Introduction
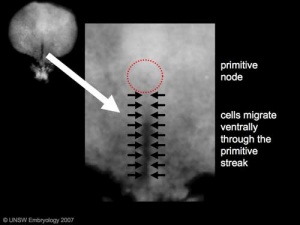
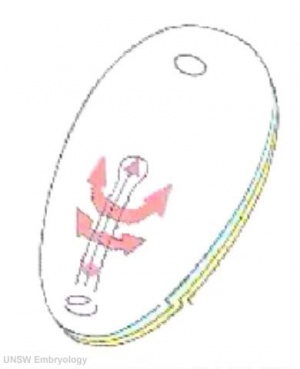
Human embryo primitive streak
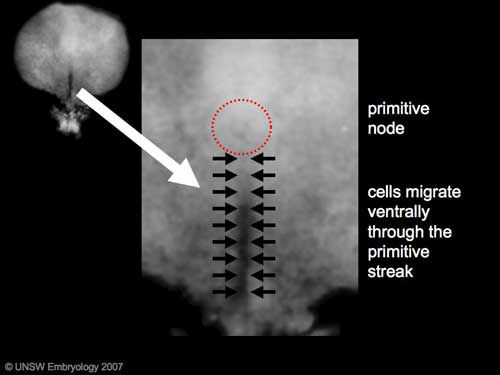
The term gastrulation means the formation of gut (Greek, gastrula = belly), but has now a more broad sense to to describe the formation of the trilaminar embryo. The epiblast layer, consisting of totipotential cells, derives all 3 embryo layers: ectoderm, mesoderm and endoderm. The primitive streak is the visible feature which represents the site of cell migration to form the additional layers.
- primitive node - region in the middle of the early embryonic disc epiblast from which the primitive streak extends caudally (tail)
- nodal cilia establish the embryo left/right axis
- axial process extends from the nodal epiblast
- primitive streak - region of cell migration from the epiblast layer forming sequentially the two germ cell layers (endoderm and mesoderm)
| Historic Embryology
|
|
Hans Spemann (1869 - 1941) identified this region in amphibia, also called the "Spemann's organiser". The same region in birds it is known as "Hensen's node" named for Victor Hensen (1835 – 1924) and is also known generally as the primitive node or knot. In humans, it is proposed that similar mechanisms regulate gastrulation to those found in other vertebrates. Currently, the molecular and physical mechanisms that regulate patterning and migration during this key event are being investigated in several different animal models.
|
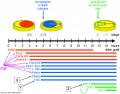
Signaling Pathway: BMP4 -> Wnt -> Activin–Nodal
| Human BMP Family
|
| Table - Human Bmp Family
|
Approved
Symbol
|
Approved Name
|
Previous
Symbols
|
Synonyms
|
Chromosome
|
| BMP1 |
bone morphogenetic protein 1 |
PCOLC |
|
8p21.3
|
| BMP2 |
bone morphogenetic protein 2 |
BMP2A |
|
20p12.3
|
| BMP3 |
bone morphogenetic protein 3 |
|
|
4q21.21
|
| BMP4 |
bone morphogenetic protein 4 |
BMP2B |
|
14q22.2
|
| BMP5 |
bone morphogenetic protein 5 |
|
|
6p12.1
|
| BMP6 |
bone morphogenetic protein 6 |
VGR |
VGR1 |
6p24.3
|
| BMP7 |
bone morphogenetic protein 7 |
|
OP-1 |
20q13.31
|
| BMP8A |
bone morphogenetic protein 8a |
|
|
1p34.3
|
| BMP8B |
bone morphogenetic protein 8b |
BMP8 |
OP-2 |
1p34.2
|
| BMP10 |
bone morphogenetic protein 10 |
|
|
2p13.3
|
| BMP15 |
bone morphogenetic protein 15 |
|
GDF9B |
Xp11.22
|
| Links: Developmental Signals - Bone Morphogenetic Protein | OMIM BMP2 | HGNC | Bmp Family | Sox Family | Tbx Family
|
|
|
|
Inhibitors - Wnt inhibitor IWP2; Nodal inhibitor (SB431542 drug TGF-beta/Smad inhibitor)
Epithelial to Mesenchymal Transition
Epithelial cells (organised cellular layer) which loose their organisation and migrate/proliferate as a mesenchymal cells (disorganised cellular layers) are said to have undergone an Epithelial Mesenchymal Transition (EMT). Mesenchymal cells have an embryonic connective tissue-like cellular arrangement, that have undergone this process may at a later time and under specific signaling conditions undergo the opposite process, mesenchyme to epithelia. In development, this process can be repeated several times during tissue differentiation.
