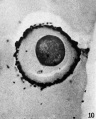
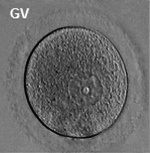
Oocyte Development
| Embryology - 6 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
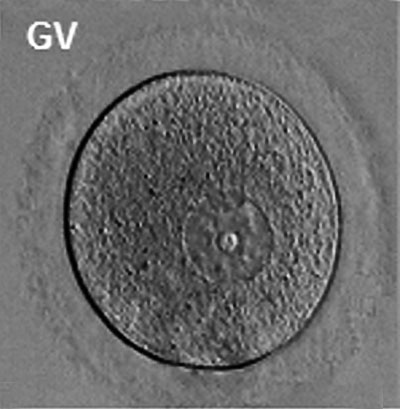
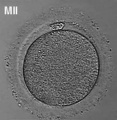
The oocyte (eggs, ova, ovum) is arrested at an early stage of the first {{meiosis))(first meiotic) division as a primary oocyte (primordial follicle) within the ovary. Following puberty, during each menstrual cycle, pituitary gonadotrophin stimulates completion of meiosis 1 the day before ovulation. Early oocytes are also classified as immature (germinal vesicle (GV) or metaphase I (MI) stage). The breakdown of the germinal vesicle indicates a resumption of meiosis and the extrusion of the first polar body (1 PB) indicates completion of the first meiotic division in human oocytes.
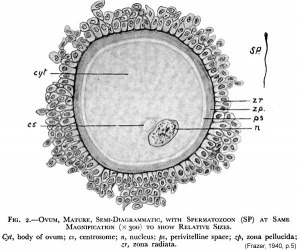
The released oocyte is surrounded by a thick specialised extracellular matrix, the zona pellucida, that in turn is covered in layers of cells, the granulosa layer.
- In an adult human female, the development of a primordial follicle containing an oocyte to a preovulatory follicle takes in excess of 120 days.
| Historic Embryology - Oocyte |
|---|
| 1834 Human and other species Ovum | 1919 Human Ovum | 1921 The Ovum | 1925 Human Graafian Follicle | 1927 Second polar body | 1929 Oocyte Size | 1930 Human large follicles | 1967 Oocyte EM | 1978 Cumulus-oocyte complex |
Genital Links: genital | Lecture - Medicine | Lecture - Science | Lecture Movie | Medicine - Practical | primordial germ cell | meiosis | endocrine gonad | Genital Movies | genital abnormalities | Assisted Reproductive Technology | puberty | Category:Genital
| ||||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Oocyte Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Movies
|
|
|
| ||||||||||||
|
|
|
|
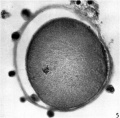
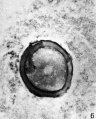
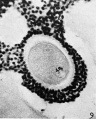
Oogenesis
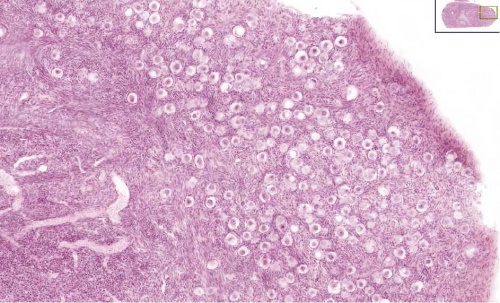
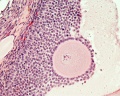
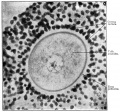
A human infant ovary histology, showing the large number of oocytes occupying the ovary cortical region. Compare this with a mature ovary and note the absence of any follicle development in the infant. These early oocytes remain at the diplotene stage of the meiosis I during development from fetal life and postnatal childhood, until puberty when the lutenizing hormone (LH) surges stimulate the resumption of meiosis.
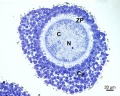
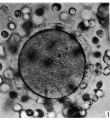
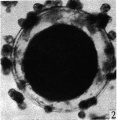
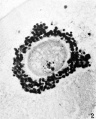
Primordial Follicle
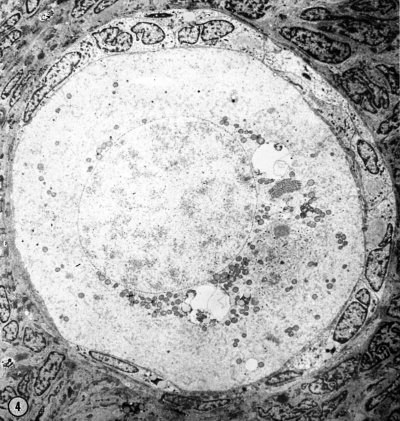
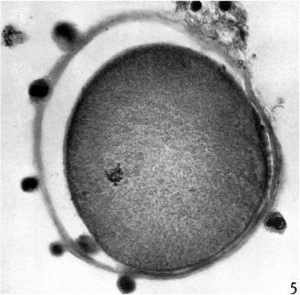
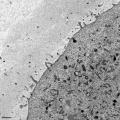
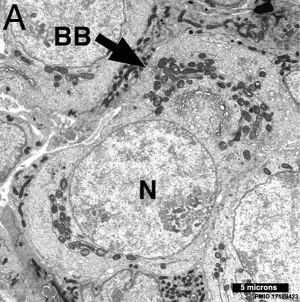
Electron Micrograph of a human oocyte in a primordial follicle. Most of the organelles are concentrated in one pole of the oocyte.[6]
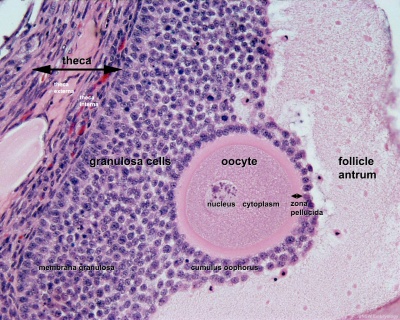
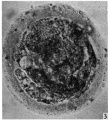
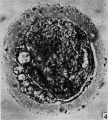
