed is about the same from the ascending aorta to the smallest arteries; the bed enlarges in the capillaries. Under such conditions (homonomous ramification) the average rate of the current is equal in all of the arteries.
1“ Thoma, Histogenese u. Histomechanik, 66.
“Thoma, Ibid., p. 86; Pathology, 275 and 276.
1‘ Thoma, Ueber den Verzweigungsmodus der Arterien. Arch. f. Entwick. d. Organimen, XII, 1901.
Whether the ramification is homonomous or heteronomous appears to me to be of little consequence, and I have pointed out the uncertainty of Thoma’s statements for my own measurement, which are quite numerous, and decidedly in favor of a heteronomous ramification. Tho1na’s assumption of homonomous ramification is based largely upon the measurements upon the aorta and its branches. From now on, however, the vascular bed enlarges, at first slowly, and more rapidly as the capillaries are approached. The bed has doubled itself in the arteries one millimeter in diameter an-d has increased about fivefold in arteries .05 mm. in diameter. A change so slight as this could barely be detected when the measurements are made in adjoining internodes. In order to obtain reliable figures the measurements must be made farther apart. For instance, it is easy to lay the intestine of the dog into a series of anatomical units to correspond with the arteries-—mesenteric arches, arches to the submucosa and arteries to the villi. If the area of the superior mesenteric artery is 7 sq. mm. and that of the ends of the main branches but 12 sq. mm., it will be seen that when a trunk divides into two branches the change in area will be but slight. But when we compute the number of villi, and this is easily done, we determine at the same time the number of terminal arteries to the villi, all of which are about of the same size. At this point, as Table I shows, the artery is .0225 in diameter and the bed is nearly 60 times the area of the superior mesenteric artery. If the ramification were homonomous down to the arteries of the villi there hould be but 17,000 villi, the number which can be counted upon 10 sq. cm. of mucous membrane. No matter how the following tables are compared, it is seen that there is a gradual widening of the vascular bed from the branches of the aorta to the capillaries.
Table I
Giving the vascular bed of the dog’s small intestine. K. S. Ges. d. Wiss., XXIV, 1887.) [1] [2] [3] [4] [3] [3] [4] (Mall, Abhandl. (1.
Area Vessels Number Diameter of Section mm. sq. mm.
Superior mesenteric . . . . . . . . . . . . . . . . . . 1 3.0 7.07 Main branches . . . . . . . . . . . . . . . . . . . . . .. 15 1.0 11.78 Terminal branches . . . . . . . . . . . . . . . . . .. 45 .6 12.72 Short intestinal arteries . . . . . . . . . . . . .. 1,440 .08 7.24 Long intestinal arteries . . . . . . . . . . . . . .. 459 .192 13.29 Long and short intestinal arteries. . . .. 20.53 Terminal branches of short intestinal arteries . . . . . . . . . . . . . . . . . . . . . . . .. 8,640 .05 16.96 Terminal branches of long intestinal arteries . . . . . . . . . . . . . . . . . . . . . . . . . 18,000 .053 39.71 Total terminal branches . . . . . . . . . . . . . . 26,640 . . . . 56.67 From the submucosa to the crypts. . . . . 4,000,000 .008 201.06 From the submucosa to the villi . . . . .. 328,500 .301 247.94 Arteries of the villi . . . . . . . . . . . . . . . . . .. 1,051,000 .0225 417.97
_ _ upper one-third . . . . . . . . .. 31,536,000 .008 1,585.17 C“P‘“"“‘°s{lower one—third . . . . . . . . .. 15,768,000 .005 309.6 Total capillaries of crypts and villi. . .. 51,804,000 . . . . 2,095.83
Veins of the villi . . . . . . . . . . . . . . . . . . . .. 2,1.02,400 .0265 1,159.57 Veins penetrating the muscularis mu cosae . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131,400 .075 580.51 Terminal branches in the submucosa... 18,000 .128 231.62 Anastomoses in the submucosa. . . . . . . .. 2,500,000 .032 2,010.62 Terminal branches of the long and short intestinal veins . . . . . . . . . . . . . 28,800 .064 92.65 Long intestinal veins . . . . . . . . . . . . . . . .. 459 .44 69.79 Short intestinal veins . . . . . . . . . . . . . . . .. 1,440 .112 14.19 Last branches of superior mesenteric vein . . . . . . . . . . . . . . . . . . . . . . . . . . .. 45 1.5 79.52 Branches of the mesenteric vein . . . . . .. 15 2.4 67.56 Mesenteric vein . . . . . . . . . . . . . . . . . . . . .. 1 6.0 28.27 MUSCLE Cons.
Direct muscle arteries . . . . . . . . . . . . . . .. 1,800 .03 1.27
Recurrent muscle arteries . . . . . . . . . . . .. 3,600 .04 2.54
Capillaries of the circularis . . . . . . . . . .. 27,000,000 .003 190.85
Capillaries of the longitudinalls . . . . . .. 9,000,000 .003 63.62
Total capillaries . . . . . . . . . . . . . . . . . . . .. 36,000,000 . . .. 254.47
Veins . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3,600 .112 35.46
Giving the vascular bed of this dog’s stomach.
Table II
pital Reports, 1, 1889.) [1] [2] [3] [4] [3] [4] [4] Vessels Aorta . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 1 Coeliac axis . . . . . . . . . . . . . . . . . . . . . . . . .. 1 Gastric . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 1 Splenic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 Hepatic . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 1 From the gastric... .. 1 To the stomach From the splenic. . . . . { 1 From the hepatic . . . . 1 Total . . . . . . . . .
From the branches of the flrst order to the stomach . . . . . . . . . . . . . . . . . .. 108
Second order . . . . . . . . . . . . . . . . . . . . . . . . 740
Third order . . . . . . . . . . . . . . . . . . . . . . . . . 5,920
To the mucosa . . . . . . . . . . . . . . . . . . . . . .. 76,960
Stellate arteries . . . . . . . . . . . . . . . . . . . . .. 615,680
Capillaries . . . . . . . . . . . . . . . . . . . . . . . . . . 22,800,000
Subepithelial venous plexus . . . . . . . . . .. 1,643,600
Interglandular plexus . . . . . . . . . . . . . . . . 431,649
Subglandular plexus . . . . . . . . . . . . . . . . . 333,090
Branches piercing the submucosa . . . . .. 12,768
Large branches in submucosa . . . . . . . .. 1,480
Veins from stomach . . . . . . . . . . . . . . . . .. 108
Pyloric . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 1
Gastro-epiploica dextra . . . . . . . . . . . . .. 1
Gastro-epiploica sinistra . . . . . . . . . . . . . . 1
Gastric . . . . . . . . . . . . . . . . . . . . . . . . . . . . .‘. 1
Pancreatico-duodenal . . . . . . . . . . . . . . . . . 1
Splenic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Portal . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 1
IN THE MUSCLE COATS.
Direct muscle arteries . . . . . . . . . . . . . . .. 108
Recurring muscle arteries . . . . . . . . . . . . 342
Arteries of intermuscular plexus . . . . .. 5,016
Capillaries of circularis . . . . . . . . . . . . . .. 25,500,000
Capillaries of longitudinalis . . . . . . . . . .. 13,000,000
Direct muscle veins . . . . . . . . . . . . . . . . . . . 108
Recurring muscle veins . . . . . . . . . . . . . .. 342
Number Diameter mm. 6.0 2.75 1.7 2.5 2.75 1.7 1.92 .486 .5 .415 .075 .025 .017 .006 .02 .037 .049 .0867 2.0 2.5 3.0 3.3 5.0 6.0 9.0 .25 .003 .003 .15 .33 241 (Mall, Johns Hopkins Hos Area of Section sq. mm.
28.27 5.92 2.27 4.91 5.92 2.27 2.89 .74 .196 6.096 14.58 23.26 26.17 37.79 139.59 645.24 493.08 445.89 577.25 75.33 44.4 21.6 3.14 4.91 7.07 8.55 19.64 28.27 63.64 .86 16.64 39.39 180.25 91.89 1.91 29.25
Table III
Giving the vascular bed of the dog’s adrenal. (flint, Welch Festschrift, Johns Hopkins Hospital Reports, IX, 1900.) Area Vessels Number Diameter of Section mm. sq. mm. [1] Arteries, first order . . . . . . . . . . . . . . . . .. 11-19 .17 .339 second order . . . . . . . . . . . . . .. 49 .15 .865 third order . . . . . . . . . . . . . . . .. 160 .13 2.123 fourth order of cortex . . . . . .; 532 .1 4.168 fourth order of medulla . . . . .. 49 .1 .384 Total fourth order . . . . . . .. 581“ .1 4.552 fifth order of cortex . . . . . . . .. 2,320 .045 4.547 fifth order of medulla. . . . . . .. 288 .08 1.445 [3] Total fifth order . . . . . . . . . . . . . . . . . 5.992 sixth order of cortex . . . . . . .. 13,920 .025 6.82 sixth order of medulla . . . . .. 3,168 .035 3.037 sixth order of capsule . . . . . .. 1,571 .018 .399 Total sixth order . . . . . . . . . . . . . . . . 10.256 capillaries of cortex . . . . . . . .. 3,724,933 .008 186.246 capillaries of medulla . . . . . .. 354,742 .007 12.650 capillaries of capsule . . . . . . .. 17,281 .007 .664 [4] Total capillaries . . . . . . . . . . . . . . . . 199.560 VENOUS TREE. Veins, seventh order, gland . . . . . . .. 12,396 .04 15.495 seventh order, capsule . . . . .. 2,156 .037 2.177 Total seventh order . . . . . .. . . . . . . . 17.672 sixth order, gland . . . . . . . . .. 2,590 .08 13.001 sixth order, capsule . . . . . . . .. 44 .1 .345 Total sixth order . . . . . . . .. . .. 13,346 fifth order, gland . . . . . . . . . .. 390 .12 4.407 fifth order, capsule . . . . . . . .. 24 .18 .610 Total fifth order . . . . . . . . .. . . . . . .. 5.011 fourth order, gland . . . . . . . .. 57 .2 1.700 fourth order, capsule . . . . . . .. 3 .25 .147 Total fourth order . . . . . . .. 1.847 third order, gland . . . . . . . . .. 4 .4 .502 second order, gland . . . . . . . . .. 2 .6 .563 flrst order, gland . . . . . . . . . . .. 2 .8 1.005 lumbar vein . . . . . . . . . . . . . . . . . . . . 4.0 12.566
- The table is rearranged and a few corrections made according to instructions from Dr. flint in a letter of April 14, 1905.
[1] [2] [3] [4] Giving the vascular bed of the dog’s lung.
Table IV
Giving the vascular bed of the dog’s spleen.
Vessels Splenic artery . . . . . . . . . . . . . . . . . . . . . . 1
Branches to spleen . . . . . . . . . . . . . . . . . . 2
first order . . . . . . . . . . . . . . . . . . . . . . . . . 15
Second order . . . . . . . . . . . . . . . . . . . . . .. 1,000
Lobuiar . . . . . . . . . . . . . . . . . . . . . . . . . . . . 80,000
Terminal . . . . . . . . . . . . . . . . . . . . . . . . . . . 40,000,000
contracted . . . . . . . . . . .. 500,000,000
Pulp Spaces (dilated . . . . . . . . . . . . . . .. 500,000,000
Interlobular veins . . . . . . . . . . . . . . . . .. 80,000,000
Interlobular veins . . . . . . . . . . . . . . . . . . 80,000
Second order . . . . . . . . . . . . . . . . . . . . . . . 1,000
first order . . . . . . . . . . . . . . . . . . . . . . . . . 15
Branches from spleen . . . . . . . . . . . . . . . .. 2
Splenic vein . . . . . . . . . . . . . . . . . . . . . . .. 1
Table V
VIII, 1893.) [1] [2] [3] [4] Vessels Pulmonary artery . . . . . . . . . . . . . . . . . . . 1 Right and left branches . . . . . . . . . . . . .. 2 Lobar arteries . . . . . . . . . . . . . . . . . . . . .. 8 first order . . . . . . . . . . . . . . . . . . . . . . . .. 24 Second order . . . . . . . . . . . . . . . . . . . . . . .. 164 Third order . . . . . . . . . . . . . . . . . . . . . . . .. 1,021 Lobular arteries . . . . . . . . . . . . . . . . . . . .. 16,000 Atrial arteries . . . . . . . . . . . . . . . . . . . .. 64,000 See arteries . . . . . . . . . . . . . . . . . . . . . . .. 128,000 Capillaries . . . . . . . . . . . . . . . . . . . . . . . . .. 600,000,000 Sac veins . . . . . . . . . . . . . . . . . . . . . . . . . .. 192,000 Atrial arteries . . . . . . . . . . . . . . . . . . . . . . 32,000 Lobular veins . . . . . . . . . . . . . . . . . . . . . .. 16,000 Third order . . . . . . . . . . . . . . . . . . . . . . . .. 1,021 Second order . . . . . . . . . . . . . . . . . . . . . . .. 164 first order . . . . . . . . . . . . . . . . . . . . .. 24 Lobar veins . . . . . . . . . . . . . . . . . . . . . .. 8 Venous trunks . . . . . . . . . . . . . . . . . . . . .. 4 19 Number Diameter mm.
2.5 1.9 .75 .1 .015 .008 .01 .02 .04 .08 .5 4.0 5.0 6.0 Number Diameter mm 15.5 11.5 5.96 3.96 2.26 1.0 .3 .115 .165 .007 .23 .45 .4 1.22 2.44 4.18 6.12 13.75 243 Area of Section sq. mm.
4.91 5.67 6.63 7.85 14.0 4021.0 39,270.0 157,080.0 100,531.0 402.0 196.0 188.0 39.26 28.27 (Miller, Journal of Morphology, Area. of Section sq. mm.
181 208 223 293 656 801 1,120 1,344 2,688 23,000 7,680 6,098 2,000 1,194 765 340 299 756 244
Table VI
Giving the vascular bed of the dog’s portal system. Vessels Number Diameter mm.
[1] Portal vein . . . . . . . . . . . . . . . . . . . . . . . .. 1 9.0 Branches of the first order . . . . . . . . . . .. 6 5.0 Branches of the second order . . . . . . . . .. 70 1.7 [2] Branches of the third order . . . . . . . . . .. 700 .8 Branches of the fourth order . . . . . . . . .. 8,000 .4 Branches of the fifth order . . . . . . . . . . .. 80,000 .15 [3] Branches of the sixth order (interlobular) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 960,000 .05 [4] Capillaries . . . . . . . . . . . . . . . . . . . . . . . .. 1,850,000,000 .008 Sixth order of hepatic vein (central)" 480,000 .09 fifth order of hepatic vein . . . . . . . . . . . . 80,000 .17 Fourth order of hepatic vein . . . . . . . . .. 8,000 .5 Third order of hepatic vein. .: . . . . . . .. 700 1.0 first order of hepatic vein . . . . . . . . . .. 70 2.0 Second order of hepatic vein . . . . . . . . .. 7 5.0 Hepatic vein . . . . . . . . . . . . . . . . . . . . . . .. 1 11.0 ARTERY. [1] Hepatic artery . . . . . . . . . . . . . . . . . . . . . .. 1 2.75 [2] Branches to the liver . . . . . . . . . . . . . . . .. 2 1.18 Branches of the flrst order . . . . . . . . . . .. 6 .8 Branches of the second order . . . . . . . .. 70 .3 Branches of the third order . . . . . . . . . .. 700 .1 [ ] Branches of the fourth order . . . . . . . . .. 8,000 .05 Branches of the fifth order . . . . . . . . . . .. 80,000 .02 Branches of the sixth order . . . . . . . . . .. 960,000 .009 [4] Capillaries . . . . . . . . . . . . . . . . . . . . . . . . ..1,850,000,000 .008 than that of both the hepatic artery and the portal vein.
Area of Section sq. mm.
