Musculoskeletal System - Muscle Development
| Embryology - 3 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
There are 3 main different types of muscle: skeletal, cardiac and smooth. This page describes skeletal muscle development, descriptions of cardiac muscle and smooth muscle development can be found in other notes. Skeletal muscle forms by fusion of mononucleated myoblasts to form mutinucleated myotubes.
- There are more than 640 skeletal muscles in the adult human body.
Differentiation/determination of mesoderm into muscle cells is thought to involve a family of basic Helix-Loop-Helix transcription factors, the first of which discovered was MyoD1. MyoD1 needs to form a dimer to be active and is maintained in an inactive state by binding of an inhibitor, Id.
Specific Skeletal Muscles: tongue | diaphragm
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Muscle Development | Skeletal Muscle Development | Myogenesis |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Myogenesis
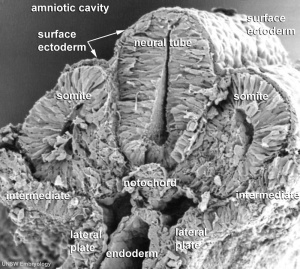
Three different types of muscle form in the body.
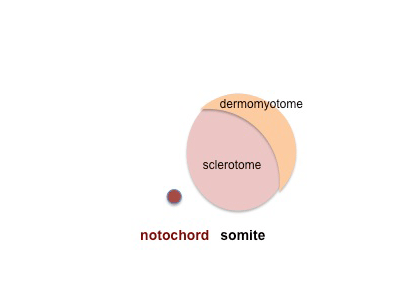
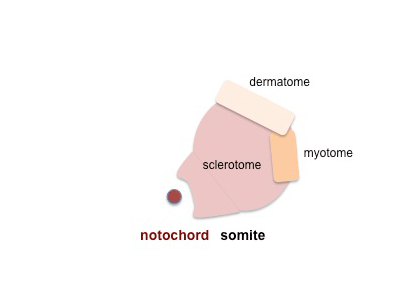
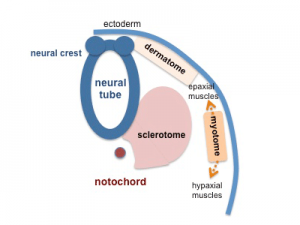
- Skeletal muscle - cells originate from the paraxial mesoderm, forming somites, then dermamyotome and finally the myotome. Myoblasts undergo frequent divisions and coalesce with the formation of a multinucleated, syncytial muscle fibre or myotube. The nuclei of the myotube are still located centrally in the muscle fibre. In the course of the synthesis of the myofilaments/myofibrils, the nuclei are gradually displaced to the periphery of the cell.
- Cardiac muscle - cells originate from the prechordal splanchnic mesoderm.
- Smooth muscle - cells originate from undifferentiated mesenchymal cells. These cells differentiate first into mitotically active cells, myoblasts, which contain a few myofilaments. Myoblasts give rise to the cells which will differentiate into mature smooth muscle cells.
Muscle Groups
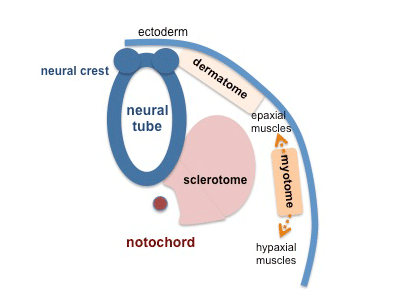
In humans, body muscles lying dorsal to the vertebral column form the epaxial muscles. The body muscles lying ventral (anterior) to the vertebral column form the hypaxial muscles.
Epaxial Muscle
Anatomical term describing skeletal muscles which lie dorsal (posterior) to the vertebral column developing from the somite. Epaxial muscles are only a small muscle group formed by the transversospinalis, longissimus, and iliocostalis muscles. At the ribcage level, the levatores costarum muscles (transverse processes of C7 to T11 vertebrae) are also involved with rib elevation during respiration.
A recent study has determined the developmental sequence of epaxial muscles in the human embryo between week 5 to 10 (see summary below).[11]
- Carnegie stage 15 - epaxial portions of myotomes become identifiable laterally to developing vertebrae.
- Carnegie stage 16 - epaxial portions fusion starting cranially to form a longitudinal muscle column,
- become innervated by spinal nerve dorsal branches.
- Carnegie stage 17 - longitudinal muscle mass segregated into medial and lateral columns (completed at CS18).
- Carnegie stage 18 - medial column segregated again into intermediate and medial columns (completed at CS20).
