Palate Development
| Embryology - 2 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
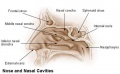
The palate anatomically separates the nasal cavity from the oral cavity and structurally has a bony (hard) anterior component and a muscular (soft) posterior component ending with the uvula. The oral side of the palate is covered with a squamous stratified (pluristratified) epithelium. The surface of the hard palate of most mammalian species is further thrown into a series of transversal palatal ridges or rugae palatinae. Both the palatal ridge number and arrangement are also species specific.
Neural crest has a major contribution to the palate development and there are a number of molecular, mechanical and morphological steps in involving the fusion of contributing structures including a key epithelial to mesenchymal transition. In palate formation there are two main and separate times and events of development, during embryonic (primary palate) and an early fetal (secondary palate). This separation of events into embryonic and fetal period corresponds closely to the classification of associated palate abnormalities.
The primary palate is formed by two parts:
- maxillary components of the first pharyngeal arch (lateral)
- frontonasal prominence (midline)
The secondary palate can also be divided in two anatomical parts:
- anterior hard palate - ossified (contributions from the maxilla and palatine bones).
- posterior soft palate - muscular.
| Palate Links: palate | cleft lip and palate | cleft palate | head | Category:Palate |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Palate Development | Palate Embryology | Embryonic Palate | Fetal Palate | Cleft Palate | levator veli palatini Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Textbooks
- The Developing Human: Clinically Oriented Embryology (8th Edition) by Keith L. Moore and T.V.N Persaud - Moore & Persaud Chapter Chapter 10 The Pharyngeal Apparatus pp201 - 240.
- Larsen’s Human Embryology by GC. Schoenwolf, SB. Bleyl, PR. Brauer and PH. Francis-West - Chapter 12 Development of the Head, the Neck, the Eyes, and the Ears pp349 - 418.
Movies
|
|
|
|
|
|
|
- Links: Movies | ultrasound
Development Overview
- week 4 - pharyngeal arch formation, first pharngeal arch contributes mandible and maxilla.
- week 6 - 7 - primary palate formation maxillary processes and frontonasal prominence.
- week 9 - secondary palate shelves fuse, separating oral and nasal cavities.
Embryonic Period
- (week 4) - pharyngeal arch formation in rostrocaudal sequence (1, 2, 3, 4 and 6)
- First pharyngeal arch - upper maxillary (pair) and lower mandibular prominences
- Late embryonic period - maxillary prominences fuse with frontonasal prominence forming upper jaw (maxilla and upper lip)
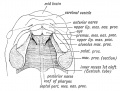
- Embryonic Palate
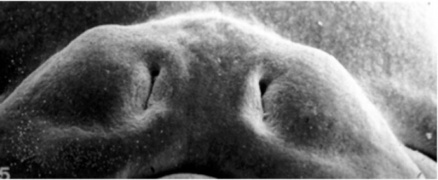
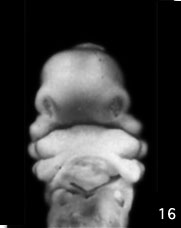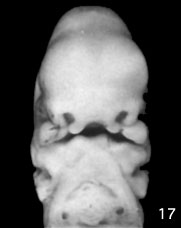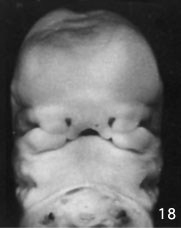
Week 5 stage 16
Week 6 stage 17
Week 6.5 stage 18
Week 7 stage 19
Week 8 stage 22
Week 8 stage 23
Week 8 stage 23
Fetal Period
|
|
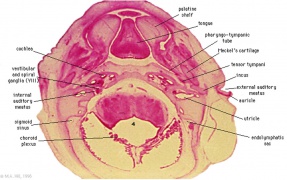
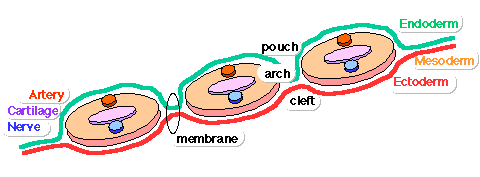
Pharyngeal Arch Development
Major features to identify for each: arch, pouch, groove and membrane. Contribute to the formation of head and neck and in the human appear at the 4th week. The first arch contributes the majority of upper and lower jaw structures.