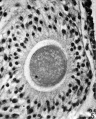
Secondary Follicle
Secondary follicle with oocyte
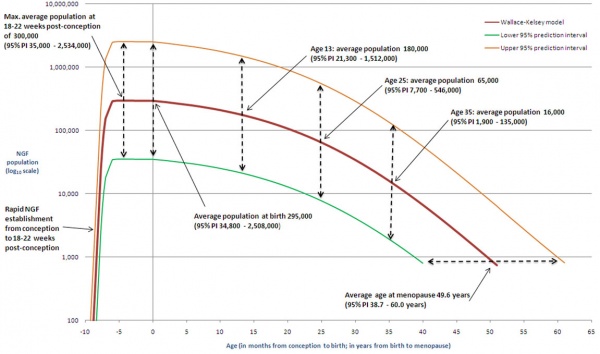
The graph below shows the changes in human germ cell numbers in the ovary with age, peaking at about 7 million (occuring in early fetal development) and then decreasing by apopotic cell death. At puberty there remain only about 400,000 and only about 10% of these will be released through reproductive life. (More? Menstrual Cycle)
Human ovary non-growing follicle model[7]
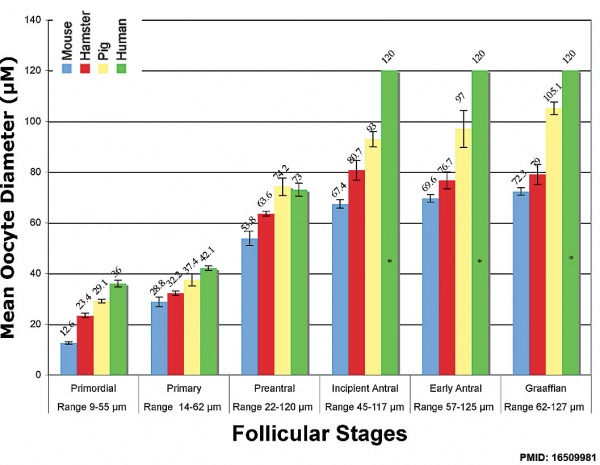
Oocyte Growth
Graph shows species comparison in oocyte size growth (diameter) at different stages of follicle development.[8] (See also Follicle size graph)
| Human Ovarian Follicle Classification | |||||
| Class | Alternate nomenclature | Type | Number of Cells | Size (diameter µm) | Size ultrasound (mm) |
|---|---|---|---|---|---|
| primordial follicle | small | 1, 2, 3 | 25 | less than 50 | |
| primary follicle | preantral | 4 5 |
26 - 100 101 - 300 |
up to 200 | |
| secondary follicle | antral small antral large antral |
6 7 |
3001 - 500 501 - 1000 |
500 1000 - 6000 |
less than 18 |
| preovulatory follicle | Graafian | 8 | greater than 1000 | greater than 6000 | 18 – 28 |
| Links: ovary | oocyte | menstrual cycle | |||||
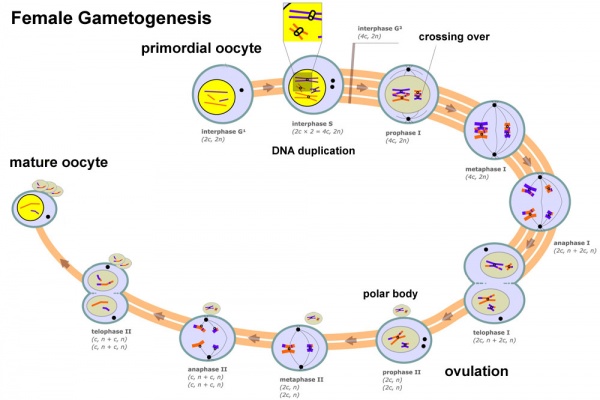
Meiosis
In females, the total number of eggs ever to be produced are present in the newborn female initially arrested at the diplotene stage of the meiosis I from fetal life through childhood until puberty, when the lutenizing hormone (LH) surges stimulate the resumption of meiosis.
- All eggs are arrested at an early stage (prophase I) of the first meiotic division as a primary oocyte (primordial follicle). Following purberty, during each menstrual cycle, pituitary gonadotrophin stimulates completion of meiosis 1 the day before ovulation.
- In meiosis 1, a diploid cell becomes 2 haploid (23 chromosomes) daughter cells, each chromosome has two chromatids. One cell becomes the secondary oocyte the other cell forms the first polar body.
- The secondary oocyte then commences meiosis 2 which arrests at metaphase and will not continue without fertilization.
- At fertilization meiosis 2 completes, forming a second polar body. Note that the first polar body may also undergo this process forming a third polar body.
- Links: meiosis
Polar Body
The polar body is a small cytoplasmic exclusion body formed to enclose the excess DNA formed during the oocyte (egg) meiosis and following sperm fertilization. The breakdown of the germinal vesicle indicates a resumption of meiosis and the extrusion of the first polar body (1 PB) indicates completion of the first meiotic division in human oocytes.
There are 2-3 polar bodies derived from the oocyte present in the zygote, the number is dependent upon whether polar body 1 (the first polar body formed during meiosis 1) divides during meiosis 2. This exclusion body contains the excess DNA from the reductive division (the second and third polar bodies are formed from meiosis 2 at fertilization). These polar bodies do not contribute to the future genetic complement of the zygote, embryo or fetus.