- lateral and intermediate columns did not separate in the lower lumbar and sacral regions.
- Carnegie stage 20 to Carnegie stage 23 - cervical portions of the three columns segregated again from lateral to medial resulting ventrolaterally in rod-like continuations of the caudal portions of the columns and dorsomedially in spade-like portions.
- lateral column - iliocostalis and splenius
- intermediate column - longissimus
- medial column - (semi-)spinalis
- Early fetal - medial (multifidus) group gain the transversospinal course during vertebral arch closure.
Hypaxial Muscle
(hypomere) Anatomical term describing skeletal muscles which lie ventral (anterior) to the vertebral column developing from the somite myotome. These muscles contribute both body (trunk) and limb skeletal muscle.
- In the trunk, these form the three anterior body muscle layers.
- In the limb, these form the extensor and flexor muscle groups.
Ventral body wall timeline[3]
- Carnegie stage 15 - hypaxial flank muscle primordium laterally in the body wall.
- Carnegie stage 18 - separate intercostal and abdominal wall muscles differentiated.
- Carnegie stage 20 - muscles expand ventromedially and caudally bilateral sternal bars fusing in the midline
- Carnegie stage 23 - rectus muscles reach the umbilicus
- 6 and 10 weeks - dorsal body wall growth closes the ventral body wall.
Head Muscle
- jaw associated muscles mainly from cranial mesoderm.
- jaw, connective tissues and tendons from neural crest cells.
Head muscle precursor myoblast summary from a review.[12]
- myoblasts for the tongue muscle, migrate like those seen in the limb.
- myoblasts for extraocular muscles, condense within paraxial mesoderm, then cross the mesoderm:neural crest interface en route to periocular regions.
- myoblasts for branchial muscle, establish contacts with neural crest populations before branchial arch formation and maintain these relations through subsequent stages of development.
See also for head muscle and connective tissue.[13]
Skeletal Muscle Stages
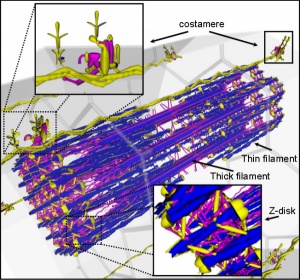
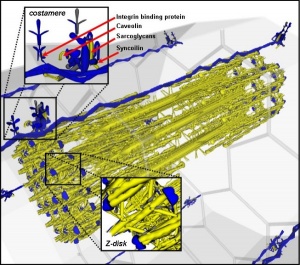
Myoblast - individual progenitor cells
Myotube - multinucleated, but undifferentiated contractile apparatus (sarcomere)
Myofibre (myofiber, muscle cell) - multinucleated and differentiated sarcomeres
- primary myofibres - first-formed myofibres, act as a structural framework upon which myoblasts proliferate, fuse in linear sequence
- secondary myofibers - second later population of myofibres that form surrounding the primary fibres.
Muscle Fibre Types
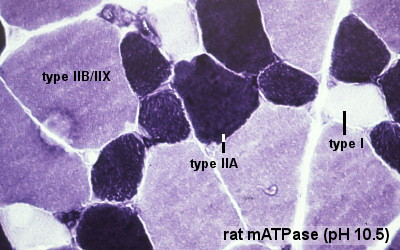
Human skeletal muscle generally consists of individual fibres with different contractile and other properties, this is the basis of classification into "types".
- type IIB, IIA, IIX, and I fibres - based only on the myosin ATPase activity.
- Type I fibres appear red, due to the presence of myoglobin.
- Type II fibres appear white, due to the absence of myoglobin and their glycolytic nature.
- A group of individual myofibres within a muscle will be innervated by a single motor neuron (motor unit).
- The electrical properties of the motor neuron will regulate the contractile properties of all associated myofibres.