- Pharynx - begins at the buccopharyngeal membrane (oral membrane), apposition of ectoderm with endoderm (no mesoderm between).
- branchial arch (Gk. branchia= gill)
- arch consists of all 3 trilaminar embryo layers
- ectoderm - outside
- mesoderm - core of mesenchyme
- endoderm - inside
Neural Crest
- Mesenchyme invaded by neural crest generating connective tissue components
- cartilage, bone, ligaments
- arises from midbrain and hindbrain region
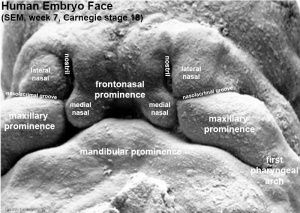
Face Development
Begins week 4 centered around stomodeum, external depression at oral membrane
5 initial primordia from neural crest mesenchyme
- single frontonasal prominence (FNP) - forms forehead, nose dorsum and apex
- nasal placodes develop later bilateral, pushed medially
- paired maxillary prominences - form upper cheek and upper lip
- paired mandibular prominences - lower cheek, chin and lower lip
Frontonasal Process
The frontonasal process (FNP) forms the majority of the superior part of the early face primordia. It later fuses with the maxillary component of the first pharyngeal arch to form the upper jaw. Failure of this fusion event during the embryonic period leads to cleft lip. Under the surface ectoderm the process mesenchyme consists of two cell populations; neural crest cells, forming the connective tissues; and the mesoderm forming the endothelium of the vascular network.
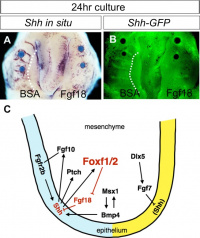
A chicken developmental model study has identified a specific surface region, the Frontonasal Ectodermal Zone (FEZ), initially induced by bone morphogenetic proteins that appears to regulate the future growth and patterning of the frontonasal process. The specific frontonasal ectodermal zone was located in the frontonasal process ectoderm flanking a boundary between Sonic hedgehog (SHH) and Fibroblast growth factor 8 (FGF8) expression domains.[12]
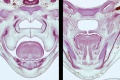
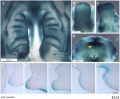
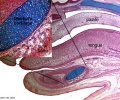
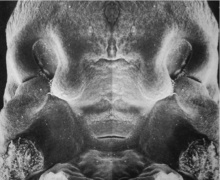
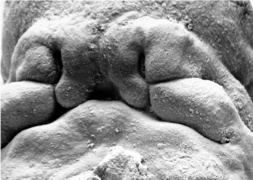
Embryonic Palate
|
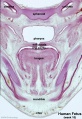
Human primary palate |
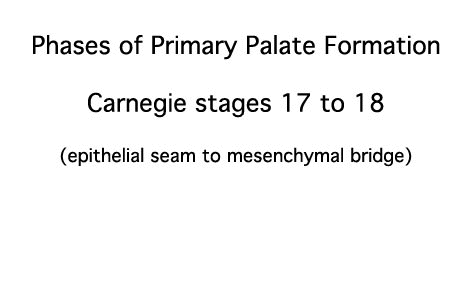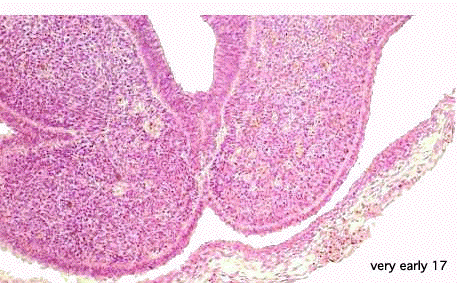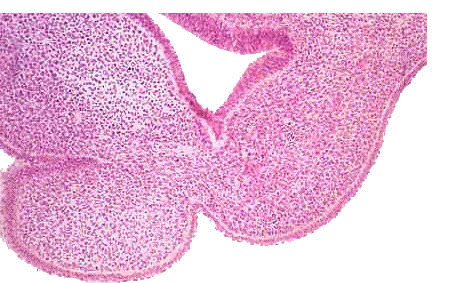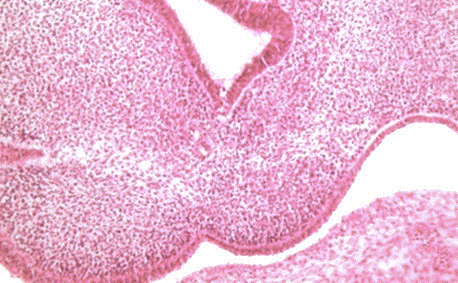
|