This process occurs at the primitive streak where epiblast cells undergo an epithelial to mesenchymal transition in order to delaminate and migrate.
Some Recent Findings
- Single-cell transcriptomic characterization of a gastrulating human embryo[1] "Here we characterize in a spatially resolved manner the single-cell transcriptional profile of an entire gastrulating human embryo, staged to be between 16 and 19 days after fertilization. We use these data to analyse the cell types present and to make comparisons with other model systems. In addition to pluripotent epiblast, we identified primordial germ cells, red blood cells and various mesodermal and endodermal cell types."
- The Physical Mechanisms of Drosophila Gastrulation: mesoderm and endoderm Invagination[2] "A critical juncture in early development is the partitioning of cells that will adopt different fates into three germ layers: the ectoderm, the mesoderm, and the endoderm. This step is achieved through the internalization of specified cells from the outermost surface layer, through a process called gastrulation. In Drosophila, gastrulation is achieved through cell shape changes (i.e., apical constriction) that change tissue curvature and lead to the folding of a surface epithelium. Folding of embryonic tissue results in mesoderm and endoderm invagination, not as individual cells, but as collective tissue units. The tractability of Drosophila as a model system is best exemplified by how much we know about Drosophila gastrulation, from the signals that pattern the embryo to the molecular components that generate force, and how these components are organized to promote cell and tissue shape changes. For mesoderm invagination, graded signaling by the morphogen, Spätzle, sets up a gradient in transcriptional activity that leads to the expression of a secreted ligand (Folded gastrulation) and a transmembrane protein (T48). Together with the GPCR Mist, which is expressed in the mesoderm, and the GPCR Smog, which is expressed uniformly, these signals activate heterotrimeric G-protein and small Rho-family G-protein signaling to promote apical contractility and changes in cell and tissue shape. A notable feature of this signaling pathway is its intricate organization in both space and time. At the cellular level, signaling components and the cytoskeleton exhibit striking polarity, not only along the apical-basal cell axis, but also within the apical domain. Furthermore, gene expression controls a highly choreographed chain of events, the dynamics of which are critical for primordium invagination; it does not simply throw the cytoskeletal "on" switch." fly
- A single-cell molecular map of mouse gastrulation and early organogenesis[3] "Across the animal kingdom, gastrulation represents a key developmental event during which embryonic pluripotent cells diversify into lineage-specific precursors that will generate the adult organism. Here we report the transcriptional profiles of 116,312 single cells from mouse embryos collected at nine sequential time points ranging from E6.5 to E8.5 days post-fertilization. We construct a molecular map of cellular differentiation from pluripotency towards all major embryonic lineages, and explore the complex events involved in the convergence of visceral and primitive streak-derived endoderm. Furthermore, we use single-cell profiling to show that Tal1-/- chimeric embryos display defects in early mesoderm diversification, and we thus demonstrate how combining temporal and transcriptional information can illuminate gene function." (See Nature 11 Dec)
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Gastrulation
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- Self-organization of a human organizer by combined Wnt and Nodal signalling[4] "In amniotes, the development of the primitive streak and its accompanying 'organizer' define the first stages of gastrulation. Although these structures have been characterized in detail in model organisms, the human primitive streak and organizer remain a mystery. When stimulated with BMP4, micropatterned colonies of human embryonic stem cells self-organize to generate early embryonic germ layers 1 . Here we show that, in the same type of colonies, Wnt signalling is sufficient to induce a primitive streak, and stimulation with Wnt and Activin is sufficient to induce an organizer, as characterized by embryo-like sharp boundary formation, markers of epithelial mesenchymal transition and expression of the organizer-specific transcription factor GSC. Moreover, when grafted into chick embryos, human stem cell colonies treated with Wnt and Activin induce and contribute autonomously to a secondary axis while inducing a neural fate in the host."