Recent research in some species suggest that the space formed by the peripheral polar body (between the oocyte and the zona pellucia) can influence the site of spermatozoa fertilization.
| First Polar Body | Second Polar Body |
|---|---|
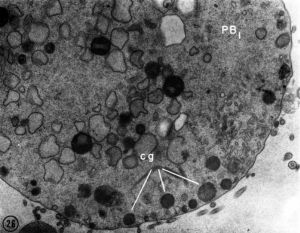
|
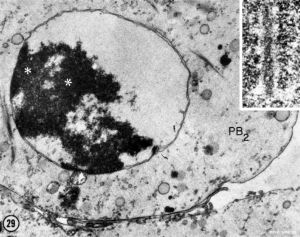
|
| First polar body (PB1) portion of the cytoplasm. Few mitochondria, large ER vesicles are collapsed, absence of the small ER vesicles. Numerous dense cortical granules (eg) are present beneath the plasma membrane of the polar body. X 24,000 | Second polar body (PB2) spheroidal nucleus consisting of dense chromatin aggregates and a highly hydrated nucleoplasm. A double nuclear membrane is present. X 15,000 |
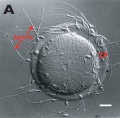
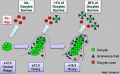
Polar Body Extrusion Model
The following cartoon model from mouse oocyte study of polar body extrusion, involving cortical cap protrusion and spindle midzone-induced membrane furrowing.[10]
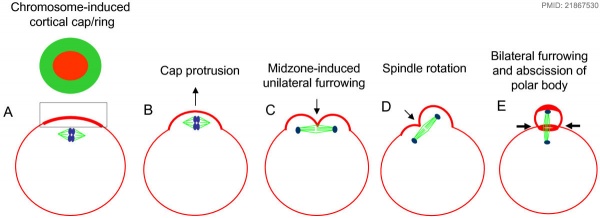
| ||||
| (A) Chromosomes induce formation of a cortical actomyosin cap/ring prior to polar body extrusion. | (B) Egg activation induces the cortical cap protrusion. | (C) The anaphase spindle midzone induces unilateral furrowing. | (D) Spindle rotation. | (E) Spindle midzone induces bilateral furrowing and abscission of polar body. |
| The squared region of the cortical cap/ring is shown on the top, an actin cap (red) surrounded by a myosin II ring (green). | ||||
Assisted reproductive techniques involving intracytoplasmic sperm injection (ICSI) have looked at the "quality" of the polar body and found that the morphology is related to mature oocyte viability and has the potential to predict oocyte fertilization rates and pregnancy achievement.[11][12]
- Links: Meiosis
Calcium Release
Oocyte calcium ion (Ca2+) release occurs after spermatozoa fusion and is part of the reactivation of meiosis (arrested at metaphase II) and the primary block to polyspermy. Earlier in oocyte meiosis, between prophase I (germinal vesicle stage) and MII, this release mechanism is developed within the cell.
Oocyte cytoplasmic changes include:
- endoplasmatic reticulum reorganization.
- IP3 receptor increase in both number and sensitivity.
- increase in calcium ion concentration.
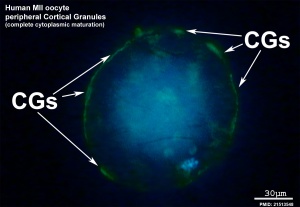
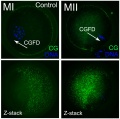
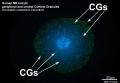
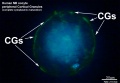
Cortical Granules
The release of cortical granules by exocytosis, the "cortical reaction", occurs following spermatozoa fertilisation and is the main block to polyspermy by modifying the zone pellucida. These granules develop from the golgi apparatus initially forming smaller vesicles that coalesce to form mature membrane bound cortical granules (0.2 to 0.6 microns in diameter) located in the cortex of unfertilized oocytes. In mammals, cortical granule production in the developing follicular oocyte is an ongoing and continuous process, with newly synthesized granules translocating to the cortex until the time of ovulation.
Cortical granules:
- vary in time of initial development between species.
- primordial follicle stage - rat and mouse.
- primary follicle stage - human, monkey, hamsters, and rabbit.
- vary in type formed in the same species.
- migration requires the microfilaments of the cell cytoskeleton.
- are evenly distributed in the cortex of unfertilised oocytes.
- contain carbohydrates, proteinases, ovoperoxidase, calreticulin, N-acetylglucosaminidase
Ovastacin
- oocyte-specific member of the astacin family
- mouse cortical granule protease (metalloendoproteases)
- cleaves zona pellucida ZP2
- female mice lacking ovastacin do not cleave ZP2 after fertilisation[13]
Oocyte-Follicle Cell Interaction
The oocyte and the surrounding granulosa cells have a complex paracrine interactions during follicle growth and development. Oocyte maturation has been shown to depend on secretory products of both the granulosa and cumulus cells.
Oocyte Factors
- promotes granulosa cell proliferation in preantral and antral follicles (GDF-9, BMP15)
- cumulus expansion and granulosa cell differentiation are dependent upon oocyte-derived factors
- BMP15 inhibits FSH-stimulated progesterone production
Oocyte Different Species
| 1929 Hartman - Species Oocyte Sizes | ||
|---|---|---|
| Animal | Most probable size of egg (μm) | Jenkinson's Table |
| Monotremata | ||
| Platypus | 1.5 mm | |
| Echidna | 3 .0 mm | |
| Marsupialia | ||
| Dasyurus | 240 | 280 |
| Didelphis | 140-160 | 130 |
| Edentata | ||
| Armadillo | 80 | |
| Cetacea | ||
| Whales | 140 | |
| Insectivora | ||
| Mole (Talpa) | 12.5 | 90 |
| Hedgehog (Erinaceus) | 100 | 60 |
| Rodentia | ||
| Mouse | 70-75 | 60 |
| Rat | 70-75 | |
| Guinea pig | 75-85 | 80 |
| Lagomorpha | ||
| Rabbit | 120-130 | 150 |
| Carnivora | ||
| Dog | 135-145 | 180 |
| Cat | 120-130 | |
| Ferret | 120 | |
| Ungulata | ||
| Horse | 135 | |
| Sheep | 120 | 150 |
| Goat | 140 | |
| Pig | 120-140 | |
| Cheiroptera | ||
| Bat | 95-105 | |
| Lemurs | ||
| Tarsius | 90 | |
| Primates | ||
| Gibbon | 110-110 | |
| M. rhesus | 110-120 | |
| Gorilla | 130-140 | |
| Man | 130-140 | |
Table 8 Reference: Hartman CG. How Large is the Mammalian Egg?: A Review. (1929) Quart. Rev. Biol., 4(3): 373-388. | ||
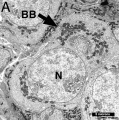
Balbini Body
The Balbiani body (Balbiani vitelline body; mitochondrial cloud) is a large organelle aggregate found in developing oocytes of many species.[15], including human.[16][17] Located asymmetrically beside the nucleus and is composed of: endoplasmic reticulum, mitochondria, Golgi, and proteins. In the human fetal ovary oocyte development, the RNA binding protein VASA protein is expressed in primordial follicles has been shown to colocalise to Balbiani's vitelline space.[18] Note VASA expression is not detectable in the adult ovary.