| Fibre Type | Type I fibres | Type II a fibres | Type II x fibres | Type II b fibres |
|---|---|---|---|---|
| Contraction time | Slow | Moderately Fast | Fast | Very fast |
| Size of motor neuron | Small | Medium | Large | Very large |
| Resistance to fatigue | High | Fairly high | Intermediate | Low |
| Activity Used for | Aerobic | Long-term anaerobic | Short-term anaerobic | Short-term anaerobic |
| Maximum duration of use | Hours | <30 minutes | <5 minutes | <1 minute |
| Power produced | Low | Medium | High | Very high |
| Mitochondrial density | High | High | Medium | Low |
| Capillary density | High | Intermediate | Low | Low |
| Oxidative capacity | High | High | Intermediate | Low |
| Glycolytic capacity | Low | High | High | High |
| Major storage fuel | Triglycerides | Creatine phosphate, glycogen | Creatine phosphate, glycogen | Creatine phosphate, glycogen |
| Myosin heavy chain, human genes |
MYH7 | MYH2 | MYH1 | MYH4 |
| Muscle Fibre Type | ||||
|---|---|---|---|---|
| Fibre Type | Type I fibres | Type II a fibres | Type II x fibres | Type II b fibres |
| Contraction time | Slow | Moderately Fast | Fast | Very fast |
| Size of motor neuron | Small | Medium | Large | Very large |
| Resistance to fatigue | High | Fairly high | Intermediate | Low |
| Activity Used for | Aerobic | Long-term anaerobic | Short-term anaerobic | Short-term anaerobic |
| Maximum duration of use | Hours | <30 minutes | <5 minutes | <1 minute |
| Power produced | Low | Medium | High | Very high |
| Mitochondrial density | High | High | Medium | Low |
| Capillary density | High | Intermediate | Low | Low |
| Oxidative capacity | High | High | Intermediate | Low |
| Glycolytic capacity | Low | High | High | High |
| Major storage fuel | Triglycerides | Creatine phosphate, glycogen | Creatine phosphate, glycogen | Creatine phosphate, glycogen |
| Myosin heavy chain, human genes |
MYH7 | MYH2 | MYH1 | MYH4 |
| Links: Muscle Development | Muscle Development Timeline | ||||
Muscle Contraction
Individual myoblasts in the developing muscle bed initial fuse together to form multi-nucleated myotubes. These myotubes then express the contractile proteins, that are organized into sarcomeres in series along the length of the myotube.
This animation shows the molecular interactions that occur within the skeletal muscle sarcomere between actin and myosin during skeletal muscle contraction.
|
Legend
|
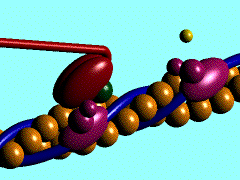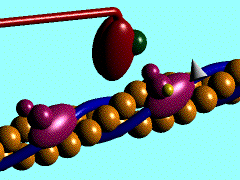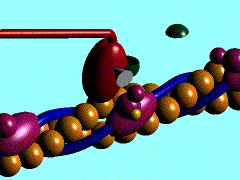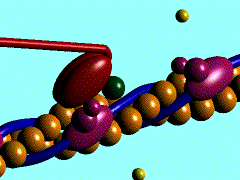
|
Adult Myotome
In both development and the adult, the group of skeletal muscles supplied by a specific segmental spinal nerve is also referred to as a "myotome". The muscle arises from a specific somite and the spinal nerve arises from a specific level of the spinal cord (identified by veretebral column).
In humans this corresponds to the following spinal nerves (from top to bottom) and muscular functions:
| Spinal Nerve | Acronym | Innervation |
|---|---|---|
| Cervical spinal nerve 3 | C3 | supply the diaphragm for breathing. |
| Cervical spinal nerve 4 | C4 | |
| Cervical spinal nerve 5 | C5 | supply the diaphragm for breathing and supply shoulder muscles and muscles to bend our elbow. |
| Cervical spinal nerve 6 | C6 | for bending the wrist back. |