- Molecular regulation of Nodal signaling during mesendoderm formation[5] "One of the most important events during vertebrate embryogenesis is the formation or specification of the three germ layers, endoderm, mesoderm, and ectoderm. After a series of rapid cleavages, embryos form the mesendoderm and ectoderm during late blastulation and early gastrulation. The mesendoderm then further differentiates into the mesoderm and endoderm. Nodal, a member of the transforming growth factor β (TGF-beta, TGF-β) superfamily, plays a pivotal role in mesendoderm formation by regulating the expression of a number of critical transcription factors, including Mix-like, GATA, Sox, and Fox. Because the Nodal signal transduction pathway is well-characterized, increasing effort has been made to delineate the spatiotemporal modulation of Nodal signaling during embryonic development. In this review, we summarize the recent progress delineating molecular regulation of Nodal signal intensity and duration during mesendoderm formation." TGF-beta
- BMP and FGF signaling interact to pattern mesoderm by controlling basic helix-loop-helix transcription factor activity[6] "The mesodermal germ layer is patterned into mediolateral subtypes by signaling factors including BMP and FGF. How these pathways are integrated to induce specific mediolateral cell fates is not well understood. We used mesoderm derived from post-gastrulation neuromesodermal progenitors (NMPs), which undergo a binary mediolateral patterning decision, as a simplified model to understand how FGF acts together with BMP to impart mediolateral fate. Using zebrafish and mouse NMPs, we identify an evolutionarily conserved mechanism of BMP and FGF mediated mediolateral mesodermal patterning that occurs through modulation of basic helix-loop-helix (bHLH) transcription factor activity. BMP imparts lateral fate through induction of Id helix loop helix (HLH) proteins, which antagonize bHLH transcription factors, induced by FGF signaling, that specify medial fate. We extend our analysis of zebrafish development to show that bHLH activity is responsible for the mediolateral patterning of the entire mesodermal germ layer."
- Review - Molecular specification of germ layers in vertebrate embryos[7] "In order to generate the tissues and organs of a multicellular organism, different cell types have to be generated during embryonic development. The first step in this process of cellular diversification is the formation of the three germ layers: ectoderm, endoderm and mesoderm. The ectoderm gives rise to the nervous system, epidermis and various neural crest-derived tissues, the endoderm goes on to form the gastrointestinal, respiratory and urinary systems as well as many endocrine glands, and the mesoderm will form the notochord, axial skeleton, cartilage, connective tissue, trunk muscles, kidneys and blood. Classic experiments in amphibian embryos revealed the tissue interactions involved in germ layer formation and provided the groundwork for the identification of secreted and intracellular factors involved in this process."
- Rho kinase activity controls directional cell movements during primitive streak formation in the rabbit embryo[8] "During animal gastrulation, the specification of the embryonic axes is accompanied by epithelio-mesenchymal transition (EMT), the first major change in cell shape after fertilization. EMT takes place in disparate topographical arrangements, such as the circular blastopore of amphibians, the straight primitive streak of birds and mammals or in intermediate gastrulation forms of other amniotes such as reptiles. Planar cell movements are prime candidates to arrange specific modes of gastrulation but there is no consensus view on their role in different vertebrate classes. Here, we test the impact of interfering with Rho kinase-mediated cell movements on gastrulation topography in blastocysts of the rabbit, which has a flat embryonic disc typical for most mammals. Time-lapse video microscopy, electron microscopy, gene expression and morphometric analyses of the effect of inhibiting ROCK activity showed - besides normal specification of the organizer region - a dose-dependent disruption of primitive streak formation; this disruption resulted in circular, arc-shaped or intermediate forms, reminiscent of those found in amphibians, fishes and reptiles. Our results reveal a crucial role of ROCK-controlled directional cell movements during rabbit primitive streak formation and highlight the possibility that temporal and spatial modulation of cell movements were instrumental for the evolution of gastrulation forms." rabbit
- Generation of organized germ layers from a single mouse embryonic stem cell[9] "Mammalian inner cell mass cells undergo lineage-specific differentiation into germ layers of endoderm, mesoderm and ectoderm during gastrulation. It has been a long-standing challenge in developmental biology to replicate these organized germ layer patterns in culture. Here we present a method of generating organized germ layers from a single mouse embryonic stem cell cultured in a soft fibrin matrix." stem cells
- Deficiency in crumbs homolog 2 (Crb2) affects gastrulation and results in embryonic lethality in mice[10] "The Crumbs family of transmembrane proteins has an important role in the differentiation of the apical membrane domain in various cell types, regulating such processes as epithelial cell polarization. The mammalian Crumbs protein family is composed of three members. Here, we inactivated the mouse Crb2 gene with gene-targeting techniques and found that the protein is crucial for early embryonic development with severe abnormalities appearing in Crb2-deficient embryos at late-gastrulation. Our findings indicate that the primary defect in the mutant embryos is disturbed polarity of the epiblast cells at the primitive streak, which affects epithelial to mesenchymal transition (EMT) during gastrulation, resulting in impaired mesoderm and endoderm formation, and embryonic lethality by embryonic day 12.5. These findings therefore indicate a novel role for the Crumbs family of proteins."
