Gastrointestinal Tract - Pancreas Development
| Embryology - 17 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
This section of notes gives an overview of how the pancreas develops as an exocrine organ associated with the gastrointestinal tract. There is a second description, similar in overview, in relation to the pancreas as an endocrine organ, see Endocrine - Pancreas Development.
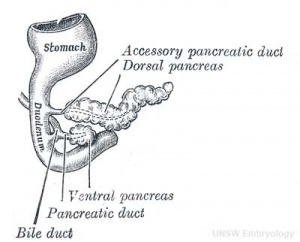
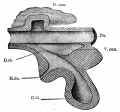
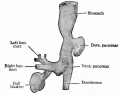
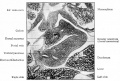
At the foregut/midgut junction the septum transversum generates 2 pancreatic buds (dorsal and ventral endoderm) which will fuse to form the pancreas. The dorsal bud arises first and generates most of the pancreas. The ventral bud arises beside the bile duct and forms only part of the head and uncinate process of the pancreas.
The digestive enzyme alpha amylase is the main exocrine enzyme produced by the pancreas (Amy2), and also by salivary glands (Amy1). Study of prenatal fetal [1] and postnatal[2] production of amylase during the first 2 years has shown that the majority of produced initially is salivary amylase. Infants and newborns also show a poor secretory response, to secretin, that is either absent or minimal at birth and is then acquired during the postnatal period.[3] These results taken together suggest that the exocrine pancreas only becomes gradually "functional" postnatally.
- Links: Endocrine Pancreas | Exocrine Pancreas
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Pancreas Embryology | Pancreas Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page. Pancreatic Duct[5] "The long-type accessory pancreatic duct represents a continuation of the main duct of the dorsal pancreatic bud. The short-type accessory pancreatic duct is probably formed by the proximal main duct of the dorsal pancreatic bud and its long inferior branch." SOX9[6] "We show that Sox9 maintains pancreatic progenitors by stimulating their proliferation, survival, and persistence in an undifferentiated state. Our finding that SOX9 regulates the Notch-effector HES1 suggests a Notch-dependent mechanism and establishes a possible genetic link between SOX factors and Notch." (More? OMIM Sox9 | Protein Sox9) |
Development Overview
- Functions- exocrine (amylase, alpha-fetoprotein) and endocrine (pancreatic islets)
- Pancreatic buds- endoderm, covered in splanchnic mesoderm
- Pancreatic bud formation - duodenal level endoderm, splanchnic mesoderm forms dorsal and ventral mesentery, dorsal bud (larger, first), ventral bud (smaller, later)
- Week 6 - Duodenum growth/rotation - brings ventral and dorsal buds together, fusion of buds
- Pancreatic duct - ventral bud duct and distal part of dorsal bud, exocrine function
- Islet cells- cords of endodermal cells form ducts, which cells bud off to form islets
- Week 7 to 20 - pancreatic hormones secretion increases, small amount maternal insulin
- Week 10 - glucagon (alpha) differentiate first, somatostatin (delta), insulin (beta) cells differentiate, insulin secretion begins
- Week 15 - glucagon detectable in fetal plasma
- Beta cells - stimulate fetal growth, continue to proliferate through to postnatal in infancy, most abundant
- Maternal diabetes mellitus - hypertrophy of fetal beta cells
Embryonic
Week 6
During week 6 about day 41 (Carnegie stage 17, GA week 8) the stomach rotation brings the smaller ventral pancreatic bud dorsally to fuse with the larger dorsal pancreatic bud.
Pancreatic Bud Rotation (animal models)
- mouse - day 13 to 14 (E13 - E14)
- pig - 38 to 52 days days post coitum (d.p.c.)
- cattle - before 45 d.p.c.