| Cervical spinal nerve 7 | C7 | for straightening the elbow. |
| Cervical spinal nerve 8 | C8 | bends the fingers. |
| Thoracic spinal nerve 1 | T1 | spreads the fingers and supplies the chest wall and abdominal muscles. |
| Thoracic spinal nerve 2 | T2 | supplies the chest wall and abdominal muscles. |
| Thoracic spinal nerve 3 | T3 | |
| Thoracic spinal nerve 4 | T4 | |
| Thoracic spinal nerve 5 | T5 | |
| Thoracic spinal nerve 6 | T6 | |
| Thoracic spinal nerve 7 | T7 | |
| Thoracic spinal nerve 8 | T8 | |
| Thoracic spinal nerve 9 | T9 | |
| Thoracic spinal nerve 10 | T10 | |
| Thoracic spinal nerve 11 | T11 | |
| Thoracic spinal nerve 12 | T12 | |
| Lumbar spinal nerve 1 | L1 | |
| Lumbar spinal nerve 2 | L2 | bends the hip. |
| Lumbar spinal nerve 3 | L3 | straightens the knee. |
| Lumbar spinal nerve 4 | L4 | pulls the foot up. |
| Lumbar spinal nerve 5 | L5 | wiggles the toes. |
| Sacral spinal nerve 1 | S1 | pulls the foot down. |
| Sacral spinal nerve 2 | S2 | |
| Sacral spinal nerve 3 | S3 | supply the bladder, bowel, sex organs, anal and other pelvic muscles. |
| Sacral spinal nerve 4 | S4 | |
| Sacral spinal nerve 5 | S5 | |
| Links: Spinal Nerve Myotomes | Spinal Nerve Dermatomes | skeletal muscle | ||
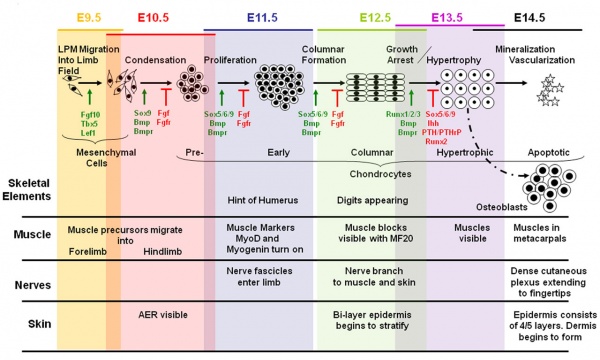
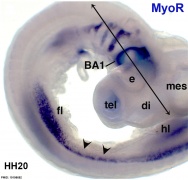
Mouse Limb Muscle
Change in cell types and tissue formation as a function of mouse developmental stage.{#pmid:22174793|PMID22174793}}
- Links: mouse
Histology Images
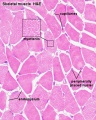
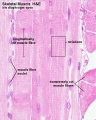
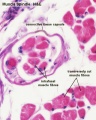
- Muscle Histology: Muscle Development | Human HE x4 longitudinal and transverse | Human HE x40 transverse | Human HE x40 longitudinal | Human HE x40 longitudinal | Human HE x4 longitudinal and transverse | Muscle Spindle HE x40 | Human HE x40 | Human HE x40 | Human HE x40 | Human HE x100 | Human HE x100 | Fetal human muscle | Myotendinous junction label | Myotendinous junction HE x40 | Whipf 1 | Whipf 2 | Whipf 3 | Tongue HE x10 transverse | Tongue x100 | Muscle spindle HE x20 | Muscle spindle HE x40
Electron Microscopy Virtual Slides
Electron micrographs below are thin longitudinal section cut through adult human skeletal muscle tissue.
|
|
| |||||||||
|
|
Image Source: Contributed by Dartmouth College Electron Microscope Facility special thanks to Chuck Daghlian and Louisa Howard. Gallery. Original images may have been altered in size contrast and labelling. (These images are in the public domain)
Puberty
- Musculoskeletal mass doubles by the end of puberty
- regulated growth by - sex steroid hormones, growth hormone, insulin-like growth factors
- accumulation of (peak) bone mass during puberty relates to future osteoporosis in old age
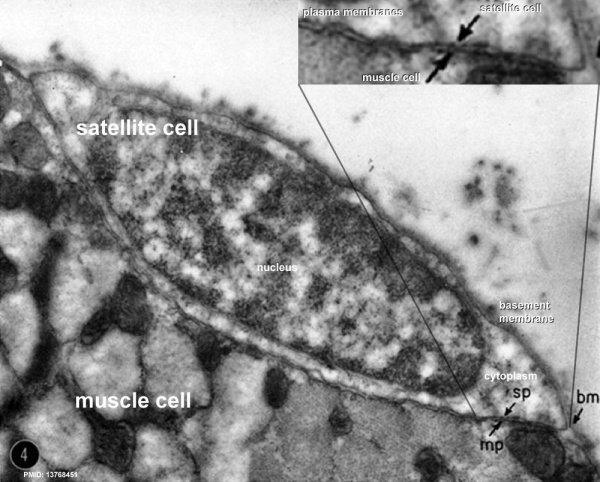
Satellite Cells
These cells remain as muscle stem cells under the basal lamina around each skeletal muscle fibre. They have a role in postnatal growth and also regeneration of muscle fibres. (see review[15])
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Satellite Cell <pubmed limit=5>Satellite Cell</pubmed> |
- Links: stem cells
Abnormalities
There can be abnormalities associated directly with muscle differentiation and function as well as those mediated indirectly by abnormalities of innervation or skeletal development and other associated systems.