64.0 118.0 159.0 352.0 1,005.0 1,414.0 1,881.0 92,900.0 2,900.0 1,816.0 1,571.0 410.0 220.0 134.0 95.0 5.9 2.2 3.0 4.9 5.5 15.7 25.0 61.0 92,900.0 " The area of the hepatic vein of a given order is about 50 per cent greater
Table VII
Giving the area and ratio of enlargement of the arterial bed of six organs. The numbers in brackets refer to the corresponding numbers in the preceding tables which mark the data used in the construction of this table.
Area of arterial bed in sq. mm. Ratio of enlargement of arterial bed »—- ¢ ,:. .,_, E',_, ,_ . . . . . . M r S3 E3 E SE 2%’ E5 5 “ Hg P‘ 2. 3 ES‘ ":3 2 2 :3‘ .3: 3: 5.5 :4 8 .4 2 . .3 '= .. ta: ":1: 5.: E 8 0 as o no 3 a: we as 0 "‘ 3 was we -: ;..u ha 3 2‘ L. _a.> 0 0 a Q 0 N O a 0 ,._, 9 *"‘ E E '7.‘ E -— E E E .5 as as E 5.5 E2 5.2 52 s :1: as 5 E: sis? E25? ég ‘ .. .. .... ....'E ....'E Intestine . . . . 7.0 12 60 2,351 5 196 39 336 Stomach . . . . 4.0 1“ 6 42 917 7 153 22 229 Adrenal . . . . . .34 . . 6 199 . . . . . 33 580 Spleen . . . . . . 5.0 1*‘ 6 14 4,021 2 670 287 804 Lung . . . . . . . 181.0 801 2,688 23,000 3 30 9 127 Liver . . . . . . . 70.0 1” 354 1,897 92.900 5 264 49 1,327
The area of the vascular trees of the six organs given in Tables I to VI is based upon careful estimations made by myself or under my direction. If the volume of the organ is carefully considered while the measurements are being taken, it is. relatively easy to gain results which are very reliable. In the intestine equal sections were measured, and in the lung and the liver the lobes were measured independently of one another. Corrosion specimens were used as much as possible; the finer corrosion and thin transparent specimens were measured under the microscope. In the liver, lung and spleen the measurements were controlled by the number of lobules and their average size. In all cases for each figure given at least ten independent measurements were taken from as many different organs. The data given in Tables I, II, III and V have been published elsewhere. Those upon the spleen are in part new and in part from my article upon the spleen.[12]Those upon the liver are entirely new.
The livers of 29 dogs of medium size averaged 175 cubic centimeters. A transverse section of the lobule or a surface measurement of the lobule of a hardened liver averages .7 mm. in diameter. If a lobule is considered a cylinder 0.7 mm. high and 0.7 mm. in diameter we will find that there are about half a million of them in the liver of a medium-sized dog.
1" Estimatlons from the coeliac axis. 1' Artery and portal vein.
On account of numerous calculations of the lobules, terminal portal veins and terminal hepatic veins, I have fixed upon 480,000 as the average number. The terminal lobule or structural unit, either portal or hepatic, is about one-third of a cubic millimeter in volume, and with this size as a basis I have estimated the number of capillaries in the liver. However, lobules are usually put up in clusters, as described by Kiernan, and the volume of such a cluster is about 2 cu. mm. But the clusters can easily be diminished or increased at will to two structural units or to a whole lobe.
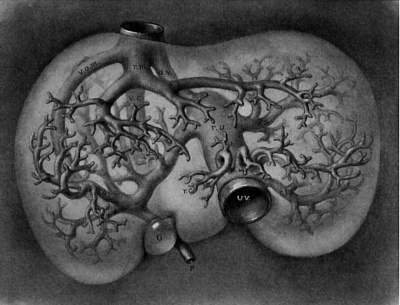
The average diameter of the portal vein is found to be 9 mm. The branches of the first order may be considered six in number and supply respectively the six lobes of the dog’s liver. They may be designated as right upper and right lower, left upper and left lower, cystic and omental branches to correspond with the terms given by Rex in his excellent paper on the morphology of the mammalian liver.[13] Each lobe usually receives two or more branches varying from 1 to 5 mm. in diameter which when bunched for each lobe will give an area represented by the single vessels from 4 to 8 mm. in diameter. So in order to round my figures without interfering with the total area of the veins of the first order I have indicated in the table that these are six in number averaging 5 mm. in diameter. This liberty is based upon measurements taken from nine corrosion specimens in celloidin and in wax, and may be controlled by the six excellent illustrations to scale given by Rex. It may be pointed out here that the left main branch ends quite abruptly in a dilatation at the point of communication with the umbilical vein; from this point veins radiate in all directions. This dilatation, the recessus umbilicalis of Rex, is even better marked in the human liver than in that of the dog (fig. 5) where it makes its appearance during the fifth week of development (fig. 25).
From the branches of the first order to the capillaries it is relatively easy to compute the number of vessels of a given order. To be sure a few of the branches which were bunched with those of the first order are of the second order, but they are so insignificant in number that they need not be considered. The main subdivisions of the branches of the first order may be collected and counted. One of average size may be selected and dissected out with the liver tissue it supplies. The volume of the whole liver divided by the volume of this piece will give a second estimation. The count and the estimation should not be far apart if both are made accurately. Slight variations will be neutralized in estimating the number of vessels of succeeding orders. The sixth order of both hepatic and portal veins is to be controlled by the volume of the structural unit. In general it is found that there are two terminal portal veins for one terminal hepatic; the former are always smaller and more slender, while the latter are tortuous and end abruptly.
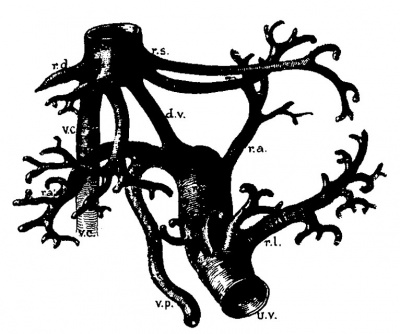
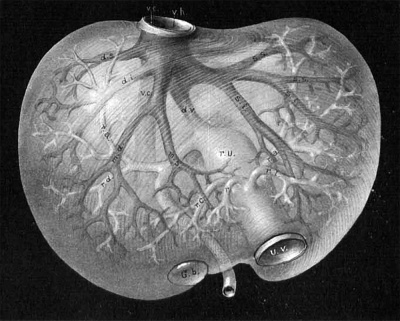
Fig. 5. Corrosion specimen of the portal tree in man. The injection was made with the liver in position. G bl, gall bladder; lt, ligamentum teres; ls. llgamentum suspensorlum; Oi, vena. cava inferior; tr. 1). p. trunk of the vena portae; r. ha, 1. ha, right and left main branches; ru, recessus umbilicalis; r. arc, ramus arcuatus; r. desc, ramus descendens; r. asc, ramus ascendens. After Rex.
It is not necessary for the blood to pass through vessels of all orders in order to reach the capillaries, for often veins of a given order have vessels of three following orders arising ide by side from the main trunk. Thus, branches of the first order have arising from them vessels of the second, third and fourth orders, and branches of the second order have arising from them veins of the third, fourth and fifth orders. The main trunk has branches as small as-the third order arising from it, and these in turn have branches as low as the sixth order arising from them. Thus in special cases blood may pass directly from the main trunk into veins of the third order and then into the terminal veins, skipping entirely the veins of the first, second, fourth and fifth orders. In general, however, most of the blood passes through veins of all orders before it reaches the capillaries. The short cut some of the blood takes is neutralized by the increased resistance due to the increased angle of the small vessel to the main trunk; very small veins always arise at right angles from large trunks, while their direction when arising from a smaller trunk is at an acute angle.
If there are 480,000 structural units in the form of small cylinders 0.7 mm. in diameter and 0.7 mm. high, it is easy to determine the number of capillaries which enter the unit in both transverse and longitudinal sections. My count in ten different injections gives 110 for the circumference of the hepatic unit and 3-3 for its height. These multiplied give 3850 as the number of capillaries which enter the hepatic lobule at its periphery. If all of the anastomoses within the lobule are estimated also, as they should be, this number is at least doubled. In order to err on the safe side I have taken the smaller number and multiplied it by the number of structural units, giving 1850 million as the number of capillaries which enter the periphery of the hepatic lobule of the liver.
I have arranged certain data (marked with brackets in Tables I to VI) in Table VII. It is at once noticed that the vascular bed is about five times larger for vessels 0.05 mm. in diameter than for those a millimeter in diameter. While the lumina of the vessels diminish 20 times, their number increases 2000 times. In the pulmontary artery and the portal vein, in both of which the blood-pressure is low, the vascular bed increases much more than in the arteries from the coeliac axis, down to the arteries one millimeter in diameter. In the intestine, stomach, adrenal and liver the increase in the vascular bed from vessels 0.05 mm. in diameter to the capillaries is much the same, averaging about 36 times; in the spleen it is much greater and in the lung it is much less. In the spleen with the numerous small slender vessels the resistance is enormous and in the lung it is insignificant, as is easily verified by making injections; it is very difficult to inject from an artery into the vein in the spleen, while in the lung melted wax can be injected through. In the spleen the area of the terminal arteries is 800 times that of the splenic artery, while if the pulp spaces be compared, the ratio is fully 30,000. That this number lies within the limit of probabilities is easily shown. The average volume of the spleen of the dogs used is 10 cc., which when distended increases to 80 cc. ; the spleen can add to itself seven times its own volume without bursting. The area of all of the pulp spaces of a moderately distended spleen is, as Table IV shows, 157,080 sq. mm., which multiplied by the length of a pulp space (0.02) gives 3 cc., a very insignificant portion of the entire volume of the spleen.
It is seen then that there are variations in the relation of the main vascular trunks to the capillaries in different organs, as was intimated by Thoma. This ratio in the spleen, determined by comparing arteries 0.05 mm. in diameter with capillary arterioles, is 287 ; in the lung it is 9; and in the liver it is at least -19. The sectional area of the capillaries of a lobule is 49 times that of the final portal twigs which supply the lobule.
All the lobules are equally favored, as has been frequently asserted and is easily proved by making injections with fluids of various consistency into any of the three vessels of the liver. In arch case all of the terminal vessels fill simultaneously. The lobule most distant from the main vessel is not less favored than the lobule near to it. Thoma’s laws have regulated the growth of the system of vessels and also have kept it adjusted.
According to Thoma’s hypothesis, whenever the capillary pressure exceeds a certain point due to an increased exchange of substance in the growing adjacent tissue, there is a new formation of capillaries. Those portions of the area vasculosa which are favored later by the circulation seem to run ahead in their development in blastoderms from 18 to 39 hours old. It is seen that accelerated growth is accompanied with the new formation of capillaries long before there could possibly be any circulation through them.[14] A similar condition is to be seen in the human embryo. The blood vessels arise in the walls of the umbilical vesicle and grow into the embryo and form the main circle of vessels within it before the heart is fully formed. Such a condition is to be seen in the embryo described by Eternod. In exceptional pathological conditions they may develop into the villi of the choriou without the formation of a heart.
Loeb[15] has shown, by an ingenious experiment, that a very complete vascular system is developed in certain fish embryos without any circulation of blood at all. He placed the eggs of Fundulus immediately after fertilization in a solution of sea water to which 111% of K Cl had been added, and found that they undergo a normal development without any heart beat; the chloride of potassium had paralyzed the heart. However, a complete vascular system is developed, being practically normal in arrangement within the embryo, as well as in the yolk-sac. The form of the vessels was Very irregular, at points forming rosettes in which narrow and dilated vessels alternated. Loeb concludes that not only did the entire vascular system develop without any circulation of blood, but also without any intravascular pressure, for had there been one, the capillaries which were developed should have been found distended. Furthermore, it is shown that the capillary buds grow independently of bloodpressure. “ Die mechanischen Ursachen fiir das Wachsthum der Gefasswande sind deshalb nicht im Gefasslumen zu suchen, sondern in allen oder einzelen Zellen der Gefasswande und die Abgabe von Aesten ist bestimmt durch inner Ursachen in den Zellen der Gefasswande oder durch Reizursachen, die von der Umgebung ausgehend, diese Zellen trefien,‘ ahnlich wie im Falle der Stolonenbildung von Hydroidpolypen.” 2‘ It is also seen from Thoma’s own illustration (fig. 3, d), that capillaries in which the pressure must be equal degenerate or multiply, as the case may be. In the transformation of fig. 3 into fig. 4, Thoma’s first law on the rapidity of the circulation must have directed all of these changes. In fact, Thoma’s hypothesis, on the budding of capillary cells, is not based upon intracapillary pressure alone, but also “vom Steffwechsel der umgebenden Gewebe, ” which can easily be harmonized with Loeb’s “Reizursachen, die von der Umgebung ausgehend, diese Zellen trefien.” In reality we can only state definitely that with the new formation of tissue new blood-vessels may grow into it, for all new tissue does not have blood vessels. Thoma’s first two laws define much better and express more clearly the ideas relating to the question brought together by Roux " in his Inaugural Dissertation. To be sure, it is all functional adaptation, fo-r the circulating blood arranges the irregular capillary anlage for a uniform circulation.
One would think that if the favored blood vessel dilates, a number of them would soon connect the arteries with the veins, and thus do away with the capillaries entirely. Thoma states that the reason why this does not take place is to be found in the great relative distance between the primary artery and vein, which is continued in their subsequent tree-like growth. From our present conceptions, it would appear, however, that occasionally an intervening capillary would dilate a little above the normal, and, being favored, would gradually become larger and larger. In fact, Brissaund and Sabourin have asserted that such anastomoses are quite common between the terminal portal and hepatic veins in many animals.[16] It seems to me that there must be other agencies that would prevent such a catastrophe. In fact, we have an abundance of examples of a reduction of enlarged capillaries whenever they occur. Thoma has given a satisfactory explanation of the closure of the ductus arteriosus and of the ductus venosus, but that is not quite to the point in this case. However, in the beginning of the capillary system of the liver, around the omphalo-mesenteric vein, we have an excellent case. This question will be taken up at greater length subsequently, so at present I shall be very brief. The liver grows around and into the omphalo-mesenteric vein, and while so doing we have a double circulation, a more direct one through the constricted vein and a more circuitous one through the capillaries of the liver. But, in spite of this, the vein is gradually eliminated, leaving only a capillary plexus. The aortic arches of amphibia are eliminated in a similar way. From them loops of capillaries grow into the external gills which gradually take the place of the artery. There are numerous other examples. Minot,[17] who has recognized the fundamental importance of the destruction of a main channel and its conversion into a system of capillaries, calls such vessels sinusoids, and the circulation through them a sinusoidal circulation. Not only are blood—vessels which are too large reduced, but it appears in the development of the blood—vessels in the tad pole’s tail as if the new blood-vessels were always growing in the direction» of the greatest resistance, for the nearest complete arch is already the shortest course from the artery to the vein ; yet another and more distant one is to be formed.