Week 8
Late Embryonic (Carnegie stage 22)
Pancreatic Duct
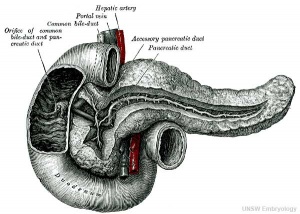
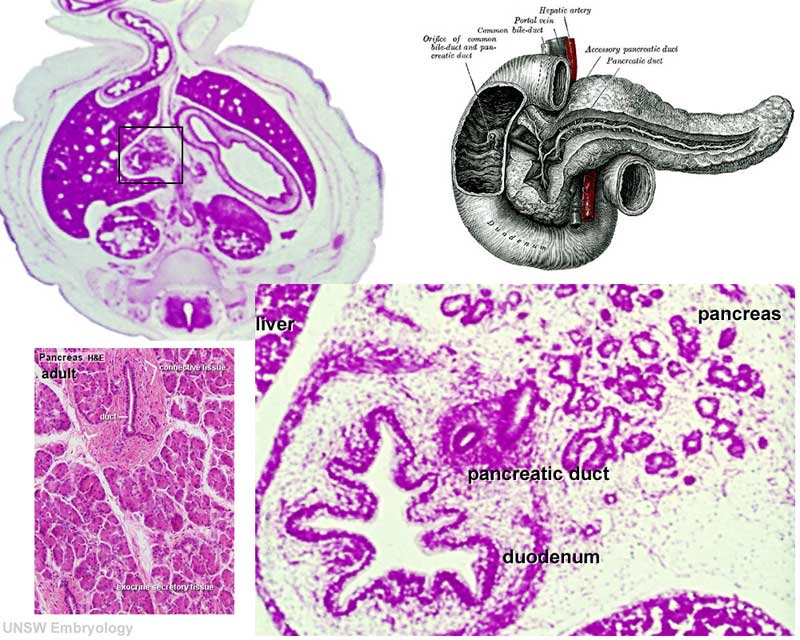
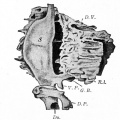
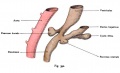
The initial formation of the pancreas as two separate lobes each with their own duct that fuses leads a range of anatomical variations in the adult exocrine pancreatic duct. Pancreatic duct five variation classification: common, ansa pancreatica, branch fusion, looped, and separated. Accessory pancreatic duct (APD, of Santorini) in the embryo is the main drainage duct of the dorsal pancreatic bud emptying into the minor duodenal papilla. In the adult it has been further classified as either long-type (joins main pancreatic duct at pancreas neck portion) and short-type (joins main pancreatic duct near first inferior branch).
Main Pancreatic Duct (MPD or Wirsung's duct) forms within the dorsal pancreatic bud and is present in the body and tail of the pancreas. Discovered by Johann Georg Wirsung (1589 - 1643) a German physician who worked as a prosector in Padua.
Accessory Pancreatic Duct (APD or Santorini’s duct) is present mainly in the head of the pancreas. Originally dissected and delineated by Giovanni Domenico Santorini (1681 - 1737) an Italian anatomist.
Endoscopic Retrograde Cholangiopancreatography (ERCP) is a medical procedure which allows an injected dye to display the duct system on an x ray (pancreatograms).
Pancreas Histology
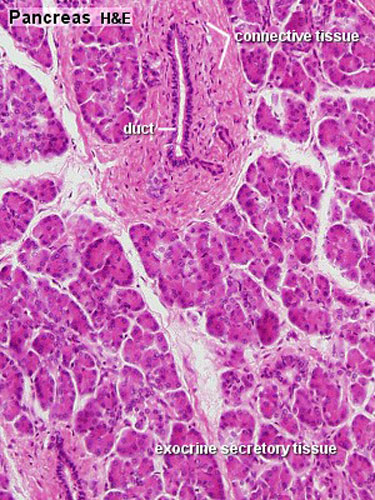
|
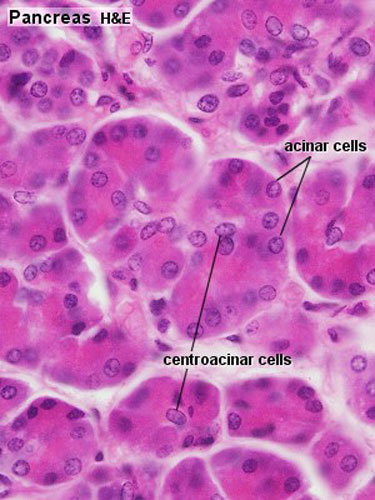
|
| (Human, H&E x10) | (H&E x40) |
Pancreas History
1642 - main pancreatic duct (MPD) discovered by Johann Georg Wirsung (1589 - 1643) a German physician who worked as a prosector in Padua. The duct is also called Wirsung's duct.
1724 - accessory pancreatic duct (APD) dissected and delineated by Giovanni Domenico Santorini (1681 - 1737) an Italian anatomist. The duct is also called Santorini's duct.
1833 - Amylase, the form enzyme also found in exocrine pancreas, isolated from a malt solution by Anselme Payen.
1893 - Islets of Langerhans named in honour of Paul Langerhans (1847-1888) by Gustave-Edouard Laguesse (1861-1927) a french histopathologist.