| Balbini Body History | |
|---|---|
Prof. Édouard-Gérard Balbiani (1823–1899)
For more information see Hertig's 1968 presentation.[16] References Balbiani EG. Recherches sur les phénomenes sexuels des Infusoires. (1864) J. de la Physiol. 4: 102-130. Balbiani EG. Recherches sur les phénomenes sexuels des Infusoires. (1864) J. de la Physiol. 4: 194- 220. Balbiani EG. Centrosome et “Dotterkern.” (1893) J. Anat. Physiol. 29: 145-180. van der Stricht O. Comparative study of mammalian ovum at the different periods of ovogenesis, according to the work of the histology and embryology laboratory of the University of Ghent. Etude comparée des ovules des mammiferes aux diflerentes periods de Yovogenese, d’aprés les travaux du laboratoire d’histo1ogie et d’embryologie de l’université de Gand. (1923) Arch. Biol., 33: 229-300. |

|
Oocyte Metabolism
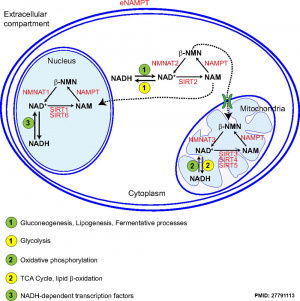
In the mouse, the secondary follicle stage through to large antral follicle stage the oocyte has a highly oxidative metabolism. In contrast, the surrounding surrounding granulosa and cumulus cells are highly glycolytic. In this second group, the cumulus cells are found to be more glycolytic than the granulosa cells.[20]
- oxidative metabolism - requires oxygen (aerobic) to make energy from carbohydrates (sugars) into pyruvate that passes into the mitochondria where it is fully oxidised by oxygen into carbon dioxide. Also called aerobic metabolism, aerobic respiration, and cell respiration.
- glycolytic metabolism - requires no oxygen (anaerobic) to make energy from carbohydrates (sugars) into pyruvate that is reduced by adenine dinucleotide hydride (NADH).
In the cat oocyte, in pre-antral oocytes mitochondria have a homogeneous distribution throughout the cytoplasm. In the antral stage they have relocated to a mainly pericortical distribution.[21]
- Links: OMIM SIRT1
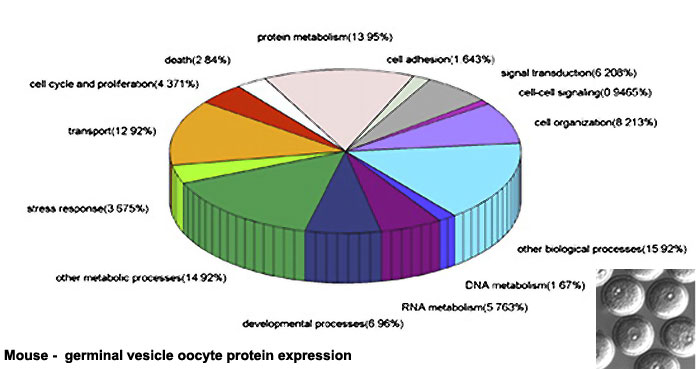
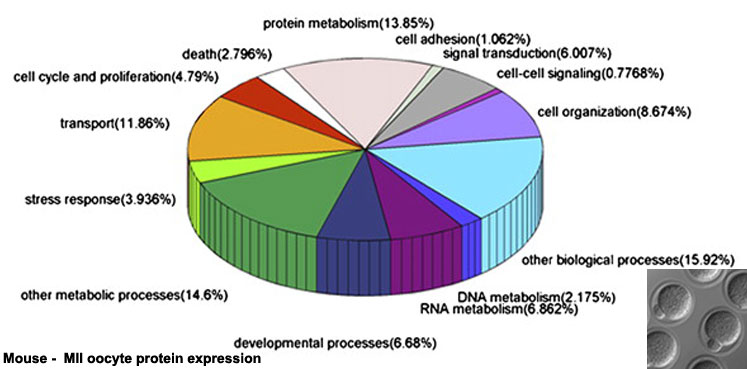
Oocyte Protein Expression
The table above shows the pattern of protein expression (as percentages of total) in the mouse germinal vesicle and MII oocyte according to 14 molecular function categories.[22]
- Links: Germinal vesicle oocyte protein expression | MII oocyte protein expression | Zygote Protein Expression | Mouse Development | Oocyte Development | Zygote
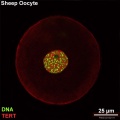
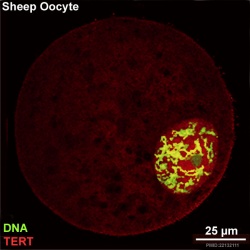
Oocyte Telomerase Reverse Transcriptase
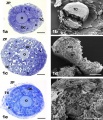
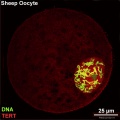
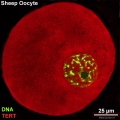
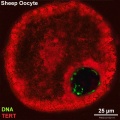
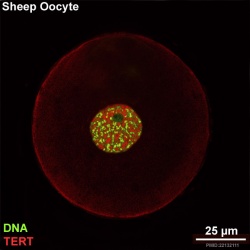
There is a redistribution of the enzyme that regulates telomere length during oocyte development. The following oocyte images are from a recent study of sheep in vitro follicle development.[23]
|
|
| preantral | early antral |
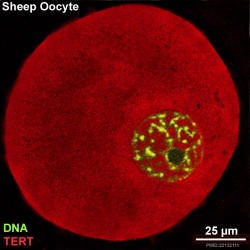
|
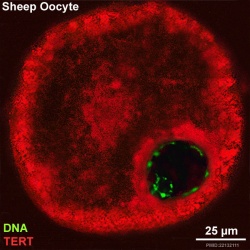
|
| early antral | preovulatory follicle |
- TERT - Red (Cy3-conjugated secondary antibody) (telomerase reverse transcriptase, TERT)
- DNA - Green (SYBR Green 14/I)
- Sheep Oocyte TERT: preantral | early antral | early antral | preovulatory follicle | Oocyte Development | Sheep Development
Abnormalities
Female Infertility Genes
| Gene abbreviation | Name | Gene Location | Online Mendelian Inheritance in Man (OMIM) |
HUGO Gene Nomenclature Committee (HGNC) |
GeneCards (GCID) | Diagnosis |
|---|---|---|---|---|---|---|
| BMP15 | Bone morphogenetic protein 15 | Xp11.22 | 300247 | 1068 | GC0XP050910 | Primary ovarian insufficiency |
| CLPP | Caseinolytic mitochondrial matrix peptidase proteolytic subunit | 19p13.3 | 601119 | 2084 | GC19P006369 | Primary ovarian insufficiency |
