Neural - Basal Ganglia Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The basal ganglia are a group of central nervous system nuclei linked to the thalamus in the base of the brain and involved in coordination of movement. In the adult brain input from the motor cortex to the basal ganglia comes through the striatum (neostriatum), that consists of the caudate and putamen.
Neural development is one of the earliest systems to begin and the last to be completed after birth. This development generates the most complex structure within the embryo and the long time period of development means in utero insult during pregnancy may have consequences to development of the nervous system.
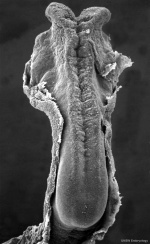
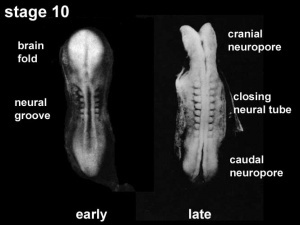
The early central nervous system begins as a simple neural plate that folds to form a groove then tube, open initially at each end. Failure of these opening to close contributes a major class of neural abnormalities (neural tube defects).
Within the neural tube stem cells generate the 2 major classes of cells that make the majority of the nervous system : neurons and glia. Both these classes of cells differentiate into many different types generated with highly specialized functions and shapes. This section covers the establishment of neural populations, the inductive influences of surrounding tissues and the sequential generation of neurons establishing the layered structure seen in the brain and spinal cord.
- Neural development beginnings quite early, therefore also look at notes covering Week 3- neural tube and Week 4-early nervous system.
- Development of the neural crest and sensory systems (hearing/vision/smell) are only introduced in these notes and are covered in other notes sections.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Basal Ganglia Development | Basal Ganglia Embryology |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page. |
Development Overview
Neuralation begins at the trilaminar embryo with formation of the notochord and somites, both of which underly the ectoderm and do not contribute to the nervous system, but are involved with patterning its initial formation. The central portion of the ectoderm then forms the neural plate that folds to form the neural tube, that will eventually form the entire central nervous system.
- Early developmental sequence: Epiblast - Ectoderm - Neural Plate - Neural groove and Neural Crest - Neural Tube and Neural Crest
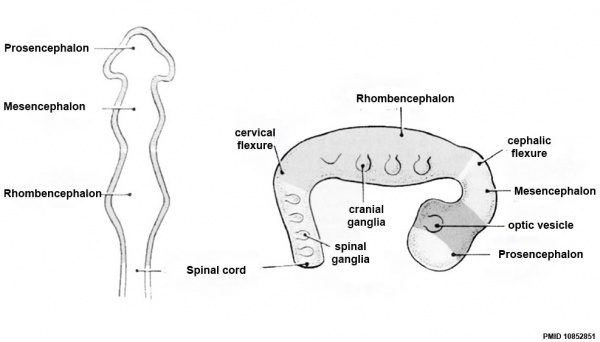
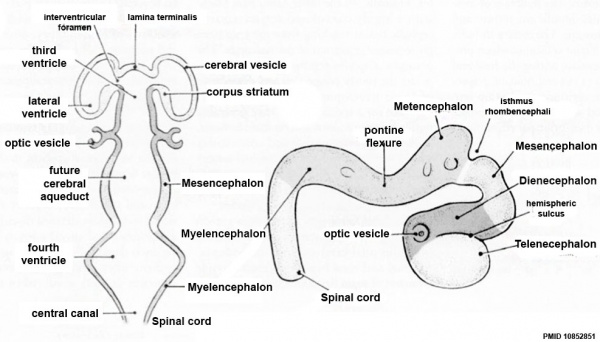
| Neural Tube | Primary Vesicles | Secondary Vesicles | Adult Structures |
|---|---|---|---|
| week 3 | week 4 | week 5 | adult |
| prosencephalon (forebrain) | telencephalon | Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles | |
| diencephalon | epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle | ||
| mesencephalon (midbrain) | mesencephalon | tectum, Cerebral peduncle, cerebral aqueduct, pons | |
| rhombencephalon (hindbrain) | metencephalon | cerebellum | |
| myelencephalon | medulla oblongata, isthmus | ||
| spinal cord, pyramidal decussation, central canal | |||
Early Brain Vesicles
Primary Vesicles
Secondary Vesicles
Adult