The first and guiding blood-vessel is the capillary which grows in all directions, forming a plexus. Secondary changes make arteries and veins of them and their laws of growth have been discovered and clearly stated by Thoma. The normal shape of the capillary is tubular with a lumen about .008 mm. in diameter. They arrange themselves into a plexus with a tendency to come in contact with every surrounding cell. However, the tissues vary considerably in this respect, the first capillaries growing to them or past them in tufts. In general, the capillary arrangement is influenced by the tissue or organ into which it grows, but its conversion into main trunks and branches is controlled by the circulation. Ultimately the arrangement is such that all capillaries of an organ are equally favored by the circulation. This means that the capillaries have about the same diameter and length with about the same amount of blood passing through them during a given period of time. If too little blood passes through them, in a lobule of the liver for instance, some of the capillaries disappear; if the circulation comes to a standstill all of the capillaries are obliterated. S0 their very life is dependent. upon a proper or normal circulation. If a capillary is too long, the resistance within it is increased, and the circulation is slowed with a subsequent reduction of length. So in order that a capillary may remain, it must have a definite lumen, a definite length and a definite amount of blood passing through it in a given ‘time. The diameter varies from .005 to .01 mm., according to the organ in which it is located. The average is .008 mm., the diameter of the mammalian red blood corpuscles. In some of my tables the diameter is given as .003 mm., but these measurements are from hardened and mounted tissues. The length of the capillary is less than half of a millimeter, through which the blood flows in less than a second. So, morphologically, a capillary is a blood-vessel .008 mm. in diameter, .5 mm. long with a renewal of blood every half-second. If this renewal of blood is permanently diminished enough of the capillaries are obliterated to reestablish the normal circulation in those that remain. If the quantity is permanently increased, according to Thoma’s first law, some of them are converted into arteries and some into veins. If the increased circulation is continued without a corresponding increase in the number of capillaries, the artery will extend into the vein just as is the case in the liver when the ductus venosus is formed.
The anlage, then, of the vascular system is the capillary; artery and vein are secondary and are difierentiated out of them by the flow of blood set in motion by the beat of the heart. As‘ the capillary bed increases the flow through the arteries increase, and the heart hypertrophies and the vascular proportion is maintained. In round numbers, in the dog, the arteries continue to grow until the rapidity of flow is 30 mm. a second in an artery .05 mm. in diameter, 150 mm. in an artery, 1 mm. and 300 mm. in the aorta. Thoma fixes the rapidity of the circulation in the aorta of man at 228 mm. a second, and over 34 mm. a second in an artery .04 mm. in diameter.
The unequal growth of different portions of an organ accounts for the unequal size of the arteries which supply them. The whole thing works from the periphery to the center. In this way a succession of organ units is formed all the way from the first divisions of the artery which supply the lobes, to its final twigs, which supply the lobules. It is seen from what has been said above that it is undoubtedly the growth of the tissue of the organ which leads the way. Into this newformed tissue the capillaries grow and they have an inherent power which makes them grow into an anastomosing tubular system. The density of the capillary plexus is influenced by the tissues into which they grow, but their length and arrangement is determined by the circulation through them. A vascular proportion is constantly maintained for each organ down to the minutest vascular twig. Capillaries through which the rate of circulation is below the normal shrink or disappear, and when it is above the normal they enlarge into either veins or arteries. A capillary too long will eventually cut itself off on account of the increased resistance to the circulation in its own walls. An increased flow of blood rarely causes an artery to empty directly into a vein, because the determining factor is nearly always to be found in the capillaries themselves. The growth of the capillaries causes some of them to change into arteries and veins, and the equilibrium is thus easily maintained. In rare instances, however, the amount of blood thrown into an organ may be increased greatly, as is the case when all of the blood from the umbilical vein is suddenly forced through the liver. It follows that the circulation through a chain of capillaries from the portal to the hepatic vein is much above the normal capillary circulation, and, as a result, the ductus venosus is formed.
Early Development of the Liver
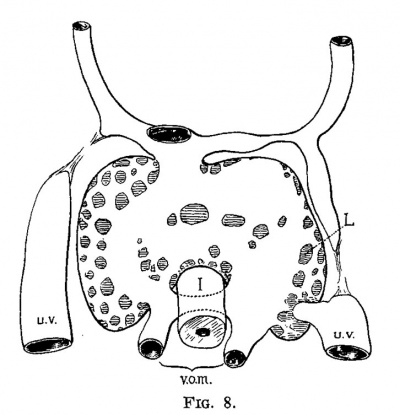
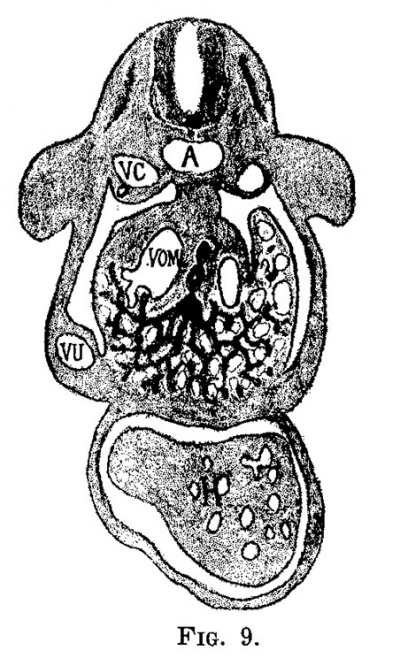
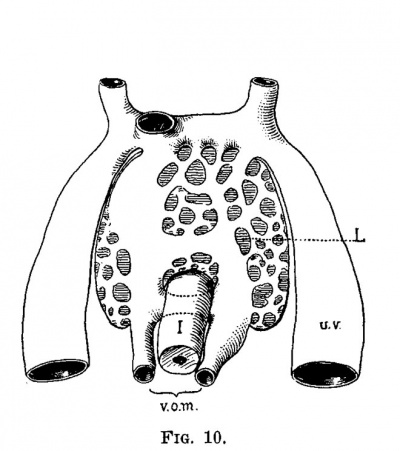
The early development of the liver has been worked out by His and others, and therefore it need not be discussed to any great extent. The liver bud, as shown in fig. 6, is well marked in an embryo at the end of the second week (2.1 mm.). It grows rapidly and then encircles the left omphalo-mesenteric vein, in the chick and in man, and both the right and the left in the dog. At the same time that the liver tissue encircles the vein it also invades it, carrying the endothelial lining ahead of the sprouts and thus forms a series of sinuses, or the sinusoids of Minot. While this process is taking place the umbilical veins are gaining much in importance and a large share of the blood which formerly returned to the heart through the omphalo-mesenteric now returns through the umbilical veins. figures '7 and 8, from an embryo 4.3 mm. long, and figs. 9 and 10, from an embryo 4.5 mm. long, illustrate this point. In the embryo 4.3 mm. long large sprouts of liver tissue have invaded the common omphalo-mesenteric veins which have also extended, forming a large ring of Minot’s sinusoids encircling the intestine as described by His. At this time the umbilical veins are broken in their course, having already passed the first stage of their growth, and from now on are destined to pass through the liver rather than past it (fig. 8). It is interesting to note that the primary sinusoidal liver—that portion which arises with the omphalo-mesenteric vein—is formed while the umbilical vein empties directly into the ductus Cuvieri. The process is at its height in an embryo of about the same age (No. 76), as shown in figs. 9 and 10. The growth of the liver around and through the omphalo-mesenteric veins is accompanied by the growth of capillaries from this vein into the new anlage. Hand in hand with this process the circulation through the omphalo-mesenteric veins is further reduced by the growth and enlargement of the umbilical veins. A double force is at work: blood is diverted by the umbilical vein which is gradually assuming greater importance and by the capillaries which supply the embryonic liver. Accordingito Thoma’s first law, the diminished rapidity of the circulation is followed by a reduction of the lumen and in order to accomplish this reduction in the present case, the liver sprouts first grow into the vein instead of around it. The operation of Thoma’s law in this case is so extensive that it reduces a main trunk to capillaries which forms a condition recognized by Minot as a sinusoidal circulation.
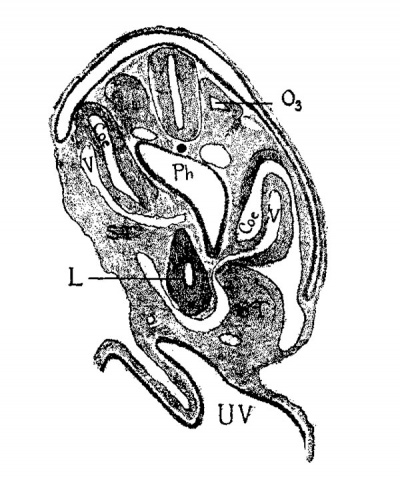
Fig. 6. Section through the third occipital myotome of a human embryo 2.1 mm. long (No. 12 of my collection). X 50. 03 third occipital myotome; coe, coelom; v, vein; st, septum transversum; 1, liver; ph, pharynx; uv, umbilical vesicle.
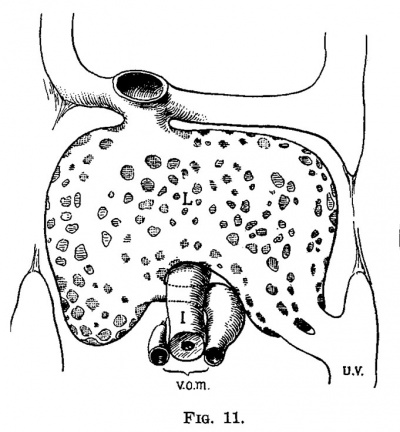
At the time the liver circulation is entirely sinusoidal, i. (3., about the end of the third week, it is composed of a single lobule with the vein entering it on one side and the collecting vein leaving it on the opposite side. The turning point between the first and second stages of development is shown in fig. 11. In the embryo represented by this figure the omphalo-mesenteric veins are broken completely into capillaries in the liver, and one umbilical vein has been transferred from the ductus Cuvieri to the lower part of the liver. The single liver lobule here is perfect; it is composed of a complete capillary network without an anaetomosing vein through it. A rough estimation of the vascular proportion shows that the area of the capillaries is fully 100 times that of the entering veins. In the next two embryos, figs. 12, 13 and 14, all of the blood from the left umbilical vein passes through the liver—the right vein having been obliterated. Within the liver it is seen that the right omphalo-mesenteric vein is open, while the main branches of the hepatic and portal veins have made their appearance. With the growth of the liver the capillary bed has increased which is naturally followed by a more rapid circulation in the distributing and collecting capillaries, and consequently they are converted into veins. All of the blood from the umbilical veins now passing through the liver increases the circulation through the small liver so much that a venous channel (right omphal0mesenteric vein) remains open, or in case it be closed it is opened up again. The two new branches within the liver care for the circulation through its left lobes, and may have been formed directly from the left omphalo-mesenteric vein. At -any rate, we see in them two permanent main trunks of the liver,—-the vena hepatica sinistra and the ramus angularis arising from the recessus umbilicalis.“ In the next stage which is found during the fifth week the right omphalo-mesenteric vein is obliterated and the ductus venosus is formed as a new and more direct channel. In place of the obliterated omphalo-mesenteric vein there are two new permanent veins, the ra.mus dextra of the hepatic vein and the ramus arcuatus et descendens of the portal system. We now have a liver of two "An excellent description of the vascular system of the mammalian liver is given by Rex (Morph. Jahrb., XIV, 1888). A much as possible I have used his nomenclature, lobules, representing the right and left lobes, with a vascular system in each identical in arrangement with that of a liver of one lobule. (fig. 11.) When the umbilical vein first shifts from its entrance into the ductus Cuvieri to the liver it has taken the course in its new position, of the least resistance, as a glance at fig. 15 shows. There is a mass, if not an excess, of capillaries in the liver at this time and this vein with its loose wall makes the change suddenly as is shown in fig. 11. This brings to the liver an excess of blood which is followed by keeping open, or opening in case it has closed, the right omphalo-mesenteric vein. The continued growth of the liver and its capillaries increases the circulation in the distributing and collecting branches which is followed by their conversion into permanent venous trunks: first those on the left side and then those on the right side. The excess of blood is still continued and on account of the shifting of the right omphalo-mesenteric vein with the growth of the right lobe of the liver the route becomes circuitous, and a new and more direct channel, the cluctus venous, is formed. This has already taken place in the specimens shown in figs. 17 and 20; in a later stage, fig. 25, the omphalo—mesenteric still remain.s open after the duetus venous is formed.
|
|
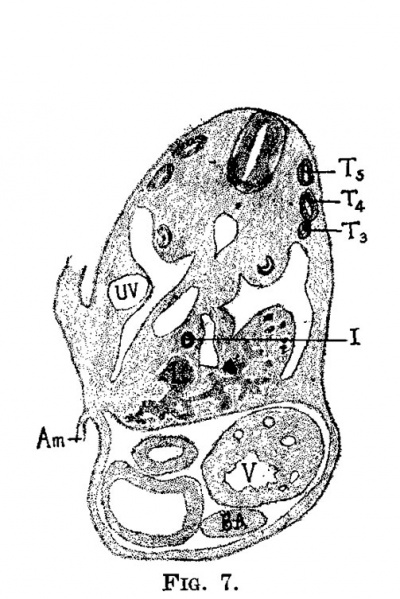
| Fig. 7. Section through a human embryo 4.3 mm. long (No. 148). X 25. T,, T., T., third, fourth and fifth thoracic myotomes; i, intestine; 1, liver; 12, ventricle; bu, bulb of the aorta; am, amnion; 1w, umbilical vein. | Fig. 8. Semidiagrammatic reconstruction of the veins of the liver of a human embryo 4.3 mm. long (No. 148). L, liver; uv, umbilical vein; vom, omphalo-mesenteric vein; i, intestine. |
|
|
| Fig. 9. Section through a human embryo 4.5 mm. long (No. 76). X 25. V0, cardinal vein; a, aorta; vom, omphalo-mesenteric vein; vu, umbilical vein; h, heart. | Fig. 10. Semidiagrammatic reconstruction of the veins of the liver of a human embryo 4.5 mm. long (No. 76). L, liver; 1w, umbilical vein; vom, omphalo-mesenteric vein; i, intestine. |
|
|
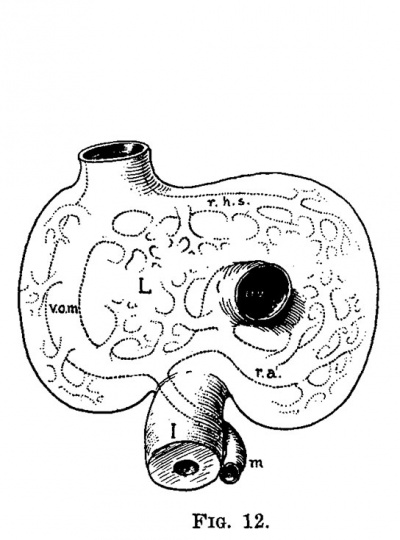
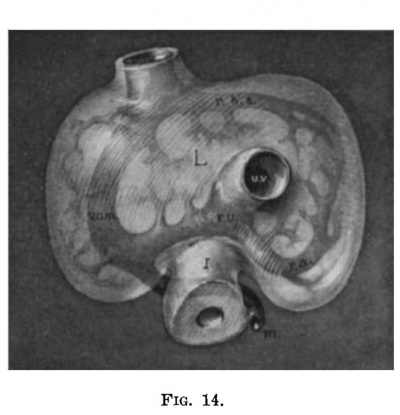
| Fig. 11. Semidiagrammatlc reconstruction of the veins of the liver of a human embryo 4 mm. long (No. 186). L, liver; uv, umbilical vein; vom, omphalo-mesenterlc vein; i, intestine. | Fig. 12. Semidiagrammatic reconstruction of the veins of the liver of a human embryo 6.6 mm. long (No. 116). L, liver; vom, right ompha1o-mesenteric vein; aw, umbilical vein; m, mesenteric vein; rhs, ramus hepatica. sinlstra; ra, ramus angularis; i, intestine. |
During all this time the vascular proportion remains normal, that is, the area of the capillaries is about 50 times that of the main portal trunk. The distributing branches are on one side of the lobule, and the collecting branches on the other. With an increase of the number of lobules, however, they are no longer set parallel, but at various angles with one another. Were they continued parallel they would have to spread as a sheet with a thickness of a millimeter, the maximum normal length of a capillary. In an embryo at the end of the fifth week, fig. 25, two new lobules have made their appearance and the two primary lobules have begun to divide. The hepatic and portal veins are telescoping; they are beginning to dovetail with each other. The new branches of the portal have gone into the field of the hepatic and the new hepatic veins have entered into the portal field. By this process, and by this process only, can a spherical vascular organ be built up maintaining a normal vascular proportion. All through the organ the terminal twigs of the distributing and of the collecting veins must not be over a millimeter apart, and this naturally keeps the units small and determines the ratio between the terminal twigs and the capillary bed.
|
|
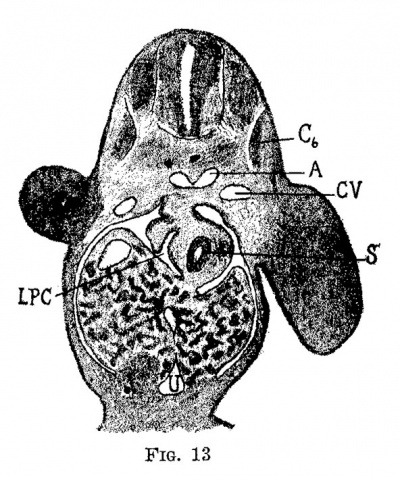
| Fig. 13. Section through the liver of a human embryo 5 mm. long (No. 80). X25. 0 sixth cervical myotome; a, aorta; cv, cardinal vein; .9, stomach u, umbilical vein; lpc, lesser peritoneal cavity. | Fig. 14. Semi-diagrammatic reconstruction of the veins of the liver of a. human embryo 5 mm. long (No. 80). L, liver; vu, umbilical vein; r. vom, right omphalo-mesenteric vein; rhs, ramus hepatic sinistra; ru, recessus umbilicalis; m, ramus angularis; m, mesenteric vein; vi, intestine. |
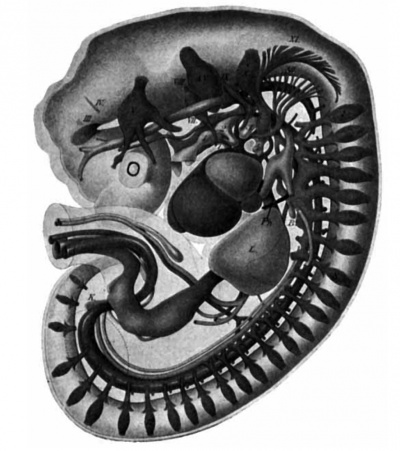
Fig. 15. Lateral reconstruction of -a human embryo 7 mm. long (No. 2). L, liver; ph, phrenic vein; 1, 2, 3, 4, branchial pouches; Roman numerals, cranial nerves; Arabic characters, spinal nerves.