- EM Links: Image - stage 16 | Image - stage 17 | Image - stage 18 | Image - stage 19
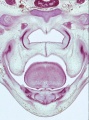
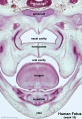
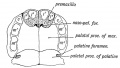
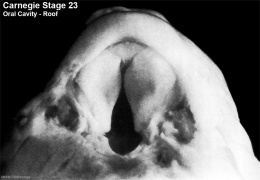
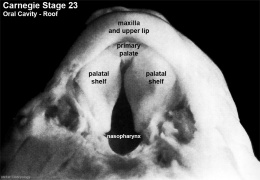
Fetal Palate
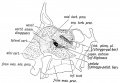
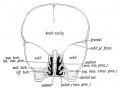
| Secondary palate, fusion in the human embryo in week 9. This requires the early palatal shelves growth, elevation, and fusion. There are many fusion events occurring during this period between each palatal shelf, to the primary palate, and also to the nasal septum.
palatal shelf elevation | secondary palate |
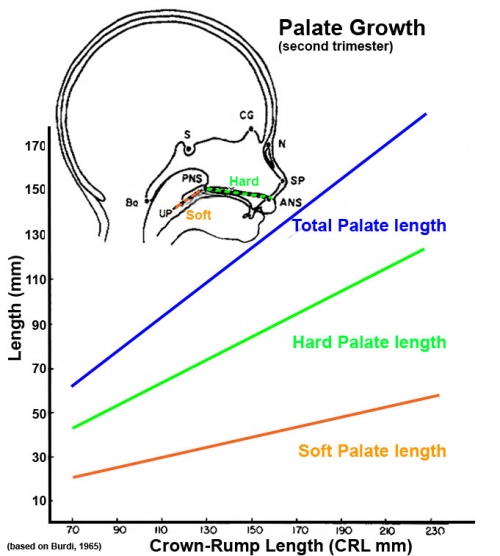
Fetal Palate Growth (second trimester)[14] |
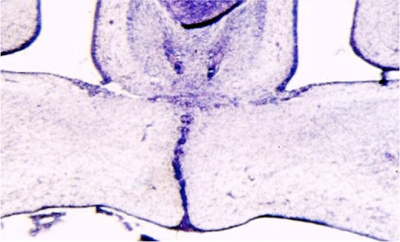
Fetal week 9 (GA week 11) hard palate fusion |
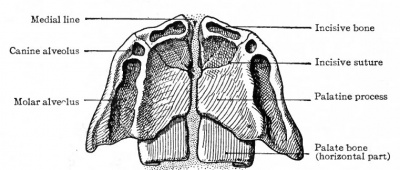
Ventral aspect of hard palate of human embryo of 80 mm |
Soft Palate
The soft palate mechanism of closure has not yet been determined, with several existing theories. A recent study[15] of embryos from the late embryonic-early fetal period (54 to 74 days post-conception) has identified the timing of soft palate closure.
- 57 days - Late embryonic (Carnegie stage 23), epithelial seam present throughout the soft palate
- 64 days - Early fetal (Week 9), epithelium only persists in the most posterior regions of the soft palate
Postnatal Head Changes
Head Growth continues postnatally with fontanelle initially allowing head distortion on birth and early growth. These bony plates remain unfused to allow growth, puberty growth of face.
The palate also grows postnatally through childhood and becomes more elevated (arched) forming the "palatine vault", with different (but insignificant) growth between the genders.[16]
- Links: Postnatal Development
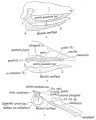
Animal Palate
Mouse Palate
- E11 - protrude from bilateral maxillary processes
- E12.5 - secondary palatal development begins
- E12.5-E14 - grow vertically along the developing tongue
- E14.5 - they elevate, meet, and fuse at the midline, to form an intact palate shelf, reflex opening and closing movements of the mouth
- E15.5 - palatal fusion is complete, mesenchymal condensation followed by osteogenic differentiation occurs.