| EIF2B2 | Eukaryotic translation initiation factor 2B subunit beta | 14q24.3 | 606454 | 3258 | GC14P075002 | Primary ovarian insufficiency |
| FIGLA | Folliculogenesis-specific BHLH transcription factor | 2p13.3 | 608697 | 24669 | GC02M070741 | Primary ovarian insufficiency |
| FMR1 | Fragile X mental retardation 1 | Xq27.3 | 309550 | 3775 | GC0XP147912 | Primary ovarian insufficiency |
| FOXL2 | Forkhead box L2 | 3q22.3 | 605597 | 1092 | GC03M138944 | Primary ovarian insufficiency |
| FSHR | Follicle stimulating hormone receptor | 2p16.3 | 136435 | 3969 | GC02M048866 | Primary ovarian insufficiency |
| GALT | Galactose-1-phosphate uridylyltransferase | 9p13.3 | 606999 | 4135 | GC09P034636 | Primary ovarian insufficiency |
| GFD9 | Growth differentiation factor 9 | 5q31.1 | 601918 | 4224 | GC05M132861 | Primary ovarian insufficiency |
| HARS2 | Histidyl-TRNA synthetase 2, mitochondrial | 5q31.3 | 600783 | 4817 | GC05P141975 | Primary ovarian insufficiency |
| HFM1 | HFM1, ATP-dependent DNA helicase homolog | 1p22.2 | 615684 | 20193 | GC01M091260 | Primary ovarian insufficiency |
| HSD17B4 | Hydroxysteroid 17-beta dehydrogenase 4 | 5q23.1 | 601860 | 5213 | GC05P119452 | Primary ovarian insufficiency |
| LARS2 | Leucyl-TRNA synthetase 2, mitochondrial | 3p21.31 | 604544 | 17095 | GC03P045405 | Primary ovarian insufficiency |
| LHCGR | Luteinizing hormone/choriogonadotropin receptor | 2p16.3 | 152790 | 6585 | GC02M048647 | Primary ovarian insufficiency |
| LHX8 | LIM homeobox 8 | 1p31.1 | 604425 | 28838 | GC01P075128 | Primary ovarian insufficiency |
| MCM8 | Minichromosome maintenance 8 homologous recombination repair factor | 20p12.3 | 608187 | 16147 | GC20P005926 | Primary ovarian insufficiency |
| MCM9 | Minichromosome maintenance 9 homologous recombination repair factor | 6q22.31 | 610098 | 21484 | GC06M118813 | Primary ovarian insufficiency |
| NOBOX | NOBOX oogenesis homeobox | 7q35 | 610934 | 22448 | GC07M144397 | Primary ovarian insufficiency |
| NOG | Noggin | 17q22 | 602991 | 7866 | GC17P056593 | Primary ovarian insufficiency |
| PMM2 | Phosphomannomutase 2 | 16p13.2 | 601785 | 9115 | GC16P008788 | Primary ovarian insufficiency |
| POLG | DNA polymerase gamma, catalytic subunit | 15q26.1 | 174763 | 9179 | GC15M089316 | Primary ovarian insufficiency |
| REC8 | REC8 meiotic recombination protein | 14q12 | 608193 | 16879 | GC14P024171 | Primary ovarian insufficiency |
| SMC1B | Structural maintenance of chromosomes 1B | 22q13.31 | 608685 | 11112 | GC22M045344 | Primary ovarian insufficiency |
| SOHLH1 | Spermatogenesis and oogenesis-specific basic helix–loop–helix 1 | 9q34.3 | 610224 | 27845 | GC09M135693 | Primary ovarian insufficiency |
| STAG3 | Stromal antigen 3 | 7q22.1 | {{Chr 608489 | 11356 | GC07P100177 | Primary ovarian insufficiency |
| SYCE1 | Synaptonemal Complex Central Element Protein 1 | 10q26.3 | 611486 | 28852 | GC10M133553 | Primary ovarian insufficiency |
| TLE6 | Transducin-like enhancer of split 6 | 19p13.3 | 612399 | 30788 | GC19P002976 | Embryonic lethalithy |
| TUBB8 | Tubulin beta 8 Class VIII | 10p15.3 | 616768 | 20773 | GC10M000048 | Oocyte maturation arrest |
| TWNK | Twinkle MtDNA helicase | 10q24.31 | 606075 | 1160 | GC10P100991 | Primary ovarian insufficiency |
| Table data source[24] (table 1) Links: fertilization | oocyte | ovary | | Female Infertility Genes | spermatozoa | testis | Male Infertility Genes | Genetic Abnormalities | ART Primary ovarian insufficiency - depletion or dysfunction of ovarian follicles with cessation of menses before age 40 years. | ||||||
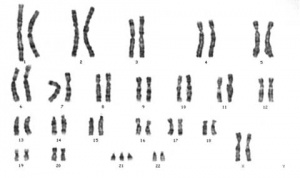
Trisomy
Meiotic non-disjunction resulting in aneuploidy, most are embryonic lethal and not seen. The potential for genetic abnormalities increase with maternal age.