In the embryo of the end of the fifth week, fig. 25, the right and left portal twigs have begun to divide, and from the recessus umbilicalis a new group of veins have formed and radiate into the middle and left lobes of the liver. On the hepatic side the left branch has divided into two trunks and two new branches have appeared: the vena cava inferior and the vena hepatica media which has its terminal right and left branches.
|
|
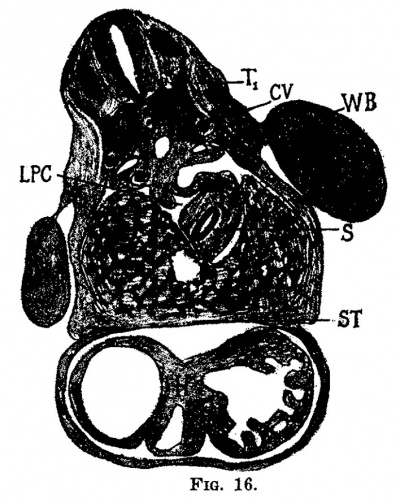
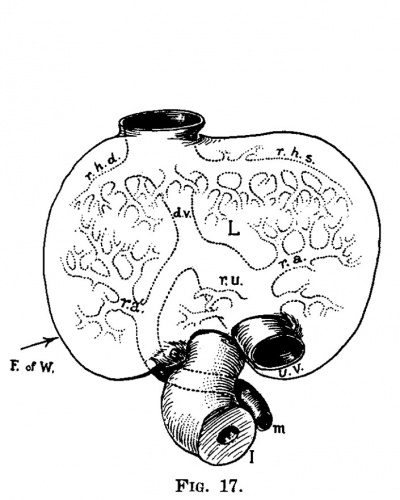
| Fig. 16. Section through the embryo 7 mm. long (No. 2). X 25. T1, flrst thoracic myotome; cv, cardinal vein; wb, Wolflian body; s, stomach; lpc, lesser peritoneal cavity; 1, liver; h, heart; st, septum transversum. | Fig. 17. Semi-diagrammatic reconstruction of the veins of the liver of the embryo 7 mm. long (No. 2). Viewed from in front. L, liver; uv, umbilical vein; m, mesenteric vein; ru, recessus umbilicilis; dv, ductuc venosus; ra, ramus angularis; ra‘, ramus arcuatus; rhd, ramus hepatic dextra; rhs, ramus hepatica sinistra. |
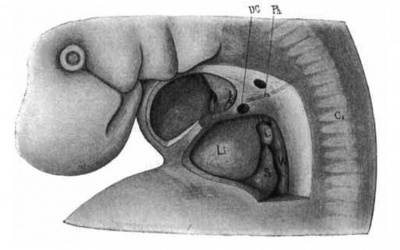
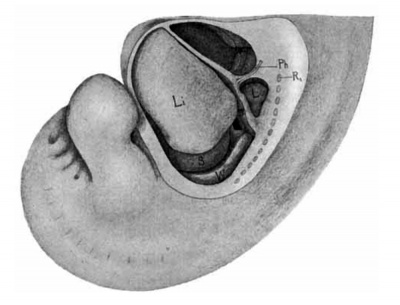
Fig. 18. Lateral view of a. model of the liver in position of a. human embryo 9 mm. long (No. 163). X 12%. 0;, eighth cervical myotome; Ii, liver; 1, lung; .9, stomach; wf, Wolflian body; ph, phrenic nerve; pc, pleuro-pericardial membrane; pp, pleuro-peritoneal membrane; dc, ductus Cuvierl.
The right omphalo-mesenteric vein is still present and the ductus venous is well marked. In this case the liver is formed of four main lobules, and with the subdivision of the middle and left hepatic veins into two branches each, six primary lobules are seen to correspond with the six primary lobes of the mammalian liver. In this case the vena hepatica dextra superior et inferior is represented by the open omphalo-mesenteric vein and the anlage of the vena cava inferior. In the next stage, figs. 26 and 28, the normal arrangement of these veins is found for the vena cava inferior really belongs to the middle lobe.
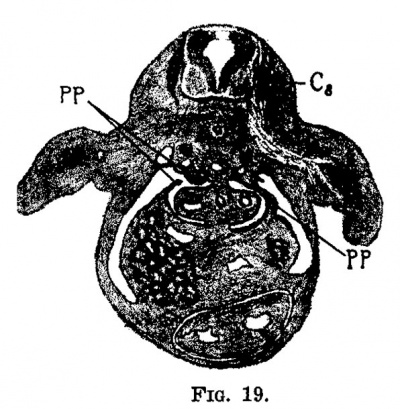
Fig.19. Section through the embryo 9 mm. long (No. 163). X 12%. 0,, eighth cervical myotome; pp, pleura-peritoneal membrane.
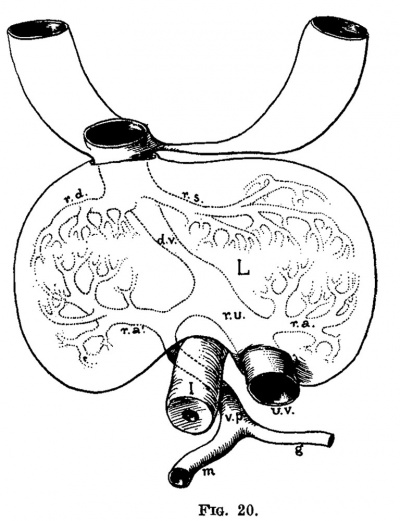
Fig. 20. Ventral view of the veins of the liver of the embryo 9 mm. long (No. 163). L, liver; t, intestine; ma, umbilical vein; op, vena portae; a, gastric vein; m, mesenteric vein; ra, ramus angularis; ra.,, ramus arcuatus; rs, ramus sinistra; rd, ramus dextra; do, ductus venosus.
With the completion of six lobules we recognize fully the adult form of the liver. Each lobule now represents one of the six lobes of the mammalian liver; each of the primary lobules is to expand into a whole lobe. The primary lobules radiate from a center and have between them the main trunks of the portal veins; each interlobular vein at this stage is to form a main trunk in the adult. At this time we have terminal vessels to follow from stage to stage, which is impossible to do in adult specimens.
Fig. 21. Lateral View of a model of the liver in position of a human embryo 11 mm. long (No. 109). ><81,é. Li, liver; 1, lung; 7, first rib; ph, phrenic nerve; s, stomach; wf, Wolflian body; pp, pleuro-peritoneal membrane.
The process of sprouting and interlacing continues at a rapid pace from now on, and for the present I shall give illustrations from the livers of two embryos of the eighth week. The first (No. 22), fig. 26, is from a wax plate reconstruction carried as far as I could conveniently, and fig. 27 is from a photograph. The second (No. 6) fig. 28, is from a graphic reconstruction which could be carried out pretty well, and figs. 29-31 are three views of a wax model of the exterior of the liver. These illustrations together show the form of the liver and the main vessels with their lobular branches. There are about 700 branches of the third order in the adult liver, and rough estimations made from figs. 27, 29-31 give about this number. The lobules in these specimens are about 0.4 mm. in diameter, considerably smaller than in the adult. In general branches of the hepatic and portal veins of the same order are as far apart as possible with a tendency to run at right angles to each other. The branches of the first order or main trunks have been present from the time of the earliest difierentiation of the liver, while those of the second and third order date from the beginning of the dovetailing process. A word more about the vena cava inferior. In its beginning it belongs entirely to the liver and is completely surrounded with liver tissues. In the adult liver two small branches empty into it in addition to the main branches mentioned above. The first is closely associated with the vena hepatica media accessoria. The other arises from the omental lobe and usually goes directly to the vena cava, but occasionally communicates with the vena hepatica sinistra. It is seen that the vena cava collects blood directly from the quadrate and Spigelian lobes.
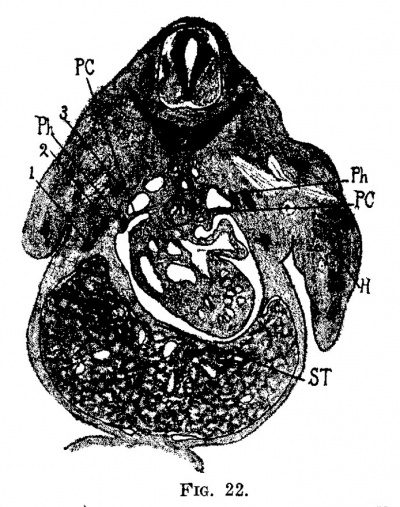
Fig. 22. Section through the body of the embryo, 11 mm. long (No. 109). X 10. The liver is attached to the septum transversum, st; 3, first rib; 1, third rib.
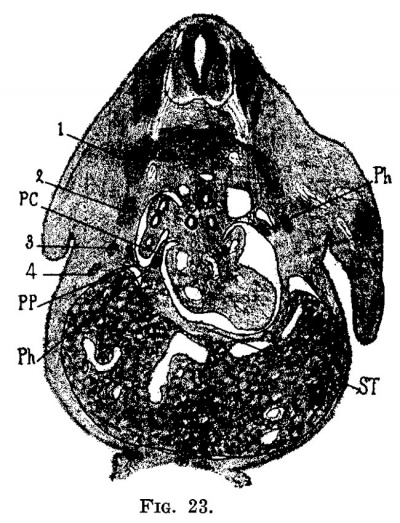
Fig. 23. Section through the embryo, deeper than in fig. 22. Just in front of the septum transversum in the liver is seen 9. section of the ramus hepatica sinistra and coming forward the ramus hepatica media.
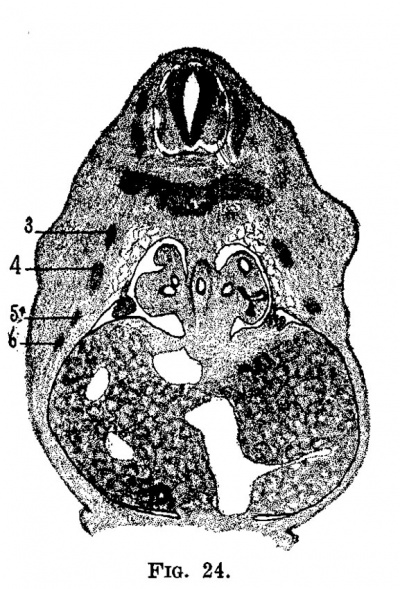
Fig. 24. Section through the same embryo showing the umbilical vein, recessus umbilicalls and the ramus arcuatus. Behind in the liver is the vena. cava, to its left the open right omphalo-mesenteric vein (see fig. 25), in front is the gall bladder and between it and the omphalo-mesenteric vein in the ramus arcuatus.
Fig. 25. Ventral view of a reconstruction of the vasicular system of the embryo 11 mm. long (No. 109). X 25. U0, umbilical vein; gm, portal vein; ra, ramus angularis; ru, recessus umbilicalls; rd, ramus descendus; ra, ramus arcuatus (possibly ramus ascendus); rc, right arborlzation of the recessus umbi1ica1is;. rl, left arborization of the recessus umbillcalis; dv, ductus venosus; vc, vena. cava; vom, omphalo-mesenterlc vein; rm, ramus media; rs, ramus sinistra.
Fig. 26. Main trunk of the liver from an embryo 20 mm. long (No. 22). From a reconstruction in wax. X 12. U12, umbilical vein; up, portal vein; ra, ramus arcuatus; ra, ramus angularis; ru, recessus umbilicalis; dv, ductus venosus; vs, vena cava; rd, rarnus dextra; rm, ramu media; rs, ramus sinitra; 11, left arborization of the recessus umbllicalis.
Fig. 27. Photograph of a section of the human embryo, 20 mm. long (No. 22). X 15. Ru, recessus umbilicalis; dv, ductus venosus; rm, ramus media. Between the two branches of the ramus media. may be seen a branch of the ramus acendus cut transversely. These branches are of the second order and the terminal branches the beginning of those of the third order. In this case the lobnles are .5 mm. in diameter.
The Hepatic Lobule and the Portal Unit
It is seen from what has been said above that the final branches of the portal and hepatic veins are always as far from one another as possible throughout all stages of their development as well as in the adult liver. At all times this distance is half the diameter of a lobule and since this is in the neighborhood of one millimeter the distance is about half a millimeter, the normal length of a capillary blood-vessel. It is also apparent, as indicated by fig. 1, that the liver breaks up into two sets of units arranged respectively around the terminal twigs of the two sets of veins.
Fig. 28. Reconstruction of the vasicular system of tne liver of a human embryo 24 mm. long (No. 6). x 20. All of the important vessels are fully formed. The stage is the same as that shown in section, fig. 27. uv, umbilical vein; vp, vena portae; ru, recessus umbilicalis; ra, rarnus arcuatus; rd, ramus descendens; m, ramus angularis; 1*, r, right arborization of ru; re, left arborization of the recessus umbilicalis; vh, vena hepetica; dv, ductus venosus; ds, vena dextra superior; dz‘, vena dextra inferior; md, vena media. dextra; ms, vena media sinistra; ss, vena sinistra superior; Si, vena sinistra inferior; vc, vena cava.
