Neural Crest - Enteric Nervous System
| Embryology - 5 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
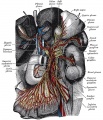
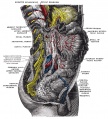
The enteric nervous system (ENS) regulates many key aspects of the gastrointestinal tract including: motility, secretion and blood flow. In the body region, neural crest cells form the entire enteric nervous system, both neurons and glia, of the gastrointestinal tract.
The neural crest are bilaterally paired strips of cells arising in the ectoderm at the margins of the neural tube. These cells migrate to many different locations and differentiate into many cell types within the embryo. This means that many different systems (neural, skin, tooth, head, face, heart, adrenal glands, gastrointestinal tract) will also have a contribution fron the neural crest cells.
Vagal neural crest cells initially migrate into the foregut splanchnic mesoderm of the developing gastrointestinal tract, these cells then migrate caudally along the gut into the midgut. A second population of sacral neural crest cells have been identified as migrating into the region of the hindgut.
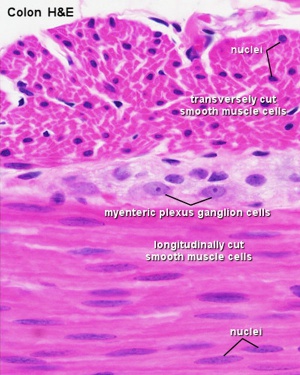
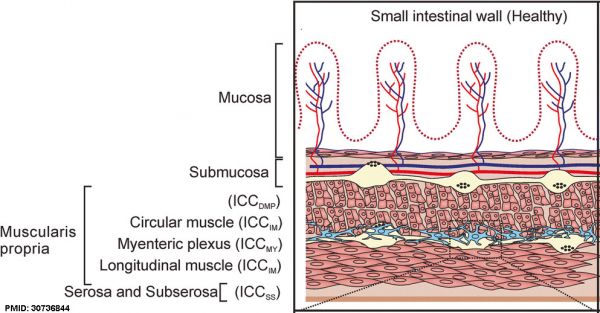
The two gastrointestinal plexuses are located between the longitudinal and circular smooth muscle layers (myenteric plexus, Auerbach's plexus) and in the submucosal layer (submucosal plexus, Meissner's plexus). Interstitial cells of Cajal (ICCs) within the myenteric plexus are pacemaker cells that control peristaltic contraction waves.
| Neural Crest Links: neural crest | Lecture - Early Neural | Lecture - Neural Crest Development | Lecture Movie | Schwann cell | adrenal | melanocyte | peripheral nervous system | enteric nervous system | cornea | cranial nerve neural crest | head | skull | cardiac neural crest | Nicole Le Douarin | Neural Crest Movies | neural crest abnormalities | Category:Neural Crest | |||
|
intestine | Gastrointestinal Tract Development
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Enteric Nervous System Development | vagal neural crest] |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Plexuses
| Myenteric plexus | Submucosal plexus |
|---|---|
| Auerbach's plexus | Meissner's plexus |
| Leopold Auerbach (1828–1897) a German anatomist and neuropathologist. | Georg Meissner (1829–1905) a German anatomist and physiologist. |
|
|
| Links: enteric nervous system | intestine | neural crest | PMID 25428846 |
Interstitial Cells of Cajal
Interstitial cells of Cajal (ICCs) are located within the gastrointestinal tract and also within the pancreas, placenta, and female reproductive tract.
Interstitial cells of Cajal (ICCs) Subtypes
- ICCdmp - deep muscular plexus region between the circular thin and thick muscle layers, only in the small intestine.
- ICCim - intramuscular located in the circular and longitudinal muscle layers, mediate motor neuronal input.
- ICCmy - myenteric plexus are the primary pacemaker cells in the small intestine, generating and propagating the electrical slow waves.
- ICCss subserosal found in the small intestine and colon (around the submucosa of the pylorus and colon).
Development Overview
This data below is a summary from a study of human enteric ganglia development[12] (ages given are gestational age GA weeks)
- week 7 - rostro-caudal neural crest cell colonization of the gut complete and differentiated into neurons and glia. Interstitial cells of Cajal (ICCs) localized in the ganglion plexus.