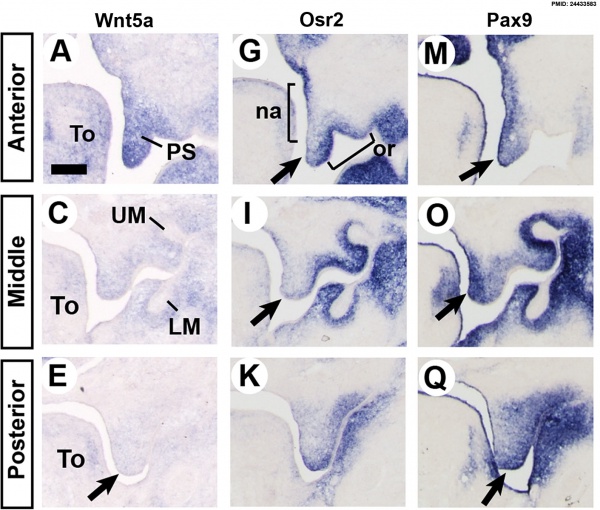
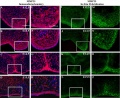
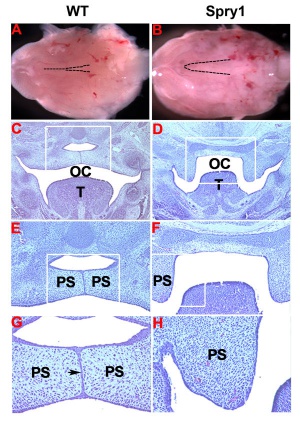
Mouse (E13.5) Palatal Shelf Wnt5a, Osr2 and Pax9 Expression.[17]
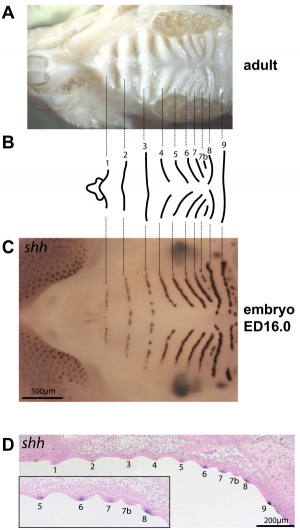
|
|
| Mouse ruga pattern (E16) | Mouse - Spry1 cleft palate |
- Links: Mouse Development | Bone Morphogenetic Protein | Wnt | Pax
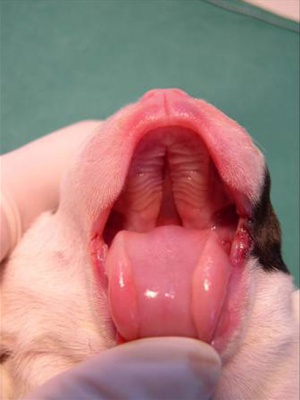
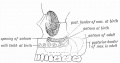
Dog Palate
Newborn dog with cleft palate
Molecular
- Links: Bone Morphogenetic Protein
Abnormalities
Note there are specific pages for both Cleft Lip and Palate and Cleft Palate.
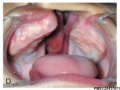
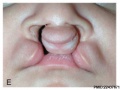
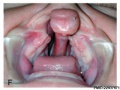
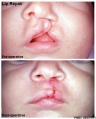
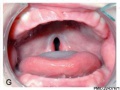
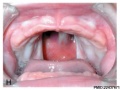
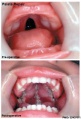
Clinical Images
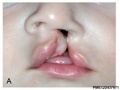
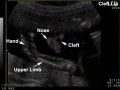
Cleft Lip/Palate
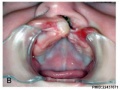
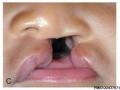
Cleft Palate
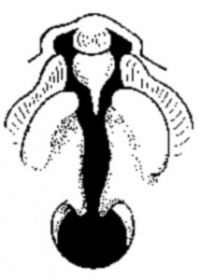
The way in which the upper jaw forms from fusion of the smaller upper prominence of the first pharyngeal arch leads to a common congenital defect in this region called "clefting", which may involve either the upper lip, the palate or both structures.