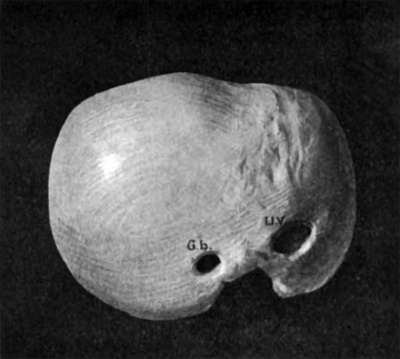
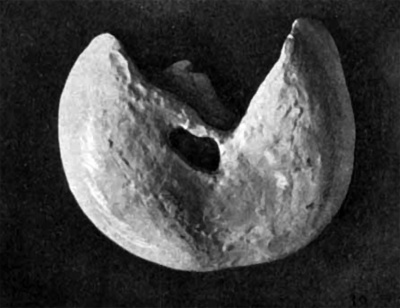
Fig. 29. Ventral view of a wax model of the embryo, 24 mm. long (No. 6) x 10. uv, umbilical vein; Gb, gall bladder.
Fig. 30. Superior view of the same liver.
That the unit arranged around the hepatic vein was finally accepted as the lobule is largely due to excessive amount of connective tissue alongthe portal twigs in the pig’s liver, 3. condition almost peculiar to this animal. Had the liver of Phoca been studied in the place of that of the 268 A Study of the Structural Unit of the Liver pig the portal unit would have been accepted, for in general either set of lobules is only occasionally well outlined and thus marked in some mammals. In the human liver, as in the dog’s, there are more terminal portal twigs than hepatic and this together with other structures which accompany the portal vein, makes it easy for practical purposes to call the hepatic unit the lobule. However, it is just as easy, if not easier, to consider the portal unit the lohule if one is so inclined.
Fig. 31. Dorsal View of the same. Gb, gall bladder; wv, umbilical vein; 8, Spigelian lobe; vc, vena cava inferior.
From the standpoint of pathology, it is easy to construct a description of the liver based upon a portal lobule. Especially marked are these lobules in venous hyperaemia, in pigmentation and in cirrhosis in which there is a marked regeneration arising from the bile ducts. Sabourin[18] has used these arguments successfully in favor of the portal lobule as the histological unit of the liver. However, his results are also not new, as may be seen by glancing over the historical note accompanying this paper. But his point is sound and shows that the liver histology may be constructed around the terminal portal veins as well, if not better, than around the terminal hepatic veins. His extensive monograph is illustrated with several hundred diagrams, many of which are fanciful, for they are defective in one respect. If a series of circles are crowded together to form hexagons and each of the angles is then used as a center of a series of superposed hexagons of the same size, the new series of circles will not form an equal layer, but will overlap each other. Thus, in his fig. 227, there are six hepatic veins surrounding one portal, while in the next figure the opposite is the case. He should have had them of equal number, having an alternating space common to both systems, the nodal point, as I call it. However, the work of Sabourin is excellent and deserves much more attention than it has received outside of France.
Fig. 32. From a corrosion in celloidin of the terminal branches of the portal and hepatic veins. X 20. The hepatic vein is larger and marked by many constrictions forming a “spiral valve.” I, interlobular veins; c, central vein; s, sublobular vein.
The typical liver lobule, as described by Kiernan,” is based upon the study of the pigs liver, and is composed of either a single lobule, or of clusters of them. It is not clear which he considers the real unit, for there are all gradations between single lobules and compoundilobules composed of at least 25 single ones. As the veins grow larger capillaries cease to arise from them, and the opened vein shows at the point of transition the bases of adjoining lobules shining through its wall; at this ”Kiernan, Phil. Trans., 1833. 2'70 A Study of the Structural Unit of the Liver point, according to Kiernan, the intralobular veins change into sublobular. This distinction is rather arbitrary and of little value, but has clung as a parasite to the text-books. In general hepatic veins with capillaries arising from them are called central or intralobular veins and larger veins are called sublobular; sublobular veins are hepatic veins from one to two millimeters in diameter.
Fig. 33. Photograph of a celloidin corrosion of the liver lobule and the portal unit. X 2. The dark clumps are the portal and the light anastomosing bodies are the lobules.
Fig. 34. Plastic diagram of a group or anastomosing lobules with the terminal branches of the portal vein, marking the centers of the portal units, added. Enlarged about 10 diameters.
Fig. 35. Diagrammatic outline of the group of lobules, shown in fig. 34, with the hepatic vein added.
I have studied carefully the hepatic lobule in the dog’s liver and found that in general I can confirm practically everything that Kiernan has said of it. Numerous injections have been made of the hepatic vein, either singly or in combination with injections of other vessels. Figure 32 is made from a celloidin corrosion specimen of both the portal and hepatic systems. The “ spiral valve ” of the hepatic vein, which is always present in the dog and the cat, is well shown in such specimens. From the study of granular injections of the hepatic vein with cinnabar, ultramarine blue, chrome yellow and baryta, it is found that the capillaries arise from veins which are about .17 mm. in diameter. This arbitrary point, fig. 32, 8, marks the beginning of the sublobular vein, although numerous capillaries still arise from it. After its walls become markedly thickened capillaries no longer arise from the sublobular vein, but in their place small veins arise which supply portions of a lobule and there fiG. 36. Photograph oi‘. a corrosion preparation of the portal and hepatic trees. X 2. At the X two of the terminal hepatic veins anastomoe. The “ special valve ” may alo be seen.
fore cannot be considered central veins. In my study I have called the hepatic veins in the neighborhood of .09 mm. in diameter intralobular veins, and those .17 mm. or a little larger sublobular veins. In Table VI they have been classed respectively under the sixth and fifth orders. The form of the hepatic lobule can be well outlined by washing vessels of the liver first with saline solution, then with alcohol, after which cinnabar and lamp-black celloidin are injected respectively into the portal and hepatic veins. With considerable pressure the capillaries are injected more or less with red or with black. figure 33 is from a specimen of this kind with red in the hepatic vein and black in the portal vein. In this specimen the lobule was but partly injected with red celloidin and in the corrosion but the center of the lobule is shown. To the extent in which 272 A Study of the Structural Unit of the Liver capillaries arise from the hepatic vein it is encircled by the red mass. It is seen that the celloidin entered the lobule only at the tips of the veins, that is from the intralobular veins. The sublobular veins are clear and have arising from them intralobular veins which again have clusters of lobules attached to them. The lobules are in clusters to correspond with the branching of the interlobular veins. The outline of one in perspective is given in fig. 34, which is reduced to a diagram in fig. 35.
Fig. 36a. Portal and hepatic veins from a corrosion preparation of the liver of the dog. X 10. The two sets of vessels were injected with black and red ceiloidin mixture respectively, after which the liver was hardened in alcohol. A block of tissue was cut into very thick serial sections which were digested for a number of days in a solution of pancreatin at 37° C. After the cells were all dissolved, leaving only the connective-tisue framework and the injected ceiloidin the section were preserved in glycerine. The minutest twigs of ceiloidin are held in place by the delicate reticulum iinriis.
While the hepatic lobules are irregular, anastomosing, and of unequal size, the portal units are regular, more spherical and of equal size. The branches of the portal vein are much more delicate and regular than those of the hepatic. A comparison between the two is shown in figs. 32 and 36, while the single portal branch is shown in fig. 37. In general the portal branches are more regular than the hepatic, as far from them as possible with a tendency to run at right angles to them. The portal veins never come to the surface of the liver and never anastomose; the terminal hepatic branches are very irregular, often come to the surface of the liver and sometimes anastomose. There is much difference of opinion regarding the statements given in the above sentence, but anyone who will take the trouble to make a few good celloidin corrosions will find them correct.
Branches of the fifth order of the portal vein are .15 mm. in diameter; those of the sixth order .05 mm. The interlobular veins are about half the diameter of the intralobular and twice as numerous. Even these often branch before they give rise to capillaries. While the hepatic vein receives capillaries down to veins marked S in fig. 32, the portal twigs of the same figure give off capillaries only at their extreme tips. Unless the extreme tips of the hepatic vein, fig. 32, 0, are taken as centers of the lobule and clusters of the tips of the portal vein, I, as centers of the portal units will these two correspond in number. The relative number is shown in fig. 38, which is from a free-hand model in clay from a celloidin corrosion. The protruding points, 0, mark the position of the tips of the central veins. The tracing, fig. 35, shows the same.
Fig. 37. Photograph of the terminal branch of the portal tree. X 2.
Not only are the centers of the portal unit marked by the tips of the 274 A Study of the Structural Unit of the Liver portal vein, but also by the tips of the hepatic artery, as well as those of the bile duct. Furthermore, it is probable that the lymphatics arise from the same point and that the nerves and connective tissue spread from it. From time to time I have found large groups of karyokinetie figures there, which, together with the pathological evidence, make this the regenerative or growing point of the liver. The portal unit is the true structural unit of the liver.
Fig. 38. Lateral view of a model of a group of lobules of the dog’s liver. The outside vessels are terminal portal branches. H, sublobular vein; 0, elevations in the lobule over the tip of the central veins within; 11, nodal points; 11, portal vein.
Growth of the Hepatic Lobule and on the Portal Unit
When I began the study of the development of the lobule of the liver twenty years ago, I thought that I had selected the simplest and most definite lobule for investigation. It seemed then easy to follow the lobule from stage to stage and thereby gain a comprehensive picture of the histogenesis of the liver. It has turned out, however, that most of the work—the hundreds of injections, experiments and sets of serial sections-has been in vain, and but the faintest sketch remains from which to picture. The great difliculty is to recognize the same things from step to step, and this is obtained with certainty only in very young livers, where all of the veins are of one order; the six central veins of the embryo become permanent main trunks in the adult. In development, the liver structure shifts distalwards, successively tearing off its capillary connections with the main veins, gradually rearranging the architecture of the lobules, often fracturing them and scattering them. Portions of each of the six primary lobules go to thousands of new lobules. None of the main trunks of a large liver, like that pictured in fig. 27, give off capillaries at birth. So we must conclude that in a child the liver structure is entirely rearranged each year which calls for a destruction and regeneration of at least a billion capillaries and towards puberty ten times this number.
In the estimation of the number of blood—vessels of the liver it has been found convenient to classify the branches of the vascular tree under six orders, designating the main trunks as the first order and those directly related to the lobule as the sixth order. It is seen by studying fig. 27, which is from an embryo of the eighth week, that but the first three orders are present with capillaries arising from all of them. By the time the liver is fully formed each of the third order, which are 700 in number, must give rise to an equal number of those of the sixth order, while the liver increases 7000 times in volume. It follows that the lobule must increase in volume as the liver grows, and, in fact, this is the case. When the lobules are well formed, as at the end of the second month, they are .5 mm. in diameter; at birth 1 mm., and in the adult 1.5 mm. Actual measurement also shows that -the weight of the liver at the end of two months is .2 gms. ; at birth, 75 gms. and in the adult 1500 gms.
As the lobules are shifting more distalwards, it is found that the capillaries have a greater and greater tendency to arise from a single order, so that when the liver is fully formed capillaries arise only from the sixth order of the branches of the portal vein, and from the fifth and sixth orders of the hepatic vein. It is evident then that the stretching of the liver is in all directions andthat there are additions to the tip of all of the blood-vessels, both within the liver and on the periphery. Not only is the liver tissue shifted more distalward, but the vessels already laid down are stretched, and from their trunks new vessels arise to supply new lobules which have arisen from fractured portions of adjoining lobules.
Toldt and Zuckerkandl[19] have advanced ideas similar to the ones I have formulated above. They state that the younger the liver the simpler is its vascular system, and that in the youngest stage the whole liver may be likened to a single lobule of the adult. In embryos four weeks old there is a distributing and collecting end to each of the lobules present and not until the eighth week is the adult form to be recognized in the structure of the liver. Furthermore, they state that in their growth the lobules split and give rise to numerous new lobules.
The process of expansion and rearrangement is expressed in the diagram, figs. 39 and 40, which may be viewed to represent either a portal fiGS. 39 and 40. Diagrammatic illustration of two stages of a growing hepatic vein. D, main vein; c, its branch; 1, 2, 3, 4, 5, the same vessels in both diagrams; a, a, new branches from the stem c; b, a new branch on the main stem, d in which the successive stages of the central vein are marked e, e’, and e”.
or an hepatic twig. As the vessels represented in fig. 39 grow, the liver tissue increases in quantity, but the liver lobules do not increase in size indefinitely, because Thoma’s first law is constantly at work and will soon break up the larger lobules into a number of smaller ones. In all cases the length of the capillaries remains constant, and when they appear to be too long and too numerous it is always found that some of them have already turned into small veins and thus mark the beginning of new lobules, or of new portal units. A more advanced step is represented in fig. 40. The lobules or units of the two stages are marked with corresponding numbers. But each of them is splitting at its end and new vessels, a, have also arisen from the main trunk. As there were no lobules at the points marked a before, the new ones must have been differentiated from adjoining lobules. The process by which this has taken place may be expressed by the twig b. At a much earlier stage all this tissue was arranged in a single lobule around the vessel (13. Then it spread over the branch 0, and finally as the vessel d elongated a new lobule, b, arose. Its central vein was marked successively by the vessels, 9, e’ and c".
It may be considered that the figs. 39 and 40 represent the process of growth of the liver in one dimension of space, which becomes much more complex when viewed in two dimensions, as is shown in figs. 41, 42 and 43. The single branch, at, fig. 41, becomes the main branch, (L, fig. 43.
Figs. 41, 42 and 43. Diagrams of three successive stages of the portal and hepatic veins in a growing liver. .1, hepatic side; d, portal side; 1), and c. successive stage of the hepatic vein; e and f, successive stages of the portal vein.
The successive orders of new branches, 1) and c, explain themselves. finally the lobule c’ is adjoined by two diiferent kinds of lobules, a‘ younger than itself, and In older. The portal vein has spread into this region, corresponding with the hepatic, and we see the branches reaching out in all directions, keeping equidistant from one another. This diagram is practically correct for most regions of the liver where there are a succession of portal and hepatic veins alternating, so that i.n the region, L, there is another vessel like a, and in the region R another like d. But when these vessels, a a.nd d, are the only ones that run out to the edge of the liver, the vessel e must cross a. into the field R, and the vessel b must 278 A Study of the Structural Unit of the Liver cross d into the field L. In fact, there is quite a regular crossing of portal and hepatic veins in many portions of the liver, and it indicates that at one time the main stems were the only vessels in that portion of the organ.
I shall not venture to describe the growth of the vascular system of the liver in three dimensions of space, but refer the reader who desires it to a good double (portal and hepatic) corrosion with the diagrams given in figs. 39 to 43. If he takes the pains to apply them to double corrosions of the livers of very young and of old animals, I think he Wlll find them of some aid. I wish, however, to add two points. first, if the vessel, b, fig. 40, is imagined cut transversely, as represented in fig. 1, it will be seen that the lobule represented by it must have arisen from three adjoining lobulcs. Secondly, if the tip of the lobules 1, 2, etc., are considered in three dimensions of space it will be found that the growing part of the lobule is always at a certain point, which is as far as possible from both portal and hepatic tips, and is marked n in figs. 1 and 38. In the diagrammatic section, fig. 1, it is seen that there is a special arrangement of the capillaries passing towards this point, and since it is so constant, can be located in any lobule and is of such great morphological significance I shall term it the nodal point of the lobule. The nodal points are always located in Kiernan’s interlobular fissures, but the fissures are not always nodal points. In fig. 38 a line drawn through the letters 0, n, e, n, c, n, 0 marks the middle of a Kiernan fissure, but only the points marked 11 are nodal points.
Meaning or the Nodal Points
In general, it is stated that the capillaries of the lobules of the liver radiate from the central vein and in so doing branch until the periphery of the lobule is reached where they communicate with the plexus of interlobular veins, as described by Kiernan. It was shown, however, by Krukenberg and others that the terminal portal twigs do not anastomose and this in itself indicates that the usual description of the capillaries of the lobule is incorrect, for there must be some kind of collecting system for that portion of the periphery of the lobule devoid of veins. Correct illustrations of the vascular arrangement of the lobule in cross section are given by Stohr and Bohm and von Davidoif without any description of them in the text.