1922 - discovery of insulin by Frederick Banting and John Macleod, two Canadian researchers, and they subsequently win the 1923 Nobel Prize in Medicine. Banting shared his part of the prize money with a younger coworker Charles Best.
1953 - glucagon, originally called "hyperglycemic glycogenolytic factor", purified by Staub, Sinn and Behrens.[8][9][10] See also book JM. Howard and W, Hess (2002) "History of the Pancreas: Mysteries of a Hidden Organ".
- Links: Nobel Lecture, September 15, 1925 | Amazon - History of the Pancreas: Mysteries of a Hidden Organ | PDF Article - Purification and Crystallization of Glucagon |
Pancreas Digestive
- Pancreatic buds- endoderm, covered in splanchnic mesoderm
- Pancreatic bud formation - duodenal level endoderm, splanchnic mesoderm forms dorsal and ventral mesentery, dorsal bud (larger, first), ventral bud (smaller, later)
- Duodenum growth/rotation -ì brings ventral and dorsal buds together, fusion of buds
- Pancreatic duct - ventral bud duct and distal part of dorsal bud, exocrine function
Functions - exocrine (amylase, alpha-fetoprotein)
Pancreatic amylase digests starch to maltose. Postnatally, a blood test to detect amylase can be used to diagnose and monitor acute or chronic pancreatitis (pancreas inflammation).
Pancreatic alpha-fetoprotein has been found to change in expression level (in rats) during developent and has been suggested to influence pancreas development.[11] "Immunolocalization for AFP revealed that a positive reactivity was detectable at E15.5 pancreas, became stronger in the cytoplasm of mesenchyme cells at E18.5, and declined after birth to a nearly undetectable level in adults."
Secretagogues
Secretagogue is a generic term for any substance that stimulates the secretion of another substance from a tissue or organ. In the exocrine pancreas, these are substances that stimulate the acini release of digestive enzymes. Note that bicarbonate and fluid secretion occurs in the pancreatic ducts and its stimulation may differ from enzyme secretion.
- secretin
- cholecystokinin
- vasoactive intestinal polypeptide (VIP)
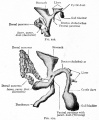
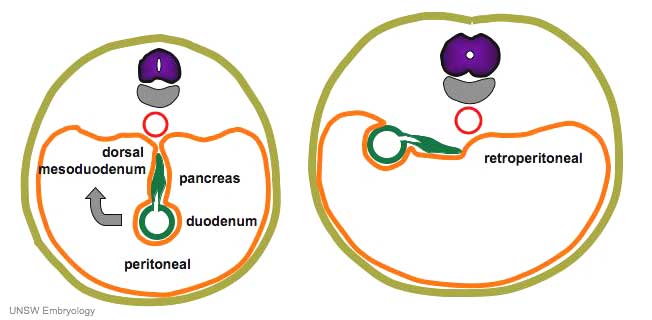
Duodenum/Pancreas Rotation
|
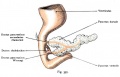
After the stomach the initial portion of the gastrointestinal tract tube is the duodenum which initially lies in the midline within the peritoneal cavity.
This region, along with the attached pancreas, undergoes rotation to become a retroperitoneal structure. This diagram shows the rotation with spinal cord at the top, vertebral body then dorsal aorta then pertioneal wall and cavity. Note this is a simplified diagram and the liver would push everything to the left during this rotation. |
Mouse Pancreas Development
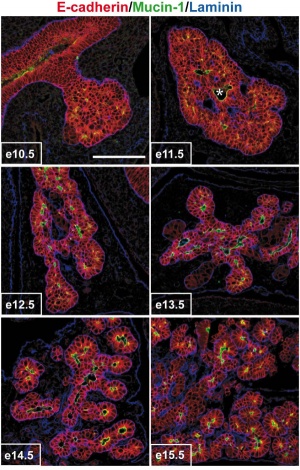
- Pancreas develops from distinct dorsal and ventral primordia.
- Dorsal pancreas - midline endoderm in posterior foregut is a single layer of epithelial cells that contacts notochord, an axial mesoderm-derived structure
- Ventral pancreas - Laterally, endoderm fated to form ventral pancreas is adjacent to both splanchnic mesoderm and aortic endothelial cells but is not in direct contact with notochord.
- The notochord and dorsal prepancreatic endoderms remain in contact until about the 13-somite stage in mice, 8.5 d postcoitum (dpc), when midline fusion of the paired dorsal aortas occurs.