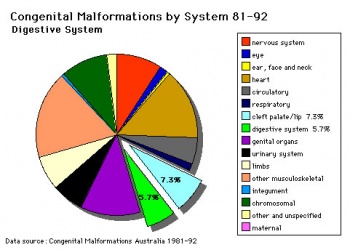
| Australian Palate Abnormalities (2002-2003) |
|---|
| Cleft lip with or without cleft palate (9.2 per 10,000 births) ICD-10 Q36.0, Q36.1, Q36.9, Q37.0–Q37.5, Q37.8, Q37.9 |
A congenital anomaly characterised by a partial or complete clefting of the upper lip, with or without clefting of the alveolar ridge or the hard palate. Excludes a midline cleft of the upper or lower lip and an oblique facial fissure (going towards the eye).
|
| Cleft palate without cleft lip (8.1 per 10,000 births) ICD-10 Q35.0–Q35.9 |
A congenital anomaly characterised by a closure defect of the hard and/or soft palate behind the foramen incisivum without a cleft lip. This anomaly includes sub-mucous cleft palate, but excludes cleft palate with a cleft lip, a functional short palate and high narrow palate.
|
|
| Global Orofacial Cleft Rate (1950 - 2015) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
This data is from a study of the published data (1950 - 2015)[19]
|
International Classification of Diseases - Cleft Palate
Cleft lip and cleft palate (Q35-Q37)
Use additional code (Q30.2), if desired, to identify associated malformations of the nose. Excludes Robin's syndrome ( Q87.0 )
| Q37 | Cleft palate with cleft lip |
| Q37.0 | Cleft hard palate with bilateral cleft lip |
| Q37.1 | Cleft hard palate with unilateral cleft lip |
| Cleft hard palate with cleft lip NOS | |
| Q37.2 | Cleft soft palate with bilateral cleft lip |
| Q37.3 | Cleft soft palate with unilateral cleft lip |
| Cleft soft palate with cleft lip NOS | |
| Q37.4 | Cleft hard and soft palate with bilateral cleft lip |
| Q37.5 | Cleft hard and soft palate with unilateral cleft lip |
| Cleft hard and soft palate with cleft lip NOS | |
| Q37.8 | Unspecified cleft palate with bilateral cleft lip |
| Q37.9 | Unspecified cleft palate with unilateral cleft lip |
| Cleft palate with cleft lip NOS |
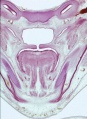
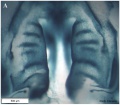
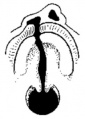
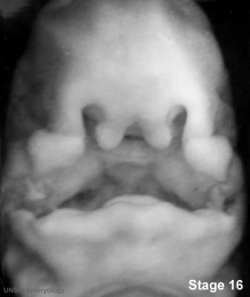
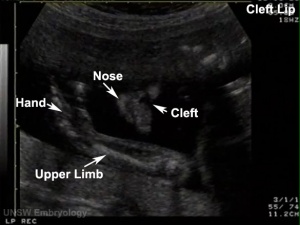
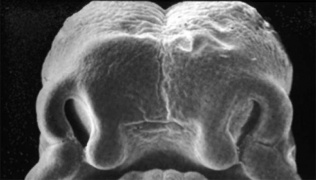
Embryonic Human Cleft Palate
|
| Stage16 (ventral view) |
Cleft Lip
|
|
| Cleft Lip Genes[20] | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Midline Cleft Lip Genes
| ||||||||||||||||||||||||||||||||||||||
|
Cleft Lip (+/− cleft palate) Genes
|
Cleft Palate
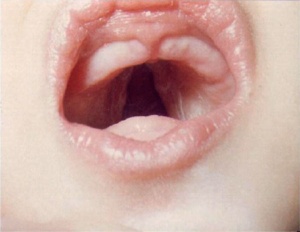
|
(Data: Congenital Malformations Australia 1981-1992 P. Lancaster and E. Pedisich ISSN 1321-8352)
Search Pubmed Now: cleft lip | cleft palate |
Cleft Risk Variants
Two genes were identified from a recent genome-wide study.[22]
- MAFB is expressed in the mouse palatal shelf.