| Autosomal chromosome aneuploidy | Sex chromosome aneuploidy |
|---|---|
|
|
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Hamilton WJ. Phases of maturation and fertilization in human ova. (1944) J Anat. 78: 1-4.
- Human Oocyte
Pincus G. and Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro. (1935) J Exp Med. 62(5): 665-75. PMID 19870440
- Rabbit Oocyte
References
- ↑ Xie Y, Wu B, Jin Y, Zhang A, Sun X, Zhang X, Gao X, Dong R, Li H & Gao J. (2018). Oocyte-specific deletion of Gsα induces oxidative stress and deteriorates oocyte quality in mice. Exp. Cell Res. , , . PMID: 30026030 DOI.
- ↑ Guo J, Zhang T, Guo Y, Sun T, Li H, Zhang X, Yin H, Cao G, Yin Y, Wang H, Shi L, Guo X, Sha J, Eppig JJ & Su YQ. (2018). Oocyte stage-specific effects of MTOR determine granulosa cell fate and oocyte quality in mice. Proc. Natl. Acad. Sci. U.S.A. , , . PMID: 29784807 DOI.
- ↑ Wang JJ, Ge W, Liu JC, Klinger FG, Dyce PW, De Felici M & Shen W. (2017). Complete in vitro oogenesis: retrospects and prospects. Cell Death Differ. , 24, 1845-1852. PMID: 28841213 DOI.
- ↑ Pires-Luís AS, Rocha E, Bartosch C, Oliveira E, Silva J, Barros A, Sá R & Sousa M. (2016). A stereological study on organelle distribution in human oocytes at prophase I. Zygote , 24, 346-54. PMID: 26170179 DOI.
- ↑ White YA, Woods DC, Takai Y, Ishihara O, Seki H & Tilly JL. (2012). Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. , 18, 413-21. PMID: 22366948 DOI.
- ↑ Hertig AT. and Adams EC. Studies on the human oocyte and its follicle. I. Ultrastructural and histochemical observations on the primordial follicle stage. (1967) J Cell Biol. 34(2):647-75. PMID 4292010
- ↑ Wallace WH & Kelsey TW. (2010). Human ovarian reserve from conception to the menopause. PLoS ONE , 5, e8772. PMID: 20111701 DOI.
- ↑ Griffin J, Emery BR, Huang I, Peterson CM & Carrell DT. (2006). Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J. Exp. Clin. Assist. Reprod. , 3, 2. PMID: 16509981 DOI.
- ↑ Zamboni L, Mishell DR, Bell JH & Baca M. (1966). Fine structure of the human ovum in the pronuclear stage. J. Cell Biol. , 30, 579-600. PMID: 6008199
- ↑ Wang Q, Racowsky C & Deng M. (2011). Mechanism of the chromosome-induced polar body extrusion in mouse eggs. Cell Div , 6, 17. PMID: 21867530 DOI.
- ↑ Ebner T, Yaman C, Moser M, Sommergruber M, Feichtinger O & Tews G. (2000). Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum. Reprod. , 15, 427-30. PMID: 10655316
- ↑ Younis JS, Radin O, Izhaki I & Ben-Ami M. (2009). Does first polar body morphology predict oocyte performance during ICSI treatment?. J. Assist. Reprod. Genet. , 26, 561-7. PMID: 19960239 DOI.
- ↑ Burkart AD, Xiong B, Baibakov B, Jiménez-Movilla M & Dean J. (2012). Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J. Cell Biol. , 197, 37-44. PMID: 22472438 DOI.
- ↑ Pepling ME, Wilhelm JE, O'Hara AL, Gephardt GW & Spradling AC. (2007). Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc. Natl. Acad. Sci. U.S.A. , 104, 187-92. PMID: 17189423 DOI.
- ↑ Cox RT & Spradling AC. (2003). A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development , 130, 1579-90. PMID: 12620983
- ↑ 16.0 16.1 Hertig AT. (1968). The primary human oocyte: some observations on the fine structure of Balbiani's vitelline body and the origin of the annulate lamellae. Am. J. Anat. , 122, 107-37. PMID: 5654499 DOI.
- ↑ Wessel GM. (2012). Clouded by terminology: Edouard-Gérard Balbiani and the mitochondrial cloud. Mol. Reprod. Dev. , 79, Fm i. PMID: 22987576 DOI.
- ↑ Albamonte MI, Albamonte MS, Stella I, Zuccardi L & Vitullo AD. (2013). The infant and pubertal human ovary: Balbiani's body-associated VASA expression, immunohistochemical detection of apoptosis-related BCL2 and BAX proteins, and DNA fragmentation. Hum. Reprod. , 28, 698-706. PMID: 23315064 DOI.
- ↑ Aguilar-Arnal L, Ranjit S, Stringari C, Orozco-Solis R, Gratton E & Sassone-Corsi P. (2016). Spatial dynamics of SIRT1 and the subnuclear distribution of NADH species. Proc. Natl. Acad. Sci. U.S.A. , , . PMID: 27791113 DOI.
- ↑ Cinco R, Digman MA, Gratton E & Luderer U. (2016). Spatial Characterization of Bioenergetics and Metabolism of Primordial to Preovulatory Follicles in Whole Ex Vivo Murine Ovary. Biol. Reprod. , 95, 129. PMID: 27683265 DOI.