- foregut neurons and glia were aggregated into ganglion plexus (myenteric region) not in submucosa.
- hind gut neurons and glia are dispersed within the mesenchyme.
- week 9 - myenteric plexus, longitudinal and circular muscle layers formed along the entire gut.
- week 12 - scattered and individual neurons and glia, and small ganglion plexuses were detected in the foregut and midgut submucosa. Muscularis mucosae formed at the foregut and midgut.
- week 14 - ganglion plexus seen in the hind gut submucosa. Muscularis mucosae formed at the hindgut.
- week 20 - ICCs preferentially localized at the periphery of the plexus.
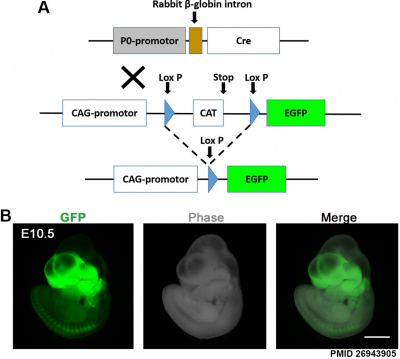
Mouse Model
Mouse enteric plexus GFP[13]
Chicken Model
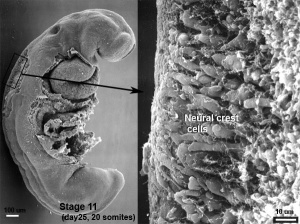
In the chicken gut, neural crest cells from both vagal (somite level 1-7) and sacral (somite level 28 and posterior) levels differentiate into the neurons and glial cells of the enteric nervous system.[14]
See also Nicole Le Douarin's research.
Historic
Auerbach's plexus
(myenteric plexus) In 1864 Auerbach first described the neural plexus lying between the longitudinal and circular smooth muscle layers of the gastrointestinal tract. The plexus has both parasympathetic and sympathetic input and is involved in the rhythmic peristaltic contractions of the gut wall. Plexus named after Leopold Auerbach (1828 – 1897) a German anatomist and neuropathologist born in Breslau.
Meissner's plexus
(submucosal plexus) Part of the enteric nervous system lying in the submucosa layer of the gastrointestinal tract is associated with mucosal secretion (secretomotor). Embryologically derived from neural crest cells. Named after Georg Meissner (1829-1905) a German histologist, physiologist and anatomist.
Neural Crest Migration
Molecular
- Impdh2 - Inosine 5′ monophosphate dehydrogenase
Abnormalities
LB16.1 Hirschsprung disease
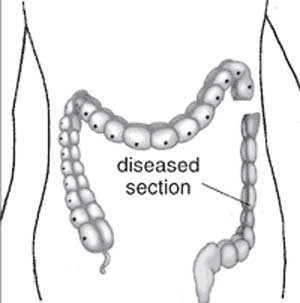
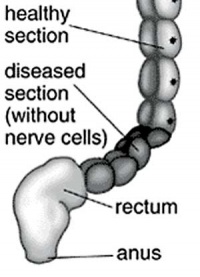
| ICD-11 LB16.1 Hirschsprung disease - This is a developmental anomaly affecting the intestinal tract characterized by congenital absence of myenteric ganglion cells (aganglionosis) in a segment of the large bowel. Due to the absence of intrinsic innervation of the muscle layers of the affected segment, there is a loss of motor function. This results in an abnormally large or dilated colon (congenital megacolon) with intestinal occlusion or constipation. This condition becomes evident shortly after birth. |
Hirschsprung disease (intestinal aganglionosis, Hirschsprung's disease, aganglionic colon, megacolon, congenital aganglionic megacolon, congenital megacolon) is a condition caused by the lack of enteric nervous system (neural ganglia) in the intestinal tract responsible for gastric motility (peristalsis). In general, its severity is dependent upon the amount of the GIT that lacks intrinsic ganglia, due to developmental lack of neural crest migration into those segments. (More? neural crest abnormalities)
Historically, Hirschsprung's disease takes its name from Dr Harald Hirschsprung (1830-1916) a Danish pediatrician (of German extraction). In 1886, he presented at the German Society of Pediatrics conference in Berlin a case of 2 infants who died of complications of bowel obstruction (H. Hirschsprung, Stuhltragheit Neugeborener in Folge von Dilatation und Hypertrophie des Colons, Jhrb f Kinderh 27 (1888), pp. 1-7). Later autopsies identified a dilatation and hypertrophy of large intestine, and the rectum appeared normally narrow. Hirschsprung suggested that the condition was an inborn disease and named it congenital megacolon.