- ABCA4 is a member of a superfamily of transmembrane proteins, and mutations in ABCA4 play a major role in the etiology of Stargardt disease and related retinopathies. Gene produces an ATP-binding cassette (ABC) superfamily trans-membrane protein
- Links: OMIM - MAFB | OMIM - ABCA4
Ten most frequently reported Birth Anomalies
- Hypospadias (More? Male movie | Genital Abnormalities - Hypospadia)
- Obstructive Defects of the Renal Pelvis (More? Renal System - Abnormalities)
- Ventricular Septal Defect (More? Cardiovascular Abnormalities - Ventricular Septal Defect)
- Congenital Dislocated Hip (More? Musculoskelal Abnormalities - Congenital Dislocation of the Hip (CDH))
- Trisomy 21 or Down syndrome - (More? Trisomy 21)
- Hydrocephalus (More? Hydrocephalus)
- Cleft Palate (More? Palate_Development)
- Trisomy 18 or Edward Syndrome - multiple abnormalities of the heart, diaphragm, lungs, kidneys, ureters and palate 86% discontinued (More? (More? Trisomy 18)
- Renal Agenesis/Dysgenesis - reduction in neonatal death and stillbirth since 1993 may be due to the more severe cases being identified in utero and being represented amongst the increased proportion of terminations (approximately 31%). (More? Renal System - Abnormalities)
- Cleft Lip and Palate - occur with another defect in 33.7% of cases.(More? Palate Development | Head Development)
(From the Victorian Perinatal Data Collection Unit in the Australian state of Victoria between 2003-2004)
Folate
A recent study of periconceptional folate supplementation using the Cochrane Pregnancy and Childbirth Group's Trials Register (July 2010) identified no statistically significant evidence of any effects on prevention of cleft palate and cleft lip at birth.[23]
References
- ↑ Xu J, Wang L, Li H, Yang T, Zhang Y, Hu T, Huang Z & Chen Y. (2019). Short stature homeobox 2 (SHOX2) regulates osteogenic differentiation and pattern formation during hard palate development in mice. J. Biol. Chem. , , . PMID: 31649032 DOI.
- ↑ Nakajima A, F Shuler C, Gulka AOD & Hanai JI. (2018). TGF-β Signaling and the Epithelial-Mesenchymal Transition during Palatal Fusion. Int J Mol Sci , 19, . PMID: 30463190 DOI.
- ↑ Dursun A, Öztürk K & Albay S. (2018). Development of Hard and Soft Palate During the Fetal Period and Hard Palate Asymmetry. J Craniofac Surg , 29, 2358-2362. PMID: 30320695 DOI.
- ↑ Hammond NL, Brookes KJ & Dixon MJ. (2018). Ectopic Hedgehog Signaling Causes Cleft Palate and Defective Osteogenesis. J. Dent. Res. , 97, 1485-1493. PMID: 29975848 DOI.
- ↑ Kishimoto H, Yamada S, Kanahashi T, Yoneyama A, Imai H, Matsuda T, Takeda T, Kawai K & Suzuki S. (2016). Three-dimensional imaging of palatal muscles in the human embryo and fetus: Development of levator veli palatini and clinical importance of the lesser palatine nerve. Dev. Dyn. , 245, 123-31. PMID: 26509917 DOI.
- ↑ Potter AS & Potter SS. (2015). Molecular Anatomy of Palate Development. PLoS ONE , 10, e0132662. PMID: 26168040 DOI.
- ↑ Mima J, Koshino A, Oka K, Uchida H, Hieda Y, Nohara K, Kogo M, Chai Y & Sakai T. (2013). Regulation of the epithelial adhesion molecule CEACAM1 is important for palate formation. PLoS ONE , 8, e61653. PMID: 23613893 DOI.
- ↑ Nelson ER, Levi B, Sorkin M, James AW, Liu KJ, Quarto N & Longaker MT. (2011). Role of GSK-3β in the osteogenic differentiation of palatal mesenchyme. PLoS ONE , 6, e25847. PMID: 22022457 DOI.