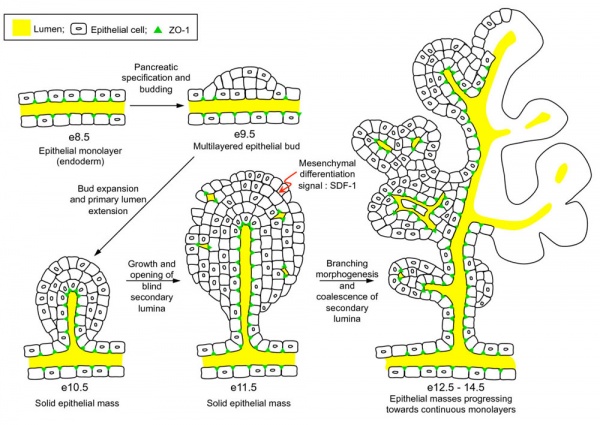
- The first indication of morphogenesis occurs at 22-25 somites in mice (9.5 dpc)
- dorsal mesenchyme condenses and underlying endoderm evaginates, forming a recognizable dorsal pancreatic bud
- the ventral bud appears later at ~30 somites (10.25-10.5 dpc). Stimulated by mesenchymal signals, pancreatic epithelial cells proliferate and branch.
- The accumulated evidence is consistent with the possibility that a unique cell gives rise to all pancreatic cell lineages. The existence of such a pancreatic "stem" cell remains debatable.
(text modified from Seung K. Kim and Matthias Hebrok Intercellular signals regulating pancreas development and function. Genes & Development 15:111-127 2001)
| Mouse Pancreas Cell Lineage
In this study[13] mouse cell types were collected at different ages E11 and E15 pancreatic progenitors, E15 acinar cells, E15 endocrine progenitors (EP), E15, E17, P1, P15, 8–12 week beta cells, P1 and 8–12 week alpha cells, and adult duct cells. The following markers were used in determining the lineages, not both endocrine and exocrine cells derive from a common precursor.
|
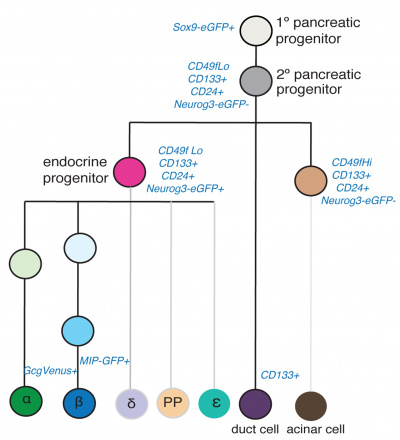
Identification of pancreas cell lineages[13] |
Duct Development
Abnormalities
See also the article on the lifetime consequences of abnormal fetal pancreatic development.[14]
ICD-11 LB21 Structural developmental anomalies of pancreas
LB21.0 Annular pancreas
LB21.0 Annular pancreas - Annular pancreas is a distinct form of duodenal atresia in which the head of the pancreas forms a ring around the second portion of the duodenum. During the neonatal period, the clinical picture is dominated by epigastric distension with vomiting, which is nonbilious as the obstruction is usually supra-vaterian. Chromosomal abnormalities are present in one-third of cases of annular pancreas, with trisomy 21 (followed by trisomy 18 and 13) being the most frequently detected anomaly. Annular pancreas is an embryopathy resulting from an anomaly occurring early (towards the fourth week) in development.
Occurs (1 in 7,000 people) where the pancreas forms as a "ring" of tissue surrounding the duodenum which is subsequently narrowed.
LB21.1 Pancreas divisum
LB21.1 Pancreas divisum - This is a congenital anomaly in the anatomy of the ducts of the pancreas in which a single pancreatic duct is not formed, but rather remains as two distinct dorsal and ventral ducts.
LB21.2 Accessory pancreas
LB21.2 Accessory pancreas - Accessory pancreas is an asymptomatic embryopathy characterized by the presence of pancreatic tissue in other sites of the body such as the splenic pedicle, gonadic pedicles, intestinal mesentery, duodenum wall, upper jejunum, or, more rarely, the gastric wall, ileum, gallbladder or spleen.
Pancreatic tissue can also be located in Meckel's diverticulum.
LB21.3 Agenesis-aplasia of pancreas
LB21.3 Agenesis-aplasia of pancreas - This refers to the failure of an organ to develop during embryonic growth and development due to the absence of primordial tissue of the pancreas.