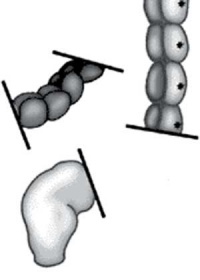
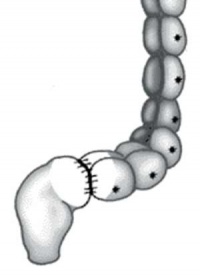
The first indication in newborns is an absence of the first bowel movement, other symptoms include throwing up and intestinal infections. Clinically this is detected by one or more tests (barium enema and x ray, manometry or biopsy) and can currently only be treated by surgery. A temoporary ostomy (Colostomy or Ileostomy) with a stoma is carried out prior to a more permanent pull-through surgery.
|

|
|
| Ostomy - Aganglionic portion removed | Stoma - intestine attached to the abdomen wall | |
|
|
|
| Short section of the colon without smooth muscle neural ganglia | Aganglionic segment removed | Reattachment |
Australian Statistics
Hirschsprung’s disease[15] (1.3 per 10,000 births) ICD-10 Q43.1
- A condition characterised by partial or complete bowel obstruction resulting from absence of peristalsis in a segment of bowel due to an aganglionic section of the bowel.
- More than two-thirds (66.7%) of the babies born with this anomaly were males.
- Women aged 40 years or older had the highest rate of affected pregnancies.
References
- ↑ Niu X, Liu L, Wang T, Chuan X, Yu Q, Du M, Gu Y & Wang L. (2020). Mapping of extrinsic innervation of the gastrointestinal tract in the mouse embryo. J. Neurosci. , , . PMID: 32690615 DOI.
- ↑ Wang XJ & Camilleri M. (2019). Hirschsprung disease: Insights on genes, penetrance, and prenatal diagnosis. Neurogastroenterol. Motil. , 31, e13732. PMID: 31609069 DOI.
- ↑ Zhang D, Rollo BN, Nagy N, Stamp L & Newgreen DF. (2019). The enteric neural crest progressively loses capacity to form enteric nervous system. Dev. Biol. , 446, 34-42. PMID: 30529057 DOI.
- ↑ Bondurand N, Dufour S & Pingault V. (2018). News from the endothelin-3/EDNRB signaling pathway: Role during enteric nervous system development and involvement in neural crest-associated disorders. Dev. Biol. , , . PMID: 30171849 DOI.
- ↑ Nagy N, Barad C, Hotta R, Bhave S, Arciero E, Dora D & Goldstein AM. (2018). Collagen 18 and agrin are secreted by enteric neural crest cells to remodel their microenvironment and regulate their migration during ENS development. Development , , . PMID: 29678817 DOI.
- ↑ Radenkovic G, Radenkovic D & Velickov A. (2018). Development of interstitial cells of Cajal in the human digestive tract as the result of reciprocal induction of mesenchymal and neural crest cells. J. Cell. Mol. Med. , 22, 778-785. PMID: 29193736 DOI.
- ↑ Uribe RA, Hong SS & Bronner ME. (2018). Retinoic acid temporally orchestrates colonization of the gut by vagal neural crest cells. Dev. Biol. , 433, 17-32. PMID: 29108781 DOI.
- ↑ Green SA, Uy BR & Bronner ME. (2017). Ancient evolutionary origin of vertebrate enteric neurons from trunk-derived neural crest. Nature , 544, 88-91. PMID: 28321127 DOI.
- ↑ Faure S, McKey J, Sagnol S & de Santa Barbara P. (2015). Enteric neural crest cells regulate vertebrate stomach patterning and differentiation. Development , 142, 331-42. PMID: 25519241 DOI.