- ↑ San Miguel S, Serrano MJ, Sachar A, Henkemeyer M, Svoboda KK & Benson MD. (2011). Ephrin reverse signaling controls palate fusion via a PI3 kinase-dependent mechanism. Dev. Dyn. , 240, 357-64. PMID: 21246652 DOI.
- ↑ Beaty TH, Murray JC, Marazita ML, Munger RG, Ruczinski I, Hetmanski JB, Liang KY, Wu T, Murray T, Fallin MD, Redett RA, Raymond G, Schwender H, Jin SC, Cooper ME, Dunnwald M, Mansilla MA, Leslie E, Bullard S, Lidral AC, Moreno LM, Menezes R, Vieira AR, Petrin A, Wilcox AJ, Lie RT, Jabs EW, Wu-Chou YH, Chen PK, Wang H, Ye X, Huang S, Yeow V, Chong SS, Jee SH, Shi B, Christensen K, Melbye M, Doheny KF, Pugh EW, Ling H, Castilla EE, Czeizel AE, Ma L, Field LL, Brody L, Pangilinan F, Mills JL, Molloy AM, Kirke PN, Scott JM, Scott JM, Arcos-Burgos M & Scott AF. (2010). A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat. Genet. , 42, 525-9. PMID: 20436469 DOI.
- ↑ Welsh IC, Hagge-Greenberg A & O'Brien TP. (2007). A dosage-dependent role for Spry2 in growth and patterning during palate development. Mech. Dev. , 124, 746-61. PMID: 17693063 DOI.
- ↑ Foppiano S, Hu D & Marcucio RS. (2007). Signaling by bone morphogenetic proteins directs formation of an ectodermal signaling center that regulates craniofacial development. Dev. Biol. , 312, 103-14. PMID: 18028903 DOI.
- ↑ Diewert VM & Lozanoff S. (1993). A morphometric analysis of human embryonic craniofacial growth in the median plane during primary palate formation. J. Craniofac. Genet. Dev. Biol. , 13, 147-61. PMID: 8227288
- ↑ BURDI AR. (1965). SAGITTAL GROWTH OF THE NASOMAXILLARY COMPLEX DURING THE SECOND TRIMESTER OF HUMAN PRENATAL DEVELOPMENT. J. Dent. Res. , 44, 112-25. PMID: 14245486 DOI.
- ↑ Danescu A, Mattson M, Dool C, Diewert VM & Richman JM. (2015). Analysis of human soft palate morphogenesis supports regional regulation of palatal fusion. J. Anat. , 227, 474-86. PMID: 26299693 DOI.
- ↑ Yang ST, Kim HK, Lim YS, Chang MS, Lee SP & Park YS. (2013). A three dimensional observation of palatal vault growth in children using mixed effect analysis: a 9 year longitudinal study. Eur J Orthod , 35, 832-40. PMID: 23314328 DOI.
- ↑ Almaidhan A, Cesario J, Landin Malt A, Zhao Y, Sharma N, Choi V & Jeong J. (2014). Neural crest-specific deletion of Ldb1 leads to cleft secondary palate with impaired palatal shelf elevation. BMC Dev. Biol. , 14, 3. PMID: 24433583 DOI.
- ↑ P. Lancaster and E. Pedisich, Congenital Malformations Australia 1981-1992, ISSN 1321-835.
- ↑ <pubmed>26742364</pubmed>
- ↑ 20.0 20.1 Dixon MJ, Marazita ML, Beaty TH & Murray JC. (2011). Cleft lip and palate: understanding genetic and environmental influences. Nat. Rev. Genet. , 12, 167-78. PMID: 21331089 DOI.
- ↑ Rastogi MV & LaFranchi SH. (2010). Congenital hypothyroidism. Orphanet J Rare Dis , 5, 17. PMID: 20537182 DOI.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedPMID20436469 - ↑ De-Regil LM, Fernández-Gaxiola AC, Dowswell T & Peña-Rosas JP. (2010). Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst Rev , , CD007950. PMID: 20927767 DOI.