| Basal ganglia frontal lobe connectivities for motor cognitive interaction[3] | |
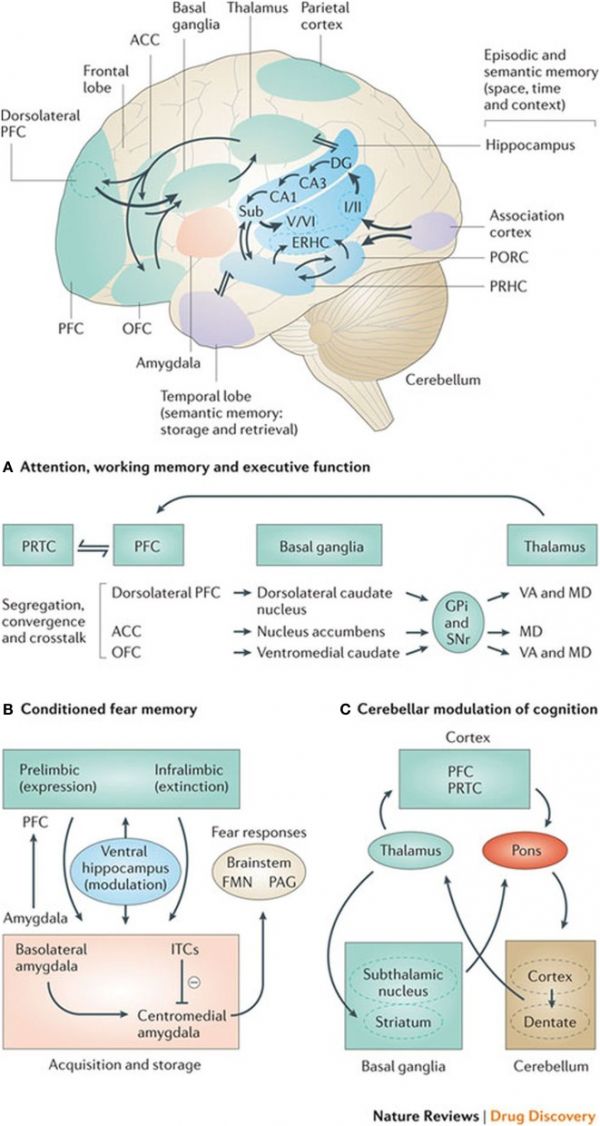
|
All regions of the cerebral cortex project to the basal ganglia, but the output of the basal ganglia are directed toward the frontal lobe, particularly the premotor and supplementary motor cortex with specific connectivities of the basal ganglia for (A) attention, working memory, and executive function (B) conditioned fear memory and (C) cerebellar and basal ganglia modulation of cognition.
(text from figure legend) |
References
- ↑ Grillner S & Robertson B. (2016). The Basal Ganglia Over 500 Million Years. Curr. Biol. , 26, R1088-R1100. PMID: 27780050 DOI.
- ↑ Pyrgaki C, Trainor P, Hadjantonakis AK & Niswander L. (2010). Dynamic imaging of mammalian neural tube closure. Dev. Biol. , 344, 941-7. PMID: 20558153 DOI.
- ↑ Leisman G, Braun-Benjamin O & Melillo R. (2014). Cognitive-motor interactions of the basal ganglia in development. Front Syst Neurosci , 8, 16. PMID: 24592214 DOI.
Reviews
Grillner S, von Twickel A & Robertson B. (2018). The blueprint of the vertebrate forebrain - With special reference to the habenulae. Semin. Cell Dev. Biol. , 78, 103-106. PMID: 29107476 DOI.
Greene ND & Copp AJ. (2009). Development of the vertebrate central nervous system: formation of the neural tube. Prenat. Diagn. , 29, 303-11. PMID: 19206138 DOI.
Moreno N, González A & Rétaux S. (2009). Development and evolution of the subpallium. Semin. Cell Dev. Biol. , 20, 735-43. PMID: 19374952 DOI.
Mazurová Y, Rudolf E, Látr I & Osterreicher J. (2006). Proliferation and differentiation of adult endogenous neural stem cells in response to neurodegenerative process within the striatum. Neurodegener Dis , 3, 12-8. PMID: 16909031 DOI.
Ulfig N, Setzer M & Bohl J. (2003). Ontogeny of the human amygdala. Ann. N. Y. Acad. Sci. , 985, 22-33. PMID: 12724145
Hamasaki T, Goto S, Nishikawa S & Ushio Y. (2003). Neuronal cell migration for the developmental formation of the mammalian striatum. Brain Res. Brain Res. Rev. , 41, 1-12. PMID: 12505644
Ulfig N. (2002). Ganglionic eminence of the human fetal brain--new vistas. Anat. Rec. , 267, 191-5. PMID: 12115267 DOI.
Jain M, Armstrong RJ, Barker RA & Rosser AE. (2001). Cellular and molecular aspects of striatal development. Brain Res. Bull. , 55, 533-40. PMID: 11543954
Articles
Milardi D, Quartarone A, Bramanti A, Anastasi G, Bertino S, Basile GA, Buonasera P, Pilone G, Celeste G, Rizzo G, Bruschetta D & Cacciola A. (2019). The Cortico-Basal Ganglia-Cerebellar Network: Past, Present and Future Perspectives. Front Syst Neurosci , 13, 61. PMID: 31736719 DOI.
Saitsu H & Shiota K. (2008). Involvement of the axially condensed tail bud mesenchyme in normal and abnormal human posterior neural tube development. Congenit Anom (Kyoto) , 48, 1-6. PMID: 18230116 DOI.
Search PubMed
Search Pubmed: Basal Ganglia Embryology | Basal Ganglia Development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- University of Wisconsin–Madison Basal Ganglia
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Neural - Basal Ganglia Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Basal_Ganglia_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G