- ↑ Songsasen N, Henson LH, Tipkantha W, Thongkittidilok C, Henson JH, Chatdarong K & Comizzoli P. (2017). Dynamic changes in mitochondrial DNA, distribution and activity within cat oocytes during folliculogenesis. Reprod. Domest. Anim. , 52 Suppl 2, 71-76. PMID: 28111812 DOI.
- ↑ Wang S, Kou Z, Jing Z, Zhang Y, Guo X, Dong M, Wilmut I & Gao S. (2010). Proteome of mouse oocytes at different developmental stages. Proc. Natl. Acad. Sci. U.S.A. , 107, 17639-44. PMID: 20876089 DOI.
- ↑ Barboni B, Russo V, Cecconi S, Curini V, Colosimo A, Garofalo ML, Capacchietti G, Di Giacinto O & Mattioli M. (2011). In vitro grown sheep preantral follicles yield oocytes with normal nuclear-epigenetic maturation. PLoS ONE , 6, e27550. PMID: 22132111 DOI.
- ↑ Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I, Lundin K, Mertes H, Morris M, Pennings G, Sermon K, Spits C, Soini S, van Montfoort APA, Veiga A, Vermeesch JR, Viville S & Macek M. (2018). Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur. J. Hum. Genet. , 26, 12-33. PMID: 29199274 DOI.
Reviews
Li R & Albertini DF. (2013). The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. , 14, 141-52. PMID: 23429793 DOI.
Sánchez F & Smitz J. (2012). Molecular control of oogenesis. Biochim. Biophys. Acta , 1822, 1896-912. PMID: 22634430 DOI.
Liu M. (2011). The biology and dynamics of mammalian cortical granules. Reprod. Biol. Endocrinol. , 9, 149. PMID: 22088197 DOI.
Articles
Search
- NCBI Bookshelf oocyte | oogenesis | oocyte development
- Pubmed oocyte | oogenesis | oocyte development
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Terms
Note there are additional specific term glossaries available listed at bottom of this table.
- antral follicle - (secondary) the stage following preantral in the decription of the sequence ovarian follicle development.
- antrum - (L. a cave), cavity; a nearly-closed cavity or bulge. In the ovary this refers to the follicular fluid-filled space within the follicle.
- atretic follicle - An ovarian follicle that fails to mature and degenerates. Also called "atresia" refering to the process of degeneration of the ovarian follicle. This process can occur at any stage of follicle development (folliculogenesis).
- clomiphene citrate - drug taken orally to promote the process of follicle/egg maturation.
- COCs - (cumulus-oocyte complexes) term used in Assisted Reproductive Technology to describe the ovulated Graafian follicle consisting of the oocyte surrounded by a packed layers of cumulus cells.
- corona radiata - Layer of follicle cells of cumulus oophorus remaining directly attached to zona pellucida of the oocyte. These cells communicate with the oocyte through the zone pellucida, also called granulosa cells.
- corpus albicans - (L. corpus = body, L. albicans = whitish); a degenerating corpus luteum in ovary.
- corpus luteum - (L. corpus = body, L. luteum = yellow) The remains of the ovulating ovarian follicle after ovulation, that acts as the initial endocrine organ supporting pregnancy and preventing menstruation (loss of the endometrial lining). de Graaf first observed it in the ovary of a cow as a yellow structure.
- cortical - (L. corticalis) at the outside (like the bark of a tree), usually combined with medulla meaning the core.
- cumulus oophorus - (L. cumulus = a little mound G. oon = egg + phorus = bearing); part of the wall of an ovarian follicle surrounding and carrying the ovum (oocyte).
- dictyate arrest - (prophase arrest) the oocyte meiosis state before puberty resumed with a surge of pituitary luteinizing hormone.
- first polar body - a small cytoplasmic exclusion body contains the excess DNA from the oocyte meiosis formed during meiosis 1.
- follicle - (L. folliculus = little bag,dim. of L. follis). A structure which develops in the ovary and contains a developing egg (oocyte).
- follicle stimulating hormone - (FSH, gonadotropin) A glycoprotein hormone secreted by anterior pituitary (adenohypophysis gonadotrophs, a subgroup of basophilic cells) and acts on gametogenesis and other systems in both males and females. Females, FSH acts on the ovary to stimulate follicle development. Males, acts on the testis Sertoli cells to increase androgen-binding protein (ABP) that binds androgens and has a role in spermatogenesis. pituitary
- follicular fluid - the fluid found in the antrum of a secondary follicle. Secreted by cells in the wall of the follicle. This fluid is released along with the oocyte at ovulation.
- germinal epithelium - cellular component covering surface of ovary, it is continuous with mesothelium covering mesovarium. Note that it is a historical misnomer, as it is not the actual site of germ cell formation.
- Graafian follicle - named after Regnier de Graaf (1641-1673), an historic Dutch physician embryologist who studied pregnancy using rabbits.
- granulosa cells - the supporting cells that surround the developing egg within the follicle thecal layers.
- homologs - maternal and paternal homologous chromosomes.
- Izumo1 - a protein located on the equatorial segment of acrosome-reacted spermatozoa recognizes its receptor Juno, on the oocyte surface, for plasma membrane binding and fusion. Named for a Japanese shrine dedicated to marriage. OMIM609278
- Juno - (folate receptor-δ; FOLR-δ) a glycophosphatidylinositol (GPI)-anchored, cysteine-rich glycoprotein on the oocyte surface for fertilisation that is the receptor of Izumo1 on the spermatozoa, for plasma membrane binding and fusion. OMIM615737
- luteinizing hormone - (LH, gonadotropin, lutropin, Interstitial Cell Stimulating Hormone, ICSH) glycoprotein hormone releasd from anterior pituitary hormone that acts on the gonad and has a role in male and female reproduction. Female, LH triggers ovulation (release of the oocyte). Male, LH stimulates testis interstital cell (Leydig cell) production of testosterone. Have been used clinically in humans for the treatment of female infertility.