- ↑ Jin S, Martinelli DC, Zheng X, Tessier-Lavigne M & Fan CM. (2015). Gas1 is a receptor for sonic hedgehog to repel enteric axons. Proc. Natl. Acad. Sci. U.S.A. , 112, E73-80. PMID: 25535338 DOI.
- ↑ Goldstein AM, Hofstra RM & Burns AJ. (2013). Building a brain in the gut: development of the enteric nervous system. Clin. Genet. , 83, 307-16. PMID: 23167617 DOI.
- ↑ Fu M, Tam PK, Sham MH & Lui VC. (2004). Embryonic development of the ganglion plexuses and the concentric layer structure of human gut: a topographical study. Anat. Embryol. , 208, 33-41. PMID: 14991401 DOI.
- ↑ Fujimura T, Shibata S, Shimojima N, Morikawa Y, Okano H & Kuroda T. (2016). Fluorescence Visualization of the Enteric Nervous Network in a Chemically Induced Aganglionosis Model. PLoS ONE , 11, e0150579. PMID: 26943905 DOI.
- ↑ Erickson CA & Goins TL. (2000). Sacral neural crest cell migration to the gut is dependent upon the migratory environment and not cell-autonomous migratory properties. Dev. Biol. , 219, 79-97. PMID: 10677257 DOI.
- ↑ Abeywardana S & Sullivan EA 2008. Congenital Anomalies in Australia 2002-2003. Birth anomalies series no. 3 Cat. no. PER 41. Sydney: AIHW National Perinatal Statistics Unit.
Reviews
Nagy N & Goldstein AM. (2017). Enteric nervous system development: A crest cell's journey from neural tube to colon. Semin. Cell Dev. Biol. , 66, 94-106. PMID: 28087321 DOI.
Hao MM, Bornstein JC, Vanden Berghe P, Lomax AE, Young HM & Foong JP. (2013). The emergence of neural activity and its role in the development of the enteric nervous system. Dev. Biol. , 382, 365-74. PMID: 23261929 DOI.
Obermayr F, Hotta R, Enomoto H & Young HM. (2013). Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol , 10, 43-57. PMID: 23229326 DOI.
Sasselli V, Pachnis V & Burns AJ. (2012). The enteric nervous system. Dev. Biol. , 366, 64-73. PMID: 22290331 DOI.
Articles
Musser MA & Michelle Southard-Smith E. (2013). Balancing on the crest - Evidence for disruption of the enteric ganglia via inappropriate lineage segregation and consequences for gastrointestinal function. Dev. Biol. , 382, 356-64. PMID: 23376538 DOI.
Luesma MJ, Cantarero I, Castiella T, Soriano M, Garcia-Verdugo JM & Junquera C. (2013). Enteric neurons show a primary cilium. J. Cell. Mol. Med. , 17, 147-53. PMID: 23205631 DOI.
Hao MM, Boesmans W, Van den Abbeel V, Jennings EA, Bornstein JC, Young HM & Vanden Berghe P. (2011). Early emergence of neural activity in the developing mouse enteric nervous system. J. Neurosci. , 31, 15352-61. PMID: 22031881 DOI.
Anderson RB, Newgreen DF & Young HM. (2006). Neural crest and the development of the enteric nervous system. Adv. Exp. Med. Biol. , 589, 181-96. PMID: 17076282 DOI.
Copenhaver PF. (2007). How to innervate a simple gut: familiar themes and unique aspects in the formation of the insect enteric nervous system. Dev. Dyn. , 236, 1841-64. PMID: 17420985 DOI.
Burns AJ & Douarin NM. (1998). The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development , 125, 4335-47. PMID: 9753687
Books
Anderson RB, Newgreen DF, Young HM. Neural Crest and the Development of the Enteric Nervous System. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-. Available from: http://www.ncbi.nlm.nih.gov/books/NBK6273/
Search PubMed
Search Pubmed: Enteric Neural Development | hirschprung's disease
Search All Databases: Enteric Neural Development
NCBI - Policies and Guidelines | PubMed | Help:Reference Tutorial
Additional Images
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 5) Embryology Neural Crest - Enteric Nervous System. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_Crest_-_Enteric_Nervous_System
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G