A careful study of the vascular arrangement of the lobules will show that the capillaries themselves are the collecting vessels due to their own anastomosing system. A diagrammatic representation of this system in a cross section of a group of lobules is shown in fig. 1. It is here shown that the shortest course between the terminal portal and hepatic veins is taken by perfectly straight capillaries, and as the region away from the straight course is approached the general direction of the capillaries becomes more and more bent. It is thus seen that the deflected capillaries from several adjoining lobules come together, forming points which can easily be seen in the sections of any liver. In general the picture is sharper in the rabbit’s liver than in that in any other animal I have examined, especially when it is taken immediately under and parallel with
Fig. 44. Terminal distribution of a portal twig entering three portal units. X 85. The capillaries all arise from the tips of the vein.
Fig. 45. Terminal hepatic vein with the capillaries arising from it. X85.
the surface of the liver in order to strike the lobules at right angles. Thus we have in the liver lobule two sets of capillaries, long ones and short ones, while according to Thomafs first law they should all be of the same length. This stumbling block caused me a great deal of trouble, for at first I saw no way to overcome the difiiculty. An elaborate model of the vascular system of the lobule seemed to show that as much fluid, 280 A Study of the Structural Unit of the Liver or more, passed through the longer and less direct capillaries than through the shorter and direct ones. The capillaries passing through the nodal points, therefore, seem to be as well favored as those taking the shorter course. In the diagram it is seen that the nodal point is fed from three sources, and on account of the great number of capillaries in it the resistance to the circulation is probably diminished. It would follow that some of the main feeding capillaries should be converted into veins, and in growing livers this is the case, but every time a new vein is formed, we have a new vascular unit, or a new lobule with two new
Fig. 46. Arrangement of the capillaries at the nodal point of a lobule. X 85. P, portal vein; h, hepatic vein.
but smaller nodal points to complicate the situation again. This process might be continued until the portal branches communicated with the hepatic, were there not some self-regulating force to check it. It appears that this force is due to the normal length of the capillary which is about .4 mm. in the direct course. In the indirect course, 12. (2., through the nodal points, this distance is about .8 mm., but each nodal point is fed from three sides instead of from one, and this arrangement may account for this increase in distance without the formation of new veins.
If the hepatic and portal veins are injected with thick granular masses——cinnabar, lamp-black or ultra-marine blue suspended in gelatin— it will be found that in successful cases these terminal veins with the capillaries arising from them are filled. figures 44 and 45 show this distribution from the tips of the portal and hepatic veins, marking the centers of the portal unit and the hepatic lobules. Both are drawn to the same scale and show the relative arrangement of their capillaries. The portal twigs give rise to capillaries only at their ends, while in the hepatic the origin of the capillaries extends much farther down the vein than is shown in the drawing. The portal vein lies of course in an interlobular space and its three main branches extend into the adjoining fissures. Together they mark the center of a portal unit. figure 46 shows the arrangement of the capillaries throughout a nodal point. The 282 A Study of the Structural Unit of the Liver course of the capillaries is in a more direct line from the portal to the hepatic, from the portal to the portal and from the hepatic to the hepatic than in other directions.
Fig. 47. Tracing of the vessels around a lobule showing the relative number of terminal and hepatic and portal branches. X 40. Portal vein slightly injected.
Fig. 48. Tracing of the terminal hepatic _and portal veins with the nodal points marked n. X 40. Hepatic veins slightly injected.
figures 47 and 48 show the first spreading of granular injections and their relation to the lobule. It is seen in both cases that the most favored vessels point toward the nodal points, 1'. e., these capillaries are a little larger than the rest of the capillaries of the lobule; they have grown sufficiently to favor the capillaries of the nodal point. figure 49 is from a more extensive injection of the hepatic vein with lamp-black gelatin. It is seen that the injected area, which is sharp, is angular in shape, with projections directed towards the nodal points. We have here again the well-known picture of the beginning of passive congestion, which incidentally marks the portal units beautifully.
Enough has been said to indicate that the growth of the lobule takes place at the nodal point. That does not mean that the cells multiply at this point, but simply that the new vessels, alternately portal and hepatic grow toward this point and break it into fragments to form new nodal points. In order to test this question a dozen sets of sections were made of the livers of growing rabbits and foetal pigs with more or less satisfactory results. The rabbit which is a favorable object for this kind of study has a lobule of constant size (.6 mm.) from birth, until it is fully grown. In the pig the lobule measures .8 mm. in embryos 4 cm. long until a number of months after birth; in the adult they are 1.4 mm. in diameter. 284: A Study of the Structural Unit of the Liver In the set of rabbits’ livers, hardened in a variety of ways, it was found that whenever the distance between two adjoining portal veins is considerably greater than the avcrage diameter of a lobnle, a small portal vein grows into the nodal point which separates them. The same is true regarding the hepatic veins as shown in fig. 50. From all appearances, the hepatic branch, 4, is a recent one, growing into the large nodal point which had pushed apart the hepatic veins 1 and 5. No doubt earlier in its development, the whole field of this figure formed one nodal point, and ii 3i 3/
Fig. 50. Section of the liver of a rabbit one day old. X 85. Hardened in fle1nming’s solution. P, portal branch; h, hepatic branch; n, nodal point; 1, 2, 3, 4, order of growth of the vessels; the next. vessel will appear at 5, and then it will grow towards the nodal point 6.
Fig. 49. Tracing to show the extent of a granular injection of the hepatic vein. X 40. The portal vein was first injected with blue gelatin and the thick granular lamp black gelatin was forced into the hepatic vein. The portal units are outlined as in chronic passive congestion.
I then the hepatic vein, I, grew into it. This was followed by portal veins, 9, then 3, then by hepatic vein 1;. At a later stage the portal vein, 2, will send a branch into nodalpoint 6, and so on. In a measure, we can corner a bit of liver tissue at the junction of two main stems, as shown in figs. 51 and 52. It is fair to assume that the tissue at this angle grows with the rest of the liver, for a time at least, and that the small vessel arising from the portal in fig. 51 and that from the hepatic in fig. 52 are new vessels growing into the nodal point, n. But the growth of the liver lobules can never be proved by the study of any section or specimen, for at all times there is an equilibrium; the cells do not multiply to be followed by the growth of veins, but they go forward side by side. All grades of veins can be seen in any sections of livers, young or old, and 286 A Study of the Structural Unit of the Liver the imagination of the investigator can patch them together, correctly or not as the case may be. But the final evidence is obtained by studying serial sections of early livers, where the same vessel can be followed from stage to stage.
Fig. 51. Section of a rabbit’s liver eleven weeks old to show the origin of a new branch from a portal trunk growing towards a. nodal point. X 40.
Fig. 52. The same as fig. 51 to show the origin of a. hepatic branch. X 40.
The very first vessels that grow into the nodal points in the human embryo are shown in fig. 25 from a specimen of the end of the fifth week. The vena cava and the vena hepatic media on the hepatic side and the ramus arcuatus, the ramus descendens and the ramus angularis from the portal side are new vessels. All of the branches of the main trunks with the branches of the second order are shown in the reconstruction, fig. 28, and in the section, fig. 27. Here the new branches of the hepatic alternate with those of the portal, each newly-formed field, as it is formed, receiving its proper branch in the next stage. The best example is the vena hepatica media in an early stage and the rest of the main trunks and their branches as they arise in order.
There are as many nodal points as there are portal units, and more than there are lobules, unless the complex lobule is cut into blocks. The arrangement is shown in fig. 38, which shows one lobule with six terminal portal veins encircling it. If it is considered that these veins are related to adjoining lobules, an estimation of two terminal portal veins to one hepatic, as I have it in my table, is not far from being the correct number. The reconstruction shows that terminal portal twigs run parallel, recur again, and run at right angles with the intralobular vein. If the portal units are imagined around the interlobular veins of fig. 38, it is seen that both apex and base of the same lobule may represent the distal ends of different units. Adjacent intralobular veins and nodal points mark the outline of the portal units.
Relation of the Hepatic Artery to the Portal Units
There always has been and still is much confusion regarding the distribution of the hepatic artery and the reason for this is very evident when it is considered that it communicates at its end directly, and possibly indirectly also, with the capillaries of the lobule. To test this question thoroughly, I have made numerous single, double and triple injections with granular and fluid substances in order to determine the courses the arterial blood may take during life.
Long ago Ferrein stated that there were two kinds of branches of the portal vein within the liver, one which conveyed blood to the lobule and the other which collected blood from the capillaries of the hepatic artery. That the collecting veins of the gall bladder and surrounding connective tissue empty into the vena portal is easily proved by a simple injection of a granular mass into this vessel. But the conclusion that all, or even a great portion, of the blood from the hepatic artery is collected by similar, but smaller, veins which enter the branches of the vena portae within the substance of the liver is a doubtful one, in my opinion. Theile, who made a careful study of these vessels, called them the rami vasculares (a term generally ascribed to Kolliker) and brought forth very meagre evidence that they are present in great number. In fact, his best proof is the observation occasionally of a vein which arises in the hilus of the liver and enters the quadrate lobe before it communicates with a branch of the portal vein.” Theile believes that the rami vasculares venosi enter very small portal branches because he was never able to see them opening into the larger branches after they had been cut open. The presence of these branches, he claims, explains why an injection into the hepatic artery enters the portal vein and why an injection into the portal vein enters the artery as well as the hepatic vein.
My own injections speak decidedly against internal roots to the portal vein, except those that arise from the gall bladder and that neighborhood, and can be recognized with the naked eye. After the arterial branches once enter the substance of the liver all of their twigs, including those of the capsule, communicate directly into the capillaries of the lobule, and from there are collected into the hepatic vein. It is usually stated that the portal branches are distributed under the capsule and collect the blood of that region, but this is incorrect. If a liver is injected with two granular masses of different colors, one into the hepatic vein and the other into the portal, it will be found that in all cases it is always the branches of the hepatic vein which come to the surface of the liver and spread out between the meshes of the arterial plexus.
From our present knowledge of the vascular system of the lobules We can easily understand how these three sets of vessels communicate freely at this point. In order to test this question in another way, I injected whipped blood i.nto the artery of a fresh liver and found that three-fourths of it came out of the portal vein and one-fourth out of the hepatic vein. In another experiment the arterial pressure was kept constantly at 100 mm. Hg. with the same result as above. Then upright glass tubes were inserted into the cannula connected with the veins, with the following result: Blood rose in the tube in the Time Portal vein Hepatic Vein 1 minute. 5 cent. .0 cent. 2 “ 9 “ 6.0 “ 3 “ 11 “ 8.5 “ 4 “ 12 “ 10.5 “ 5 “ 12.5 “ 11.5 “ Further experiments show that at a given pressure it takes about as long for a liter of whipped blood to flow from the portal vein to the hepatic vein as in the opposite direction. Together these tests show that the artery communicates more freely with the portal vein than with the hepatic, apparently speaking in favor of Ferrein’s venous rootlets to the portal vein. If it is considered that under normal conditions there is a high blood-pressure in the portal vein, higher than in any other vein, it is not remarkable that an injection into the artery should flow more freely from the portal vein than from the hepatic vein.
" Theile, Handwbrterbuch d. Physiologie, II, 1844, p. 342.
inserted into the cannulute. 5 cent. .0 cent. 2 “ 9 “ 6.0 “ 3 “ 11 “ 8.5 “ 4 “ 12 “ 10.5 “ 5 “ 12.5 “ 11.5 “ Further experiments show that at a given pressure it takes about as long for a liter of whipped blood to flow from the portal vein to the hepatic vein as in the opposite direction. Together these tests show that the artery communicates more freely with the portal vein than with the hepatic, apparently speaking in favor of Ferrein’s venous rootlets to the portal vein. If it is considered that under normal conditions there is a high blood-pressure in the portal vein, higher than in any other vein, it is not remarkable that an injection into the artery should flow more freely from the portal vein than from the hepatic vein.
There seems to be no definite way to settle this question, except by making sections of injected specimens. If a cannula is tied into the hepatic vein and a single spurt of an aqueous solution of Prussian blue is made into it, it is found that numerous minute blue spots appear below the peritoneal surface of the liver. Sections of such specimens show that all of the fluid has entered the substance of the liver at the centers of portal units. If the injection is pushed a little :Earther—until the lobules are outlined—a large portion of the blue enters the terminal portal veins from the common capillary plexus of the lobule. As soon as this has taken place the fluid backs into the larger veins, forms secondary injections of other units and the picture becomes confused. In order to obviate this, I injected the artery with Prussian blue gelatin in which were suspended a large number of granules of cinnabar. The blue again flowed over into the portal and the hepatic veins, but the granules all lodged in the capillaries in the periphery of the lobule. Very few granules were found in any of the terminal portal twigs. This experiment shows at least that the bulk of the granules reach the capillaries of the lobule without passing through the portal vein. But an insignificant number of red granules are found lodged in the capillaries Within the capsule of Glisson, and these encircle the bile ducts.
The first experiment, with but a. spurt of Prussian blue into the artery, shows that the long delicate arteries give rise to capillaries which form a plexus around the bile ducts and then enter the center of the portal units together with a terminal portal twig. Now these arterial capillaries communicate with the capillaries of the lobule, as do all of those that encircle the bile ducts. At no point does any of the injected mass enter the portal branches within the liver unless it is as a backward injection, in a direction the blood does not circulate normally. To test this question thoroughly the whole portal tree was first plugged with a thick granular injection mass made by mixing lamp-black or baryta with gelatin. The venous tree thus plugged did not cut off the capillaries, but prevented a second injection into the artery from spreading in the vein. In general it was found that baryta gelatin for the portal vein and aqueous Prussian blue for the artery gave the most satisfactory result. All the vessels and capillaries injected with either fluid are always sharply defined. In such an experiment it is possible to force the blue fluid through all of the capillaries arising from the artery and in case they collect within the capsule of Glisson which empty into the portal vein, their beginnings should be marked. But at no place were such veins found; the blue had only percolated through the white mass in the interlobular veins. The capillary plexus around the bile ducts communicated directly with the capillary plexus of the lobule. This condition seemed to be true down to the largest bile duct. At no time did I find a collecting vein within the substance of the liver, and I must, therefore, declare the rami vasculares venosi as mythical.
Fig. 53. Picture to show the termination of the hepatic artery. X 40. P, portal vein; h, hepatic artery. The portal vein was first plugged with a granular mass and then an aqueous solution of Prussian blue was injected into the artery. The extent of the capillary injection with the blue is shown.
It is evident by studying good injections of the artery after the portal vein has been plugged that the artery communicates with the lobule throughout the extent of the vessels of the sixth order and possibly in part with those of the fifth order (fig. 53). All the capillaries in the capsule of Glisson in this region communicate only with those of the lobule and they do not communicate at all with the portal vein. In those regions of the liver immediately around portal veins of the fourth order it is always found that in their immediate neighborhood there are both arteries and veins of the sixth order which ramify again, as stated above. So down to and including vessels of the fourth order all of the capillaries of the artery communicate directly with the capillaries of the lobule. By consulting the table giving the number of vessels of the various orders it is seen that this observation excludes the possibility of any veins of Ferrein arising from over a million terminal arteries and leaves them to arise from the walls of the main trunks of the first three orders; probably there are no more veins of Ferrein that empty into the vena portae than can be seen with the naked eye.
The hepatic artery then supplies the gall bladder and the hilum of the liver, and the veins from this region communicate with the portal vein. The branches of the artery then enter the lobes of the liver with the branches of the portal vein and the bile ducts. Here the artery gives off a few branches to the bile ducts which form a capillary plexus around them, after which it communicates with the capillary plexus of the lobule. By far the greater number of arteries enter the centers of the portal units and communicate at once with the capillaries; they supply the periphery of the lobules. There are about a million of these very small terminal arteries, one for each portal unit which then spread out toward the nodal points and the hepatic veins. The branches that spread over the capsule of the liver supply the subcapsular portal units and then communicate with the hepatic veins. Some of the hepatic veins perforate the subperitoneal lobules as described by Kiernan; none of the portal twigs reach the surface. The great bulk of the arterial blood is equally distributed from the centers of the portal units and is fully mixed with portal blood before it reaches the nodal points or the hepatic veins.