- Zebrafish eve1 regulates the lateral and ventral fates of mesodermal progenitor cells at the onset of gastrulation[11] "Our data show that Eve1 functions together with Ved, Vent and Vox in a transcriptional network to prevent the spread of anti-Bmp gene activity from the dorsal side, leading to the establishment of the Bmp gradient activity along the dorsoventral axis to induce distinct transcriptional outputs in mesodermal progenitor cells (MPCs) to maintain the lateral and ventral MPC fates during gastrulation."
|
Gastrulation Movies
Animation showing the secondary spread of mesoderm following the migration of endoderm through the primitive streak.
|
|
|
|
|
|
| mesoderm spread
|
mouse cilia
|
quail extracellular matrix
|
Planar cell movement
|
Mesoderm migration
|
Human Gastrulation
The site of gastrulation, the primitive streak is visible during week 3 on the epiblast dorsal surface of the embryonic disc.
Stage 7
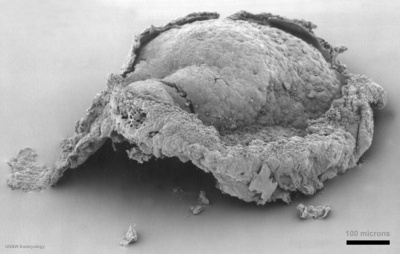
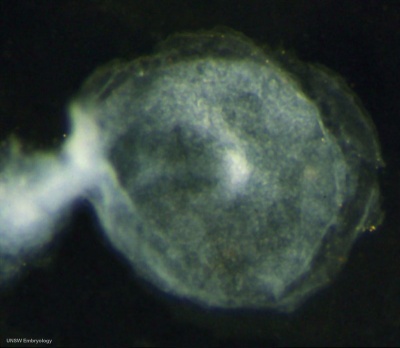
- Links: Carnegie stage 7
Stage 8
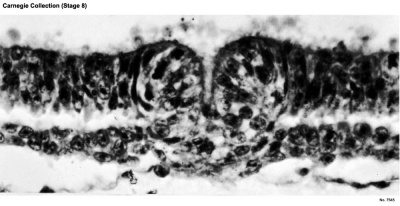
Primitive pit
|
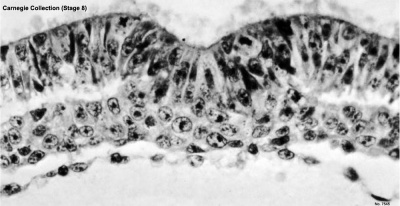
Primitive groove and primitive streak
|
Nodal cilia and neurenteric canal
- Links: Carnegie stage 8
Molecular
Germ Layer Markers
Stem cell researchers have used the following markers to identify early differentiation of cells in the three germ layer in embryoid bodies.[12]
Signaling - BMP and FGF
- BMP and FGF signaling interact to pattern mesoderm by controlling basic helix-loop-helix transcription factor activity[6] "The mesodermal germ layer is patterned into mediolateral subtypes by signaling factors including BMP and FGF. How these pathways are integrated to induce specific mediolateral cell fates is not well understood. We used mesoderm derived from post-gastrulation neuromesodermal progenitors (NMPs), which undergo a binary mediolateral patterning decision, as a simplified model to understand how FGF acts together with BMP to impart mediolateral fate. Using zebrafish and mouse NMPs, we identify an evolutionarily conserved mechanism of BMP and FGF mediated mediolateral mesodermal patterning that occurs through modulation of basic helix-loop-helix (bHLH) transcription factor activity. BMP imparts lateral fate through induction of Id helix loop helix (HLH) proteins, which antagonize bHLH transcription factors, induced by FGF signaling, that specify medial fate. We extend our analysis of zebrafish development to show that bHLH activity is responsible for the mediolateral patterning of the entire mesodermal germ layer."