Journals
- The Cleft Palate-Craniofacial Journal Homepage | Available issues
Reviews
Indian J Plast Surg. 2009 October; 42(Suppl):Cleft Lip and Palate Issue
Nakajima A, F Shuler C, Gulka AOD & Hanai JI. (2018). TGF-β Signaling and the Epithelial-Mesenchymal Transition during Palatal Fusion. Int J Mol Sci , 19, . PMID: 30463190 DOI.
Tarr JT, Lambi AG, Bradley JP, Barbe MF & Popoff SN. (2018). Development of Normal and Cleft Palate: A Central Role for Connective Tissue Growth Factor (CTGF)/CCN2. J Dev Biol , 6, . PMID: 30029495 DOI.
Weng M, Chen Z, Xiao Q, Li R & Chen Z. (2018). A review of FGF signaling in palate development. Biomed. Pharmacother. , 103, 240-247. PMID: 29655165 DOI.
Lan Y, Xu J & Jiang R. (2015). Cellular and Molecular Mechanisms of Palatogenesis. Curr. Top. Dev. Biol. , 115, 59-84. PMID: 26589921 DOI.
Suzuki A, Sangani DR, Ansari A & Iwata J. (2016). Molecular mechanisms of midfacial developmental defects. Dev. Dyn. , 245, 276-93. PMID: 26562615 DOI.
Abramyan J & Richman JM. (2015). Recent insights into the morphological diversity in the amniote primary and secondary palates. Dev. Dyn. , 244, 1457-68. PMID: 26293818 DOI.
Bush JO & Jiang R. (2012). Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development , 139, 231-43. PMID: 22186724 DOI.
Meng L, Bian Z, Torensma R & Von den Hoff JW. (2009). Biological mechanisms in palatogenesis and cleft palate. J. Dent. Res. , 88, 22-33. PMID: 19131313 DOI.
Dudas M, Li WY, Kim J, Yang A & Kaartinen V. (2007). Palatal fusion - where do the midline cells go? A review on cleft palate, a major human birth defect. Acta Histochem. , 109, 1-14. PMID: 16962647 DOI.
Ferguson MW. (1988). Palate development. Development , 103 Suppl, 41-60. PMID: 3074914
Hay ED. (1995). An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) , 154, 8-20. PMID: 8714286
Articles
Hammond NL, Brookes KJ & Dixon MJ. (2018). Ectopic Hedgehog Signaling Causes Cleft Palate and Defective Osteogenesis. J. Dent. Res. , 97, 1485-1493. PMID: 29975848 DOI.
Sun L, Wang J, Liu H, Fan Z, Wang S & Du J. (2017). A Comprehensive Study of Palate Development in Miniature Pig. Anat Rec (Hoboken) , 300, 1409-1419. PMID: 28296336 DOI.
Steding G & Jian Y. (2010). The origin and early development of the nasal septum in human embryos. Ann. Anat. , 192, 82-5. PMID: 20149609 DOI.
Xiong W, He F, Morikawa Y, Yu X, Zhang Z, Lan Y, Jiang R, Cserjesi P & Chen Y. (2009). Hand2 is required in the epithelium for palatogenesis in mice. Dev. Biol. , 330, 131-41. PMID: 19341725 DOI.
Search PubMed
Search Pubmed: palate development | cleft palate development |
Additional Images
Historic
Terms
| Palate Development (expand to see terms) |
|---|
|
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Prof Virginia Diewert - Professor of Orthodontics, University of British Columbia, who recently visited the Lab and helped with content, organisation and development of the Palate Development section.
- NIH FACEBASE - Comprehensive craniofacial data and resources.
- Medline Plus - Cleft Lip and Palate
- Better Health Channel - Cleft palate and cleft lip
- March of Dimes Birth Defects Foundation - Cleft Palate
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 2) Embryology Palate Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Palate_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G




![Stage 16]]](/embryology/images/thumb/3/34/Stage16_em01.jpg/120px-Stage16_em01.jpg)
![Stage 17]]](/embryology/images/thumb/b/bb/Stage17_em01.jpg/120px-Stage17_em01.jpg)
![Stage 18]]](/embryology/images/thumb/1/1c/Stage18_em01.jpg/120px-Stage18_em01.jpg)