Duchenne Muscular Dystrophy
The most common occuring in Boys and in Duchenne Muscular Dystrophy (DMD). This cause of the disease was discovered in 1988 as a mutation in dystrophin, a protein that lies under the muscle fiber membrane and maintains the cell's integrity. As skeletal muscles have little prenatal load or use it is not until postnatally that muscle wasting occurs, usually in the anti-gravity muscles first. This is a progressive disease usually detected between 3-5 years old.
- X-linked dystrophy
- large gene encoding cytoskeletal protein - Dystrophin
- progressive wasting of muscle, die late teens
Becker Muscular Dystrophy
(BMD) Similar to DMD but allows muscles to function better than in DMD, slower progression, make a shortened form of the mutated protein. Named after Peter Emil Becker, a German doctor who first described this variant in the 1950s.[16]
Autosomal Recessive Muscular Dystrophy
Dystroglycan, a protein that associates with both dystrophin and membrane molecules, is a candidate gene for the site of the mutation in autosomal recessive muscular dystrophies. A knockout mouse has been generated that has early developmental abnormalities.
Myotonic Dystrophy
An inherited disorder in which the muscles contract but have decreasing power to relax. With this condition, the muscles also become weak and waste away. The myotonic dystrophy gene, found on chromosome 19, codes for a protein kinase that is found in skeletal muscle, where it likely plays a regulatory role. The disease is "amplified" through generations probably by a similar GC expansion associated with Huntington disease.
Facioscapulohumeral muscular dystrophy (FSHD)
- characterized by the progressive weakness and atrophy of a specific subset of skeletal muscles.
- mostly affects the muscles of the face, scapula, and upper arms.
- involvement of specific muscles that it is often used clinically to distinguish FSHD from other forms of muscular dystrophy.
References
- ↑ Warmbrunn MV, de Bakker BS, Hagoort J, Alefs-de Bakker PB & Oostra RJ. (2018). Hitherto unknown detailed muscle anatomy in an 8-week-old embryo. J. Anat. , , . PMID: 29726018 DOI.
- ↑ Chal J & Pourquié O. (2017). Making muscle: skeletal myogenesis in vivo and in vitro. Development , 144, 2104-2122. PMID: 28634270 DOI.
- ↑ 3.0 3.1 Mekonen HK, Hikspoors JP, Mommen G, Köhler SE & Lamers WH. (2015). Development of the ventral body wall in the human embryo. J. Anat. , 227, 673-85. PMID: 26467243 DOI.
- ↑ Lee KY, Singh MK, Ussar S, Wetzel P, Hirshman MF, Goodyear LJ, Kispert A & Kahn CR. (2015). Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism. Nat Commun , 6, 8054. PMID: 26299309 DOI.
- ↑ Mayeuf-Louchart A, Lagha M, Danckaert A, Rocancourt D, Relaix F, Vincent SD & Buckingham M. (2014). Notch regulation of myogenic versus endothelial fates of cells that migrate from the somite to the limb. Proc. Natl. Acad. Sci. U.S.A. , 111, 8844-9. PMID: 24927569 DOI.
- ↑ Powell GT & Wright GJ. (2011). Jamb and jamc are essential for vertebrate myocyte fusion. PLoS Biol. , 9, e1001216. PMID: 22180726 DOI.
- ↑ Murphy M & Kardon G. (2011). Origin of vertebrate limb muscle: the role of progenitor and myoblast populations. Curr. Top. Dev. Biol. , 96, 1-32. PMID: 21621065 DOI.
- ↑ Tao Y, Neppl RL, Huang ZP, Chen J, Tang RH, Cao R, Zhang Y, Jin SW & Wang DZ. (2011). The histone methyltransferase Set7/9 promotes myoblast differentiation and myofibril assembly. J. Cell Biol. , 194, 551-65. PMID: 21859860 DOI.
- ↑ Ropka-Molik K, Eckert R & Piórkowska K. (2011). The expression pattern of myogenic regulatory factors MyoD, Myf6 and Pax7 in postnatal porcine skeletal muscles. Gene Expr. Patterns , 11, 79-83. PMID: 20888930 DOI.
- ↑ Mei H, Ho MK, Yung LY, Wu Z, Ip NY & Wong YH. (2011). Expression of Gα(z) in C2C12 cells restrains myogenic differentiation. Cell. Signal. , 23, 389-97. PMID: 20946953 DOI.
- ↑ Mekonen HK, Hikspoors JP, Mommen G, Eleonore KÖhler S & Lamers WH. (2016). Development of the epaxial muscles in the human embryo. Clin Anat , 29, 1031-1045. PMID: 27571325 DOI.