- Links: Induced Stem Cells | Alpha-Fetoprotein
Animal Models
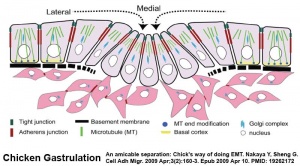
Chicken Gastrulation
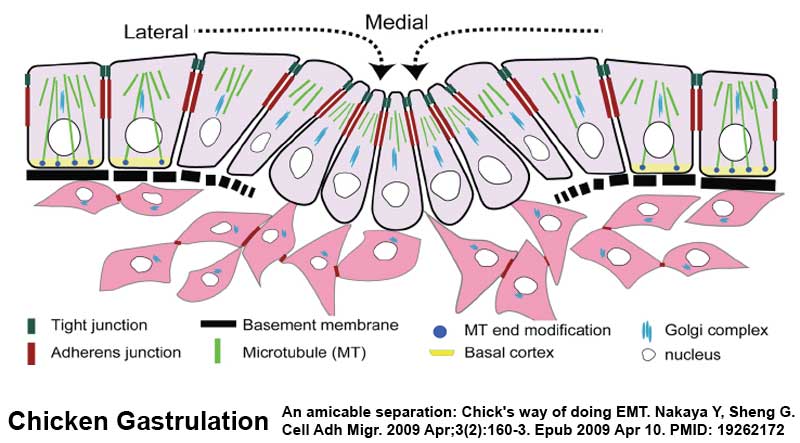

- Links: chicken
Frog Gastrulation
|
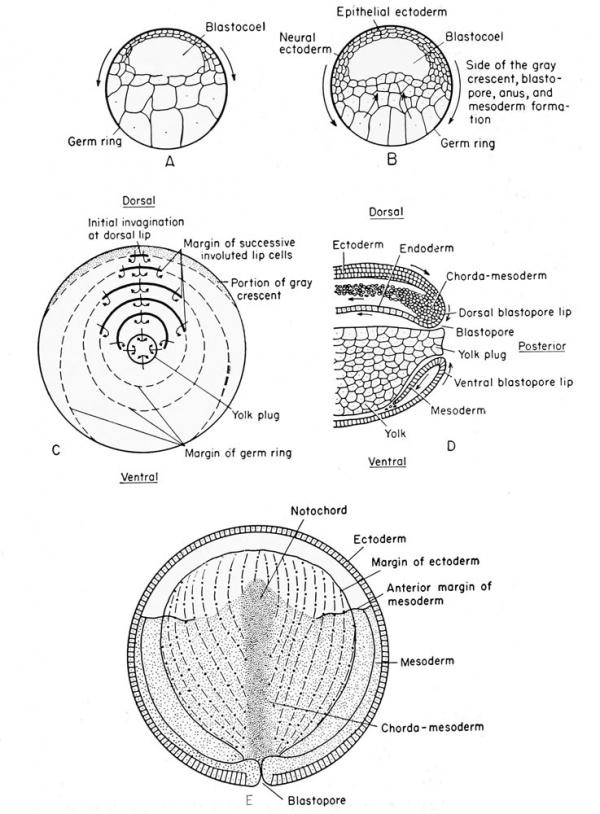
Frog Gastrulation[13]
A The blastula stage, prior to any gastrulation movement.
B Movement of the blastula cells preliminary to gastrulation.
C Blastoporal view of successive phases of gastrulation; (solid line) lip of blastopore, {dotted line) germ ring, to be subsequently incorporated into the blastoporal lips.
D Lateral view of sagittal section during late gastrulation showing the origin of the mesial notochord, and the lateral mesoderm from the proliferated chorda-mesoderm cells at the dorsal lip.
E Composite drawing to illustrate the germ layer relations in the later gastrula of the frog. The medullary plate (ectoderm) is not indicated; {alternate dots and dashes) notochord, {heavy stippling) notochord, {sparse stippling) mesoderm, (cellular markings) ectoderm.