Relation of the Connective Tissue to the Structural Unit
From the very earliest appearance of the liver, from the time the sprouts of epithelial cells invade the omphalo-mesenteric vein and break it into sinusoids, it is extremely difficult to demonstrate connective tissue cells within the liver substance. In all cases the endothelial cells lining the capillaries of the lobule come in apposition with the liver cells, and there are no nuclei between them. The cells which have been described as connective tissue cells have been abundantly proved to be von Kupffer’s stellate cells, and these are, according to Von Kupffer’s last paper, the endothelial cells of the capillaries.[20] It is impossible to demonstrate sharp outlines to the lining cells of the capillaries of the liver lobule with nitrate of silver. Successful injections show the markings in the portal vein until it reaches the lobule and there they stop. From now on the endothelial cells form an extensive syncytium with large openings through which the blood plasma comes in direct contact with the liver cells. However, there is a framework of fibers which has been described from time to time during the last fifty years as delicate, naked fibers which encircle the capillaries as they pass through the lobule.[21] This adventitia capillaris of His can be demonstrated by brushing fresh sections, but clear pictures were not obtained until special methods were invented for this purpose by Oppel[22] and by myself.[23] Oppel isolated the net—work by his special precipitation method which showed the thickness of the fibrils and their relation to the surrounding tissue. By my method all the cells were destroyed by digesting fresh sections in pancreatin, leaving only the fibrils which for special reasons were classed with the reticulum fibrils of the lymph node as well as those of other organs.[24] It is now generally admitted that the Gitterfasem and my reticulum are identical and that they form the framework of the lobule.[25] That they are the same is shown by digesting a section upon the glass slide by the method of Spalteholz when pictures of reticulum identical in form and arrangement with the Gitterfasem are obtained. Upon the network of fibrils of reticulum which encircle the capillaries the syncytium of endothelium lies. We have, therefore, but three elements within the liver lobule, liver cells, a syncytium of endothelial cells, and a network of reticulum between them.
I have been unable to find any of the fibrils of the reticulum of the liver in embryo pigs less than 2 cm. long. If frozen sections are made of fresh livers at this stage it will be found that they are very delicate and can be crushed under the coverglass very easily. When such preparations are stained by allowing a solution of magenta to run under the coverglass, it is seen that a network of stained fibrils lies between clumps of liver cells. In any such sections it is easy to determine that all of the fibrils of the young reticulum surround the capillaries and are in intimate connection with Kupifer’s endothelial cells. The fibrils, or rather the syncytium is delicate, and can be broken easily by slight pressure upon the coverglass. Frozen sections are easily broken into granules by shaking them slightly in water, showing that the reticulum is not strong. When digested for a short time, at room temperature the liver cells disintegrate, leaving only the delicate syncytium to which many small granules adhere. Pressure upon the cover glass shows that the reticulum is very elastic. Acetic acid does not cause it to swell and become transparent.
It is not difficult to obtain fresh specimens with all of the capillaries surrounded with this delicate reticulum with the endothelial nuclei imbedded in it. The continuity of the endothelial cells with the embryonic reticulum is complete, and thus forces us to the conclusion that the fibrils are developed from the endothelial cells in the same manner as I have shown that they are developed from the connective—tissue syncytium elsewhere.
This observation, however, is entirely out of harmony with the development of connective tissues in general, for they arise from mesenchyme, while the reticulum of the liver arises from the angioblast. However, the reticulum fibrils are in no way connected with the liver cells and there is no third group of cells in this neighborhood. It is possible that these fibrils reach into the lobule from distant interlobular spaces where connective tissue cells may be found.
If the liver of a dog is carefully crushed with the fingers in a stream of water all of the lobules are gradually destroyed and the portal and hepatic trees may be separated. At the end of the tips of the portal tree there are small enlargements which correspond with the portal units; no corresponding lobules are found to adhere to the tips of the hepatic veins. The stronger tissue — the capsule of Glisson — is thus isolated, while the framework of the lobule is destroyed. Sections of the tips of the two systems of vessels show that more connective tissue extends to the periphery of the lobule along the portal vein than along the hepatic vein. However, in nearly all animals it is not the entire lobule which is surrounded by an increased amount of connective tissue, but only that which marks the center of the portal unit.
From the study of the connective tissue of the liver by various methods, it is found that it is impossible to draw a line of separation between the interlobular and the intralobular tissues. One appears to be continued into the other. However, there are stronger bundles which take a direct course toward the hepatic vein, not always following the capillaries. These are the so-called radial fibers. More delicate fibrils communicate with them and form a dense network around the capillaries. In general the fibrils radiate from the centers of the portal unit towards the nodal points as well as towards the terminal hepatic veins. They are of course arranged as are the capillary blood-vessels, which were discussed above.
While the liver is growing it is evident that with the destruction and transformation of the lobules and portal units the reticulum must be constantly tearing and shifting. This is possible with such a delicate embryonic tissue, and it may be that the connective tissue around the portal vein in a given stage lay within a lobule in an earlier stage. Veins of the third order are terminal in embryos about 2 cm. long, i. 43., they are interlobular and intralobular, while later on these same veins become main trunks. In the pig’s liver there is no indication of any connective tissue capsule of the lobule before birth, and it probably does not appear until the liver is fully formed. Sections of the liver of pigs two months old how the lobule outlined by marked bands of cell radiating from the centers of the portal units to the centers of the nodal points. Possibly at this time the reticulum between the lobules is a little denser than that within the lobule. However this may be, the capsule of Glisson is but slightly marked, having within it but few nuclei of connective tissue cells. It is evident that the connective tissue of the liver must be studied anew from the standpoint of an ever-changing lobule during development.
The Lymphatic Ducts
Hand in hand with the development of the capsule of Glisson, the lymphatic ducts grow into the liver. According to Professor Sabin, they arise from the receptaculum in embryo pigs and grow along the trunks of the artery and portal vein. How rapidly they grow and function has not yet been determined, but it is certain that in the cat and the dog they do not extend beyond the center of the portal unit. I have been able to trace them in numerous specimens beyond portal vessels .06 mm. in diameter, and this naturally puts them into the center of the unit. One great obstacle in the way of studying the finer lymphatics is that the amount of connective tissue and the size of the portal vein seem to vary in inverse ratio, and when the veins of the sixth order are distended to their maximum the surrounding connective tissue is compressed, and consequently the lymph ducts are completely obliterated. It is not easy, therefore, to obtain clear pictures of the lymphatics from their very beginning down to the main trunks.
The origin of the lymphatics of the liver was first definitely determined by MacGillavry,” who studied this subject under the direction of Ludwig. Long before the work of MacGillavry it had been observed that ligature of the bile duct was followed by passage of bile over into the lymphatics, and the artificial filling of the lymphatics naturally followed, by injecting a colored fluid into the bile duct. Sections of liver, in which the lymphatics had been filled with Prussian blue, or with asphalt, sh owed that the fluid injected into the bile ducts leaves them at the periphery of the lobule to enter spaces surrounding the blood capillaries, the so—called perivascular lymph spaces. These spaces communicate at the periphery of the lobule, that is, in the center of the portal unit, directly with the interlobular lymph channels. Frequently there is an extravasation of the injection mass into the blood capillaries of the lobule.
These observations were subsequently confirmed by numerous competent investigators, using the method employed by MacGillavry as well as that of direct injection of Prussian blue into the walls of the portal and hepatic veins. In successful injections made in this way it is found that the Prussian blue injected enters the center of the portal unit and from there radiates and encircles its blood capillaries.” Such injections, however, are always accompanied with numerous extravasations of the injected material into the surrounding tissues, and often there is a secondary injection into the blood capillaries. This fact has raised an objection to the direct injection of the lymphatics from the bile capillaries. It appear more probable, the opponents say, that the extravasation of bile, or the injected material into the center of the portal unit enters the lymphatic radicals of the capsule of Glisson, and from them the larger lymph channels and the perivascular spaces of the capillaries are filled. Furthermore the injected mass may pass from the pericapillary spaces directly into the capillaries, thus accounting for their frequent injection.
"MacGillavry, Wiener Sitzungsben, 1864.
‘° Budge, Ludwig’s Arbeiten, 1875.
According to fleischl,“ all the bile is taken up by the lymphatics after ligature of the bile duct, and in case the thoracic duct is also ligated no bile or only a trace of bile ever reaches the blood. The observation of fleischl has been confirmed by Kunkel,“ Kufferath “’ and Harley.“ It is extremely difficult to understand why the bile does not enter the blood capillaries in case it passes from the bile capillaries over into the perivascular spaces before it reaches the interlobular spaces after ligature of the bile duct. A further objection to the idea that the perivascular spaces first take up the bile, after ligature of the duct, is the fact that fluids injected into the bile duct pass with ease over into the lymphatics but only with difficulty into the bile capillaries. In all cases it appears as if the main origin of the lymphatics is at the center of the portal unit and that the radicals communicate freely with the perivascular lymph spaces. Furthermore, it appears that the course the bile takes after ligature of the bile duct, or of a fluid injected into the bile duct in passing to the lymphatics, is well within the center of the portal unit and not within the lobule. This idea is greatly strengthened since we know that the Walls of the capillaries of the lobule are extremely porous, being composed of a dense basketlike layer of reticulum fibrils ‘” upon which lie the endothelial or Kupl?cr’s syncytial cells. This layer of reticulum fibrils encircling each capillary has been isolated by Oppel “ and by myself " and is sufliciently described above. The capillary walls then are very pcrvious, blood plasma passing easily from them out into the perivascular spaces to bathe the liver cells.
It is well known that a large quantity of lymph is constantly passing from the liver, much more than from any other organ. That this lymph comes directly from the blood is indicated by its high per cent of proteid matter, nearly equal to that of the blood, and from two to three times that of the lymph from other parts of the body.
'1 fleischl, Ludwig’s Arbeiten, 1874.
“Kunkel, Ludwig’s Arbeiten, 1875.
“Kufferath, Arch. fiir Physlo1., 1880.
“Harley, Archiv fiir Physiol., 1893.
”Kupffer, Arch. 1?. Mik. Anat., 54.
"Oppel, Arch. Anz., 1890.
" Mall, Abhandl. d. K. S. Ges. d. Wiss., XVII, 1891. See also Johns Hopkins Hospital Bulletin, XII, 1901. 296
The course the lymph takes from the blood capillaries to the lymph radicals, i. e., its natural course, can easily be marked by injecting colored gelatin into any of the blood-vessels of the liver. I have usually found it most convenient to inject the gelatin into the portal vein, but it is just as easy to fill the lymphatics by injecting either the hepatic artery or hepatic vein. In all cases the colored fluid reaches the main lymph channels in the same way. The colored gelatin flows with great ease from the capillaries at the center of the portal units as well as from those around the smaller hepatic veins into the lymphatic-s. After the lymphatics have all been filled it is well to inject a small quantity of fluid of different color into the blood-vessels. A much better method of making double injections is to mix red granules with a glue gelatin or blue granules with a red gelatin, the fenestrated lining membrane of the capillary acting as a sieve which allows the fluid to pass but holds back the granules, as is the case with the blood corpuscles and plasma in life.
Fig. 54. Section through the center of a portal unit of a cat. X 500. Stained by Van Gieson’s method. The hepatic artery was injected with cinnabar gelatin, and the portal vein with Prussian-blue gelatin. L, lobule of liver; c, capillaries; a, artery; 1, lymph vessel; plv, persivacular lymph space; pll, perilobular lymph space; w, bundles of white fibrous tissue between which are loose connective tissue fibrils and cells.
When the portal vein is injected with Prussian-blue gelatin at a low pressure, it is found that in a few minutes the lymphatics are all filled with the blue mass. Livers injected in this way are best hardened in formalin and then cut by the freezing method, for alcohol causes the gelatin to shrink. Such sections show that the blue fluid has entered the lymphatics at the center of the portal unit. The specimens are more instructive when the injection is stopped just as the first lymphatics are filling with the colored gelatin. By following the larger portal veins and lymphatics back into the liver substance it is found that the interlobular connective tissue is entirely filled with blue where the lymphatics are injected, but only partly colored blue when they are not. In other words, the blue extravasates from the capillaries at the center of the portal unit and invades the connective tissue to reach the beginning of the lymphatics, when of course it is carried rapidly from the liver. The nearest course from the capillaries to the lymphatics is at the center of the portal unit where the amount of connective tissue is small, for as colored fluid begins to enter lymph channels only the tips of the capsule of Glisson are entirely tinged, While the larger portal spaces are encircled by a zone of color. Furthermore, it is found that in certain instances, where the injection was too brief, that the blue did not enter the lymphatics at all. In such specimens, all of the interlobular spaces are surrounded by a zone of colored gelatin which does not enter the main lymph channels.
A successful injection of the lymphatics i illustrated in fig. 54. The granular blue enters the capillaries of the lobule, c, with ease, and from them the liquid blue is filtered through the capillary walls to enter the perivascular lymph space, pvl. This space communicates at the center of the portal unit directly with a large lymph space between the liver cells and the capsule of Glison, which may be called the perilobular lymph space. These spaces, pll, in turn communicate with the lymph radicals.
It is further shown by injecting the liver with aqueous Prussian blue that there are no capillaries between the periphery of the lobule and the interlobular connective tissue. The liver cells come in contact with the capsule of Glisson. An injection of brief duration with blue gelatin soon fills the perilobular lymph spaces, so that it appears as if all groups of liver cells at the periphery of the lobule were separated from the interlobular connective tissue with capillaries. In case cinnabar granules are mixed with the blue a few of these granules are found in the perivascular and perilobular lymph spaces, the openings in the walls of the capillaries being large enough to allow a few of the smaller granules to escape. As the injection is extended the blue invades the connective tissue spaces from the lymphatic radicals more and more until a lymph channel is reached, when of course it rapidly fills all of the larger ducts. Were there a direct channel from the perilobular lymph spaces the blue would flow through it at once without further filtration through the interlobular connective tissue spaces. The course the cinnabar granules take also speaks against a direct channel between the perilobular lymph spaces and the interlobular lymph channels. A few of the granules enter the perilobular lymph spaces, but none of them reach the main lymph channels. All of my specimens Without exception force me to the conclusion that there are no direct channels connecting the perivascular and perilobular lymph spaces with the lymphatics proper other than the ordinary spaces between the connective—tissue fibrils of the capsule of Glisson. These spaces, however, are relatively large, permitting of a rapid transfusion through them.
Injections with a hypodermic syringe into the walls of the smaller portal veins naturally fill the surrounding lymphatic vessels, and when no valves are in the way the injected fluid passes to the origin of the vessels, or lacunae, which are located in the center of the portal units. From here the fluid passes through the main connective-tissue spaces into the perilobular and perivascular lymph spaces, and frequently from them into the blood capillaries. When the injection of the lymphatics is made through the bile ducts I have always found that there is an extravasation at the center of the portal unit, although the bile capillaries are often injected to the nodal points. The extravasation does not take place from the bile capillaries, but only from the duct as it communicates with the capillaries as well as from the larger bile ducts. Such extravasations naturally are then taken up by the lymphatics and carried from the liver. If after ligature of the bile duct the bile enters the perivascular lymph space within the lobule it may still be carried to the lymphatics, as the direction of the current of lymph is constantly from the blood capillaries to the lymphatics.
That the blood capillaries of the liver communicate more freely with the lymphatics than do the bile ducts is proved by injecting the bile duct and the portal vein with fluids of different color under the same pressure at the same time. In all the experiments I made the fluid injected into the vein appeared in the lymphatics first. In many instances beautiful injections of the lymphatics were obtained from the vein while the fluid injected into the bile duct did not extravasate at all, showing at least that the veins communicate with the lymphatics much more freely than do the bile ducts.