- meiosis - oocyte reductive (diploid to haploid) cell division, with 1 round of DNA replication is followed by 2 rounds of chromosome segregation. The process beginning in the fetus and only completed at fertilization.
- mesovarium - mesentry of the ovary formed from a fold of the broad ligament that attaches the ovary.
- medullary - (L. medius = in the middle) relating to the medulla; pith, marrow, inner portion of an organ. Usually combined with cortex (cortical) meaning the outer layer.
- oocyte - (Greek, oo = egg, ovum) The term used to describe the haploid egg or ovum formed within the ovary (female gonad) and released to enter the uterine tube and be transported to the uterus. The mature oocyte is the cell released from the ovary during ovulation.
- oocyte retrieval - (egg retrieval) A clinical in vitro fertilization (IVF) procedure to collect the eggs contained in the ovarian follicles.
- oogenesis - (Greek, oo = egg + genesis = origin, creation, generation) process of diploid oogonia division and differentiation into an haploid oocyte (egg) within the ovary (female gonad). Mammalian meiosis will only be completed within the oocyte if fertilization occurs.
- oogonia - (Greek, oo = egg) diploid germ cells within the ovary (female gonad) which provide the primary oocytes for oocyte (egg) formation. In humans, all oogonia form primary oocytes within the ovary before birth.
- oolemma - (zona pellucida, vitelline membrane).
- oophorus - (Greek, oo = egg + phorus = carrying, egg-bearing) cumulus oophorus, used to describe the granulosa cells within the follicle that tether or link the oocyte to the wall of the follicle.
- ovarian reserve - Clinical term for the number of oocytes (non-growing follicles) available for possible fertilization at the different times during female reproductive life. A blood test for Anti-Mullerian Hormone (AMH) levels is used clinically as a measure of the ovarian reserve. human graph
- ovastacin - an oocyte released enzyme following fertilization that cleaves ZP2 protein to prevent polyspermy.
- ovulation - release of the oocyte from the mature follicle. In humans generally a single oocyte is released from a cohort of several maturing follicles.
- ovum - oocyte, note that historically this same term was also used to describe the early stages following fertilisation.
- polar body - small cytoplasmic exclusion body contains the excess DNA from the oocyte meiosis reductive division. The first polar body formed during meiosis 1, the second and sometimes third polar bodies are formed from meiosis 2 at fertilization.
- polyspermy - abnormal fertilization by more that a single spermatozoa, may generate a hydatidiform mole.
- preantral follicle - (primary) the stage following primordial in the description of the sequence ovarian follicle development.
- primary follicle - (preantral) the stage following primordial in the description of the sequence ovarian follicle development.
- primordial follicle - the first stage in the description of the sequence ovarian follicle development. Present in the ovary from birth, located in the stroma of the ovary cortex beneath the tunica albuginea. The primordial follicle is the oocyte and the surrounding follicular cells.
- primordial germ cell - oocyte present in the primordial follicle ovary from birth, located in the stroma of the ovary cortex beneath the tunica albuginea. The primordial follicle is the oocyte and the surrounding follicular cells.
- second polar body - a small cytoplasmic exclusion body contains the excess DNA from the oocyte formed during meiosis 2 at fertilization.
- secondary follicles - the stage following primary in the description of the sequence ovarian follicle development.
- stromal cells - in the ovary, cells surrounding the developing follicle that form a connective tissue sheath (theca folliculi). This layer then differentiates into 2 layers (theca interna, theca externa). This region is richly vascularized and involved in hormone secretion.
- superovulation therapy - a fertility drug treatement (oral clomiphene citrate and/or injectable FSH with or without LH) aimed at stimulating development/release of more than one follicle during a single menstrual cycle.
- tertiary follicle - (preovulatory, Graffian) the stage following secondary in the description of the sequence ovarian follicle development.
- theca folliculi - stromal cells in the ovary, cells surrounding the developing follicle that form a connective tissue sheath. This layer then differentiates into 2 layers (theca interna, theca externa). This region is vascularized and involved in hormone secretion.
- theca externa - stromal cells forming the outer layer of the theca folliculi surrounding the developing follicle. Consisting of connective tissue cells, smooth muscle and collagen fibers.
- theca interna - stromal cells forming the inner layer of the theca folliculi surrounding the developing follicle. This vascularized layer of cells respond to LH (leutenizing hormone) synthesizing and secreting androgens which are processed into estrogen.
- transzonal projection - (TZP) ovarian follicle term describing the cellular membraneous extension from the granulosa cell through the zona pellucida to the oocyte cell membrane where it forms gap junctions or adherens junctions allowing signalling and adhesion between the two cells.
- tunica albuginea - dense connective tissue layer lying near the ovary surface, a layer of simple squamous to cuboidal epithelial covers this layer. A similar named structure is found in male genital system.
- uterus - site of embryo implantation and development. Uterine wall has 3 major layers: endometrium, myometrium, and perimetrium. Endometrium can be further divided into the functional layer (shed/lost during menstruation) and basal layer (not lost during menstruation).
- zinc sparks following fertilization the oocyte releases a burst of zinc atoms in brief bursts (zinc sparks) has a role in zonal pellucida induced structural changes (hardening) along with ovastacin cleavage of ZP2 protein.
- zona hardening - following fertilization the structural changes that occur to the zona pellucida to prevents further spermatozoa binding acting as a block to polyspermy.
- zona pellucida - extracellular layer lying directly around the oocyte underneath follicular cells. Has an important role in egg development, fertilization and blastocyst development. This thick extracellular matrix consists of glcosaminoglycans and 3 glycoproteins (ZP1, ZP2, ZP3). (More? Zona pellucida)
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
| Cell Division Terms (expand to view) | ||
|---|---|---|
meiosis | mitosis
| ||
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 6) Embryology Oocyte Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Oocyte_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G