|
- Links: frog
Zebrafish Gastrulation
Kupffer's vesicle (ciliated organ of asymmetry, primitive node) a transient epithelial fluid-filled sac located midventrally posterior to the yolk cell or its extension. The vesicle has been described as equivalent to the primitive node for establishing embryo left-right (L-R) axis.[14]
- Links: zebrafish
Drosophila Gastrulation
| Drosophila gastrulation[2]
|
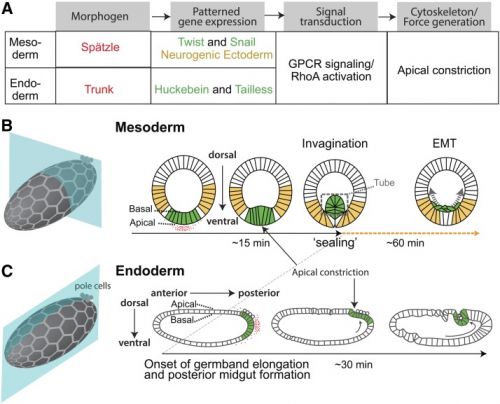
|
A Flow chart showing the regulation of cell shape changes that accompany Drosophila gastrulation. Coloured text matches the colours in (B and C).
B Cartoon showing mesoderm invagination in Drosophila. Hexagonal mesh illustrates plane of epithelium, but cell size is not to scale. Red shows pattern of active Spätzle. Green illustrates presumptive mesoderm cells, which express Twist and Snail in response to high levels of Dorsal. Yellow cells show region of ectoderm, which is specified by low levels of Dorsal.
C Cartoon showing posterior endoderm invagination. Hexagonal mesh illustrates plane of epithelium, but cell size is not to scale. Red shows distribution of the signaling ligand Trunk. Green illustrates presumptive endoderm cells expressing Huckebein and Tailless. Arrow shows the direction of germband elongation.
|
- Links: Drosophila
Additional Images
Chicken neural induction markers
Chicken primitive streak cell migration
References
- ↑ Tyser RCV, Mahammadov E, Nakanoh S, Vallier L, Scialdone A & Srinivas S. (2021). Single-cell transcriptomic characterization of a gastrulating human embryo. Nature , , . PMID: 34789876 DOI.
- ↑ 2.0 2.1 Martin AC. (2020). The Physical Mechanisms of Drosophila Gastrulation: Mesoderm and Endoderm Invagination. Genetics , 214, 543-560. PMID: 32132154 DOI.
- ↑ Pijuan-Sala B, Griffiths JA, Guibentif C, Hiscock TW, Jawaid W, Calero-Nieto FJ, Mulas C, Ibarra-Soria X, Tyser RCV, Ho DLL, Reik W, Srinivas S, Simons BD, Nichols J, Marioni JC & Göttgens B. (2019). A single-cell molecular map of mouse gastrulation and early organogenesis. Nature , 566, 490-495. PMID: 30787436 DOI.
- ↑ Martyn I, Kanno TY, Ruzo A, Siggia ED & Brivanlou AH. (2018). Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature , 558, 132-135. PMID: 29795348 DOI.
- ↑ Wei S & Wang Q. (2018). Molecular regulation of Nodal signaling during mesendoderm formation. Acta Biochim. Biophys. Sin. (Shanghai) , 50, 74-81. PMID: 29206913 DOI.
- ↑ 6.0 6.1 Row RH, Pegg A, Kinney B, Farr GH, Maves L, Lowell S, Wilson V & Martin BL. (2018). BMP and FGF signaling interact to pattern mesoderm by controlling basic helix-loop-helix transcription factor activity. Elife , 7, . PMID: 29877796 DOI.
- ↑ Kiecker C, Bates T & Bell E. (2016). Molecular specification of germ layers in vertebrate embryos. Cell. Mol. Life Sci. , 73, 923-47. PMID: 26667903 DOI.
- ↑ Stankova V, Tsikolia N & Viebahn C. (2015). Rho kinase activity controls directional cell movements during primitive streak formation in the rabbit embryo. Development , 142, 92-8. PMID: 25516971 DOI.
- ↑ Poh YC, Chen J, Hong Y, Yi H, Zhang S, Chen J, Wu DC, Wang L, Jia Q, Singh R, Yao W, Tan Y, Tajik A, Tanaka TS & Wang N. (2014). Generation of organized germ layers from a single mouse embryonic stem cell. Nat Commun , 5, 4000. PMID: 24873804 DOI.
- ↑ Xiao Z, Patrakka J, Nukui M, Chi L, Niu D, Betsholtz C, Pikkarainen T, Pikkarainan T, Vainio S & Tryggvason K. (2011). Deficiency in Crumbs homolog 2 (Crb2) affects gastrulation and results in embryonic lethality in mice. Dev. Dyn. , 240, 2646-56. PMID: 22072575 DOI.