It is seen from the above description that the lymphatics of the liver do not drain all portions of the liver lobule, but only those portions that are formed by the centers of the portal units. There are no lymphatics at the center of the lobules nor at the nodal points. At the center of the portal units there is a very free communication between the blood capillaries and the lymph radicals in the tips of the capsule of Glisson. So the lymph circulation is marked at this point, 12. e., the center of the portal structural unit, while in the rest of the unit it is insignificant or wanting altogether.
Relation on the Bile Ducts to the Structural Units
All of our knowledge of the growth of the liver lobule indicates that the multiplication of the cells is in those portions of its periphery which mark the centers of the portal units. However, this statement is extremely difficult to prove. It is probable that the bile ducts communicate with the capillaries of the lobule throughout the whole length of the ducts of the sixth order, much as is the relation of the hepatic artery with the blood capillaries of the lobule, and unlike that of the portal vein, which gives rise to capillaries only at its tip.
Much has been done to gain a clear understanding of the development, growth and regeneration of the liver cells, but the results are very meager, for only in rare instances are karyokinetic figures found in them. Frequently, however, cells with two nuclei are found and these appear to be scattered quite evenly throughout the lobule. I have studied many sets of serial sections of livers in all stages of development and have nearly always failed to find karyokinetic figures. In the few specimens in which cell divisions were present. they were in groups of several hundred around the terminal bile ducts. MacCallum “ has also found a specimen in which there were numerous karyokinetic figures at the periphery of the lobule together with indications that the cells are being destroyed around the central vein. Ponfick" has shown that such figures are very numerous in the early stages of regeneration after removal of a large portion of the liver. In his specimens the dividing cells were found distributed evenly throughout the lobule which, on account of its growth, has become much enlarged with a disarrangement of the radiating strands of cells. Recently Schaper” has discussed the question from a broad scientific standpoint and concludes that when the regeneration of the liver tissue forms typical lobules, the growth has taken place entirely within the minuter bile ducts. This conclusion is admitted by MacCallum “ for only those cases in which
“Maccallum, W. G., Jour. Amer. Med. Assoc., 1904.
“Ponflck, Vir. Arch., CXXVIII, Supplement, Heft., 1895.
“Schaper, Arch. f. Entwlcklungsmechanik, XIX, 1905.
“Maccallum, Johns Hopkins Hospital Reports, X. 300
the liver cells have all been destroyed; then the epithelial cells of the gall ducts take upon themselves the more complicated process of regeneration of liver tissue. At any rate, it is now well known that liver cells often contain more than one nucleus and that in a variety of pathological disturbances as well as in normal development the bile ducts have a tremendous power of growth. The aberrant bile ducts have been known since the time of Ferrein, and probably represent liver tissue Which was present and active at some earlier stage of development.” It has been proved quite conclusively by Toldt and Zuckerkandl in their excellent study on the growth of the liver that degeneration takes place in one part of the organ while itis growing large in another portion. The vasa aberrantia mark those portions which have degenerated as along the left lateral ligament and the region of the vena cava and the gall bladder. For example, the gall bladder in its growth encroaches upon the substance of the liver and causes its atrophy. The liver lobule in degenerating is often reduced to small islands which have the portal vein on one side of them and the hepatic on the other, returning to its early embryonic state. It is probable that a similar but diifuse degeneration is taking place in many portions of the growing liver, for vessels which are of equal size in a given step are often found very unequal subsequently. It is also known that pressure by foreign bodies, by exostoses and by the ribs in excessive lacing may produce atrophy of the liver which is always marked by aberrant bile duets, with hypertrophy elsewhere in the organ.
The striking experiments of Ponfick first showed us to what extent the liver may regenerate. His valuable communication also illustrates most beautifully that liver lobules do not hypertrophy, but sprout and give rise to newlobules, a conclusion which he thinks he disproves. Ponfick finds that after a portion of the liver has been removed the lobules in the remaining portion coalesce, and are not sharply defined as they should be in their hypertrophy (p. 86). He repeatedly states that it is difficult to find enlarged lobules, but in their stead he finds heart-shaped or clover-leaf-shaped lobules (pp. 104, 107), exactly what is to be expected in a growing liver. However, he does state that when the liver hypertrophies evenly in all directions the circumferences of the lobules are increased, two, three, or even four, times. This statement he illustrates with a figure of a lobule (fig. 2) which is compound and on account of the large veins in it must be from its base, a condition which may be found in any liver which is not growing. His figure 7 which is enlarged to the same scale as figs. 1 and 2, and fig. 3, which is on a larger scale, are not given to show that the lobules hypertrophy when the liver regenerates and are therefore found to be about of normal size. It is proved by Ponfick’s experiments, it seems to me, that in regeneration of fiG. 56. fiG. 57.
"Toldt and Zuckerkandl, Sitzungsber. d. Wiener Akademle, LXXII, 1876.
Figs. 55, 56 and 57. Three views of a. model or the liver of a human embryo, 171A; mm. long (No. 9). X 16. Gb, gall bladder; wv. umbilical veins.
the liver, the lobules do not enlarge, but sprout and give rise to new lobules, as is the case in the growing liver.
By comparing the livers of three embryos (figs. 29-31 and 55-60) it is seen that only their upper surfaces are regular in form from stage to stage; the processes extending into the abdominal cavity are irregular, to fit into the spaces that there are for them to grow into. Thus, in its fiG. 59. Fm. 60.
Figs. 58, 59 and 60. Three views of a model of the liver of a human embryo, 24 mm. long (No. 10). X 8.
growth the liver may atrophy at one portion and expand in another, the aberrant bile ducts marking those portions of the liver which have been shifted ; they are present in those portions of the liver which had to make Way for encroaching organs. Not only ‘must large masses of the liver disappear entirely, but also smaller areas throughout the liver, especially along the trunks of the main vessels, as the liver is growing from its center towards its periphery. Hand in hand with this change, the plexus of bile ducts which surrounds the main trunks of the portal vein shifts towards the periphery, leaving only a single vessel in its place, which, of necessity, becomes very variable, as has been pointed out by Rex.
In a liver which has been Well formed, as in the rabbit’s liver shortly after birth, the cells radiate from the terminal bile ducts towards the nodal points and the central veins, as indicated by the lines in fig. 1. The point of juncture between the bile ducts and liver cells is not sharp and the younger the liver the more difficult it is to determine it. In fact, in young embryos it is extremely difficult to follow the bile ducts into the structural units, 12. e., to the lobules. Injections show that the younger the specimen the more extensive is the plexus of bile ducts around the terminal veins, which indicates that an intermediate tissue, neither true lobule tissue nor true bile ducts encircle the terminal portal veins in growing livers, as is shown beautifully in a pig two months after birth. When the liver is finished, this tissue is reduced to a minimum. When the liver begins to regenerate, it becomes conspicuous again as “ newlyformed bile ducts.” With a plexus of bile ducts encircling a portal vein of the sixth order throughout its whole extent we have the most intimate connection between the bile ducts and the center of the portal unit, from which additions can be added to the unit. As the cells are added they seem to pile up in the nodal points, for the distance between the terminal portal and hepatic veins does not increase but remains constant. Hand in hand with the growth of the nodal points the capillaries follow, and on account of their increased number the resistance to the circulation through them is diminished, and, according to Thoma’s first law, veins from both sides are extended into them ; these alternate, as the observations above described have demonstrated.
To obtain an additional key by which we may unravel the growth and architecture of the liver units numerous tests have been made in the Anatomical Laboratory of the Johns Hopkins University, by Hendrickson, Sudler, Johnson, Sabin, Hill, and myself, with more or less satisfactory results. At present we are able to follow in a connected way the formation of the bile ducts and capillaries in embryos from 5 cm. long upward. Possibly at some later date they may be followed back to their earliest appearance. Furthermore, it is probable that some simple methods will soon be found, by which the history of the blood-vessels can be determined much better than it is given now, and since it appears that the development of the artery and bile ducts are parallel the study of the one will help to clear up the other.
Figs. 61, 62 and 63. Golgi specimens of the livers of human foexuses. 5 cm. and 10 cm. long, and at term. X 53. After Hendrickson.
Bile capillaries and ducts can be outlined beautifully by Golgi’s method in human embryos 5 cm. long," or longer, as figs. 61 to 63 show. But it is difficult to interpret these specimens, for it is not easy to determine which vessel is a portal vein, unless reconstructions are made, which is often out of the question. In the earliest stage, fig. 61, the capillaries encircle both hepatic and portal veins, the vessel to the left being an hepatic vein. The same is probably true in an older embryo, fig. 62, while in a foetus at birth the bile duct pictured lies at the junction of two portal veins. When the terminal ducts are arranged in order, as shown in figs. 64-67, it is seen that the first bile ducts are formed around the portal veins from bile capillaries. Longitudinal sections, figs. 68-72, indicate the same. This interpretation of the specimens, which was fiG. 66. fiG. 67.
Fros. 64, 65, 66 and 67. Golgi specimens of the livers of foetal pigs, 5, 6, 7 and 8 cm. long. X 53. The portal twigs are shown in transverse section. After Hendrlckson.
first given by Hendrickson“ is rational, and subsequent observations, which I have been able to make from some of Mr. Eben Hill’s skillful injections of the bile ducts in the embryo, corroborate Hendrickson’s view. The untimely death of Dr. Hendrickson made his preliminary report his final publication upon this subject, and it is now a pleasure to me to carry out in part one of his desire. The obstacle at the time of his..pub1ication was a lack of knowledge of the vascular tree and complete p.gtures of the bile ducts, especially in young embryos. Mr. Hill has supplied the latter by filling the bile ducts of a pig’s embryo 10 cm. long. In the early stages diluted Higgin’s India ink was injected directly into the stomach from which it flowed over into the intestine and backed up into the bile duct. Within the liver it filled the main trunks which correspond with those of the portal vein and then filled a capillary network around portal branches of the first order. An illustration of this specimen is given in fig. 73. This figure shows that the vessels pictured in figs. 65-67 are probably of the second order, while that in 64 is of the first order and, therefore, represents a main trunk.
"‘Hendrlckson, Johns Hopkins Hospital Bulletin, 1898.
Figs. 68, 69, 70, 71 and 72. Golgi specimens showing the terminal portal twigs with their surrounding bile ducts and capillaries in longitudinal ection in fatal pigs 8, 16, 19, 21 cm. long, and in the adult pig. X 53. After Hendrickson.
The bile duct-system can be injected with greater ease in older embryos, for in embryos over 15 cm. long the injection may be made directly through the gall bladder. It is unnecessary to give all intermediate stages, for fig. 74, which is from an embryo 20 cm. long, helps to tell the whole story. With it may be compared figs. 68-71, for one gives the main trunks and the terminal plexus and the other gives the terminal plexus and the bile capillaries. In the adult liver the artery communicates With the lobule throughout the extent of the vessels of the sixth order; the connection between bile ducts and bile capillaries is probably even more extensive. No bile capillaries arise from bile ducts of the fourth order, the liver tissues in their immediate neighborhood being drained by the plexus of the fifth and sixth orders. So in the center of the portal unit the branch of the portal vein ramifies, while along a portion of its axis the artery and duct spread out. The unit is bounded on its periphery by a number of intralobular veins and nodal points, as shown in fig. 1.
Fig. 73. Bile ducts of a pig, 10 cm. long, injected with India ink. X 60; II, portal branches of the second order; III, portal branches of the third order. From a. specimen made by Mr. Eben Hill.
In the development of the liver the shifting of the peripheral plexus of bile ducts is of great importance and helps to clinch much that has been said above about the development of the liver. In an embryo 10 cm. long this plexus encircles portal branches of the second order (fig. 73). In an embryo twice as long the plexus has passed the veins of the third order and now encircle completely those of the fourth or fifth orders. So as the liver tissue is shifting towards the periphery Fro. 74. Injected bile ducts of the liver of a pig, 20 cm. long. X 50. B, bile duct; 12, portal vein; h, hepatic vein; II, III, IV, respective order of the branches. From one of Mr. Hill's specimens.
branches which were once central in every respect, are reduced entirely to main trunks, and throughout this process of growth the structural units remain practically of one size. Thus from one vein encircled by one structural unit a million are formed in the dog. Throughout this growth the vascular proportion is constant. Within the center of the unit the duct expands into a plexus from which regeneration takes place. The periphery of the unit is marked by nodal points which in one sense are embryonic units.
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - A Study of the Structural Unit of the Liver. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_Study_of_the_Structural_Unit_of_the_Liver
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
- ↑ See Kiernan, Phil. Trans., 1833; Theile Handworterbuch der Physiologie, II, 1844; and Oppel, Lehrbuch der Vergleichenden Mikroskopichen Anatomie der Wirbelthiere, III, 1900.
- ↑ Sabourin, Le Progres Méd., VI, 1883; Recherches 1’anat. normale et pathol. de la glands billaire de 1’homme, 1888; Rev. Méd., XXI.
- ↑ Brissaund et Sabourin, Compt. rend., Soc. de b1ol., XL, 1888.
- ↑ Berdal, Elements d’histologie normale, Paris, 1894.
- ↑ Mall, Zeit. f. Morph. u. Anthropol., II, 1900.
- ↑ MacCal1um, J. B., English translation of Scymonowicz’s Histology.
- ↑ Lewis, F. T., Anat. Anz., XXV, 1904.
- ↑ Roux, Ueber die Leistungsfiihigkeit der Princlplen der Descendenzlehre zur Erklfirung der Zweckmiissigkeiten des thierischen Organismus, Breslau, 1880, and Gesan:-.me1te Abhandlungen, I, 1895, p. 134.
- ↑ Roux, Elnleitung zum Archiv fiir Entwickelungsmechanic der Organlsmen. Leipzig, 1894, p. 17.
- ↑ Thoma, Untersuchungen ueber die Histogenese und Histomechanik des Gefasssystems. Stuttgart, 1893.
- ↑ Thoma, Text-book of General Pathology and Pathological Anatomy. Translated by Bruce. London, 1896.
- ↑ Mall, Zeitsch. fur Morphol. u. Anthropol., II, 1900.
- ↑ Rex. Morph. Jahrbuch, XIV, 1888.
- ↑ Thoma, 1. c., p. 28. "Loeb, Pfluger’s Archiv., LIV, 1893.
- ↑ Loeb, 1. c., p. 531. *5 Roux, Ges. Abha.nd1., I, p. 19.
- ↑ See Oppel, Lehrbuch, III, p. 984. Brissaund and Sabourin are undoubtedly in error regarding this statement. I have made and examined hundreds of injections made with granules which would pass through small arteries but not through the capillaries, and have never seen such an anastomosis. Further, celloidin corrosions, which often include part of the capillaries of the lobule, never show such connections.
- ↑ Minot, Proc. Bast. Soc. Nat. Hist, XXIX, 1900.
- ↑ Sabourin, Recherches, etc., de la Glande Biliaire. Paris, 1888.
- ↑ Toldt and Zuckerkandl, Sltzungsber. d. K. Akad. d. Wiss., Bd. 72. Wien, 1876.
- ↑ Kupffer, Arch. f. Mik. Anat., LIV, 1899.
- ↑ The extensive literature upon this subject may be found collected with a. critical discussion in Oppel’s Lehrbuch, III, 1900.
- ↑ Oppel, Anat. Anz., V, 1890; VI, 1891.
- ↑ Mall, Abhandl. d. K. S. Gescell. d. Wiss., XVII, 1891; and Johns Hopkins Hospital Reports, I.
- ↑ See also Mall, On the development of the connective tissues from the connective-tissue syncytium. Amer. Jour. Anat., I, 1902.
- ↑ Hoehl, Arch. f. Anat., 1897; and Oppel, Lehrbuch, III, p. 1009, 1900.