- ↑ Noden DM & Francis-West P. (2006). The differentiation and morphogenesis of craniofacial muscles. Dev. Dyn. , 235, 1194-218. PMID: 16502415 DOI.
- ↑ Grenier J, Teillet MA, Grifone R, Kelly RG & Duprez D. (2009). Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE , 4, e4381. PMID: 19198652 DOI.
- ↑ 14.0 14.1 Waardenberg AJ, Reverter A, Wells CA & Dalrymple BP. (2008). Using a 3D virtual muscle model to link gene expression changes during myogenesis to protein spatial location in muscle. BMC Syst Biol , 2, 88. PMID: 18945372 DOI.
- ↑ Baghdadi MB & Tajbakhsh S. (2018). Regulation and phylogeny of skeletal muscle regeneration. Dev. Biol. , 433, 200-209. PMID: 28811217 DOI.
- ↑ BECKER PE & KIENER F. (1955). [A new x-chromosomal muscular dystrophy]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr , 193, 427-48. PMID: 13249581
Reviews
Baghdadi MB & Tajbakhsh S. (2018). Regulation and phylogeny of skeletal muscle regeneration. Dev. Biol. , 433, 200-209. PMID: 28811217 DOI.
Michailovici I, Eigler T & Tzahor E. (2015). Craniofacial Muscle Development. Curr. Top. Dev. Biol. , 115, 3-30. PMID: 26589919 DOI.
Abmayr SM & Pavlath GK. (2012). Myoblast fusion: lessons from flies and mice. Development , 139, 641-56. PMID: 22274696 DOI.
Murphy M & Kardon G. (2011). Origin of vertebrate limb muscle: the role of progenitor and myoblast populations. Curr. Top. Dev. Biol. , 96, 1-32. PMID: 21621065 DOI.
Mok GF & Sweetman D. (2011). Many routes to the same destination: lessons from skeletal muscle development. Reproduction , 141, 301-12. PMID: 21183656 DOI.
Albini S & Puri PL. (2010). SWI/SNF complexes, chromatin remodeling and skeletal myogenesis: it's time to exchange!. Exp. Cell Res. , 316, 3073-80. PMID: 20553711 DOI.
Buckingham M & Vincent SD. (2009). Distinct and dynamic myogenic populations in the vertebrate embryo. Curr. Opin. Genet. Dev. , 19, 444-53. PMID: 19762225 DOI.
Cossu G & Biressi S. (2005). Satellite cells, myoblasts and other occasional myogenic progenitors: possible origin, phenotypic features and role in muscle regeneration. Semin. Cell Dev. Biol. , 16, 623-31. PMID: 16118057 DOI.
Articles
Wang S, Zhang B, Addicks GC, Zhang H, J Menzies K & Zhang H. (2018). Muscle Stem Cell Immunostaining. Curr Protoc Mouse Biol , 8, e47. PMID: 30106515 DOI.
Tzahor E. (2015). Head muscle development. Results Probl Cell Differ , 56, 123-42. PMID: 25344669 DOI.
Tao Y, Neppl RL, Huang ZP, Chen J, Tang RH, Cao R, Zhang Y, Jin SW & Wang DZ. (2011). The histone methyltransferase Set7/9 promotes myoblast differentiation and myofibril assembly. J. Cell Biol. , 194, 551-65. PMID: 21859860 DOI.
Philipot O, Joliot V, Ait-Mohamed O, Pellentz C, Robin P, Fritsch L & Ait-Si-Ali S. (2010). The core binding factor CBF negatively regulates skeletal muscle terminal differentiation. PLoS ONE , 5, e9425. PMID: 20195544 DOI.
Bhatnagar S, Kumar A, Makonchuk DY, Li H & Kumar A. (2010). Transforming growth factor-beta-activated kinase 1 is an essential regulator of myogenic differentiation. J. Biol. Chem. , 285, 6401-11. PMID: 20037161 DOI.
Grenier J, Teillet MA, Grifone R, Kelly RG & Duprez D. (2009). Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE , 4, e4381. PMID: 19198652 DOI.
Search PubMed
June 2010 " Skeletal Muscle Development" All (19316) Review (2515) Free Full Text (5587)
Search Pubmed: Skeletal Muscle Development
Additional Images
- Muscle Images
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 3) Embryology Musculoskeletal System - Muscle Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Musculoskeletal_System_-_Muscle_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G