- ↑ Seebald JL & Szeto DP. (2011). Zebrafish eve1 regulates the lateral and ventral fates of mesodermal progenitor cells at the onset of gastrulation. Dev. Biol. , 349, 78-89. PMID: 20950598 DOI.
- ↑ Pekkanen-Mattila M, Pelto-Huikko M, Kujala V, Suuronen R, Skottman H, Aalto-Setälä K & Kerkelä E. (2010). Spatial and temporal expression pattern of germ layer markers during human embryonic stem cell differentiation in embryoid bodies. Histochem. Cell Biol. , 133, 595-606. PMID: 20369364 DOI.
- ↑ Rugh R. Book - The Frog Its Reproduction and Development. (1951) The Blakiston Company.
- ↑ Gao W, Xu L, Guan R, Liu X, Han Y, Wu Q, Xiao Y, Qi F, Zhu Z, Lin S & Zhang B. (2011). Wdr18 is required for Kupffer's vesicle formation and regulation of body asymmetry in zebrafish. PLoS ONE , 6, e23386. PMID: 21876750 DOI.
Reviews
Tseng WC, Munisha M, Gutierrez JB & Dougan ST. (2017). Establishment of the Vertebrate Germ Layers. Adv. Exp. Med. Biol. , 953, 307-381. PMID: 27975275 DOI.
Kiecker C, Bates T & Bell E. (2016). Molecular specification of germ layers in vertebrate embryos. Cell. Mol. Life Sci. , 73, 923-47. PMID: 26667903 DOI.
Nowotschin S & Hadjantonakis AK. (2010). Cellular dynamics in the early mouse embryo: from axis formation to gastrulation. Curr. Opin. Genet. Dev. , 20, 420-7. PMID: 20566281 DOI.
Chenoweth JG, McKay RD & Tesar PJ. (2010). Epiblast stem cells contribute new insight into pluripotency and gastrulation. Dev. Growth Differ. , 52, 293-301. PMID: 20298258 DOI.
Thiery JP, Acloque H, Huang RY & Nieto MA. (2009). Epithelial-mesenchymal transitions in development and disease. Cell , 139, 871-90. PMID: 19945376 DOI.
Roszko I, Sawada A & Solnica-Krezel L. (2009). Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin. Cell Dev. Biol. , 20, 986-97. PMID: 19761865 DOI.
Rohrschneider MR & Nance J. (2009). Polarity and cell fate specification in the control of Caenorhabditis elegans gastrulation. Dev. Dyn. , 238, 789-96. PMID: 19253398 DOI.
Heisenberg CP & Solnica-Krezel L. (2008). Back and forth between cell fate specification and movement during vertebrate gastrulation. Curr. Opin. Genet. Dev. , 18, 311-6. PMID: 18721878 DOI.
Watters C. (2005). Video views and reviews: gastrulation and the fashioning of animal embryos. Cell Biol Educ , 4, 273-8. PMID: 16344860 DOI.
Viebahn C. (2001). Hensen's node. Genesis , 29, 96-103. PMID: 11170350
Leptin M. (1999). Gastrulation in Drosophila: the logic and the cellular mechanisms. EMBO J. , 18, 3187-92. PMID: 10369659 DOI.
Articles
Kim YY, Moon JS, Kwon MC, Shin J, Im SK, Kim HA, Han JK & Kong YY. (2014). Meteorin regulates mesendoderm development by enhancing nodal expression. PLoS ONE , 9, e88811. PMID: 24558432 DOI.
Chuai M, Hughes D & Weijer CJ. (2012). Collective epithelial and mesenchymal cell migration during gastrulation. Curr. Genomics , 13, 267-77. PMID: 23204916 DOI.
Halacheva V, Fuchs M, Dönitz J, Reupke T, Püschel B & Viebahn C. (2011). Planar cell movements and oriented cell division during early primitive streak formation in the mammalian embryo. Dev. Dyn. , 240, 1905-16. PMID: 21761476 DOI.
Vasiev B, Balter A, Chaplain M, Glazier JA & Weijer CJ. (2010). Modeling gastrulation in the chick embryo: formation of the primitive streak. PLoS ONE , 5, e10571. PMID: 20485500 DOI.
Books
Search Pubmed
Search Pubmed Now: gastrulation
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 1) Embryology Gastrulation. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Gastrulation
- What Links Here?
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G