LB21.4 Partial agenesis of pancreas
LB21.4 Partial agenesis of pancreas - Partial agenesis of the pancreas is characterized by the congenital absence of a critical mass of pancreatic tissue. The severity of the disease depends on the amount of functional pancreatic tissue present. Pancreatic agenesis is commonly associated with other malformations, in particular pancreaticobiliary duct anomalies, leading to acute or chronic pancreatitis, hyperglycemia (50% of cases), or, more rarely, polysplenia.
Diabetes Mellitus
Maternal diabetes (and hyperglycaemia) have been shown to lead to increased fetal islet hyperplasia of the insulin producing beta cells and insulin secretion.
Intrauterine growth restriction
Intrauterine growth restriction can lead to a delayed development of the insulin producing beta cells and low insulin secretion.
Tumours
Serous Cystadenoma (endocrine tumour), Somatostatinoma (tumour of delta cell origin), intraductal papillary-mucinous neoplasm
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
References
- ↑ Ogita S, Noma H, Kawamura T, Shimura K, Ohnishi M, Ishiko O & Sugawa T. (1978). Differentiation of amylase isozyme in amniotic fluid with fetal age. Am J Obstet Gynecol , 131, 63-8. PMID: 645785 DOI.
- ↑ Tye JG, Karn RC & Merritt AD. (1976). Differential expression of salivary (Amy1) and pancreatic (Amy2) human amylase loci in prenatal and postnatal development. J. Med. Genet. , 13, 96-102. PMID: 933119
- ↑ Lebenthal E & Lee PC. (1980). Development of functional responses in human exocrine pancreas. Pediatrics , 66, 556-60. PMID: 6159567
- ↑ Angelo JR & Tremblay KD. (2018). Identification and fate mapping of the pancreatic mesenchyme. Dev. Biol. , 435, 15-25. PMID: 29329912 DOI.
- ↑ Kamisawa T & Okamoto A. (2008). Pancreatographic investigation of pancreatic duct system and pancreaticobiliary malformation. J. Anat. , 212, 125-34. PMID: 18194203 DOI.
- ↑ Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G & Sander M. (2007). SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc. Natl. Acad. Sci. U.S.A. , 104, 1865-70. PMID: 17267606 DOI.
- ↑ Archie JG, Collins JS & Lebel RR. (2006). Quantitative standards for fetal and neonatal autopsy. Am. J. Clin. Pathol. , 126, 256-65. PMID: 16891202 DOI.
- ↑ STAUB A, SINN L & BEHRENS OK. (1953). Purification and crystallization of hyperglycemic glycogenolytic factor (HGF). Science , 117, 628-9. PMID: 13056638
- ↑ STAUB A, SINN L & BEHRENS OK. (1955). Purification and crystallization of glucagon. J. Biol. Chem. , 214, 619-32. PMID: 14381399
- ↑ BROMER WW, SINN LG, STAUB A & BEHRENS OK. (1957). The amino acid sequence of glucagon. Diabetes , 6, 234-8. PMID: 13427628
- ↑ Liu L, Guo J, Yuan L, Cheng M, Cao L, Shi H, Tong H, Wang N & De W. (2007). Alpha-fetoprotein is dynamically expressed in rat pancreas during development. Dev. Growth Differ. , 49, 669-81. PMID: 17880577 DOI.
- ↑ Hick AC, van Eyll JM, Cordi S, Forez C, Passante L, Kohara H, Nagasawa T, Vanderhaeghen P, Courtoy PJ, Rousseau GG, Lemaigre FP & Pierreux CE. (2009). Mechanism of primitive duct formation in the pancreas and submandibular glands: a role for SDF-1. BMC Dev. Biol. , 9, 66. PMID: 20003423 DOI.
- ↑ 13.0 13.1 Benitez CM, Qu K, Sugiyama T, Pauerstein PT, Liu Y, Tsai J, Gu X, Ghodasara A, Arda HE, Zhang J, Dekker JD, Tucker HO, Chang HY & Kim SK. (2014). An integrated cell purification and genomics strategy reveals multiple regulators of pancreas development. PLoS Genet. , 10, e1004645. PMID: 25330008 DOI.
- ↑ Holemans K, Aerts L & Van Assche FA. (2003). Lifetime consequences of abnormal fetal pancreatic development. J. Physiol. (Lond.) , 547, 11-20. PMID: 12562919 DOI.
Reviews
Zakowski JJ & Bruns DE. (1985). Biochemistry of human alpha amylase isoenzymes. Crit Rev Clin Lab Sci , 21, 283-322. PMID: 2578342 DOI.
Articles
Tye JG, Karn RC & Merritt AD. (1976). Differential expression of salivary (Amy1) and pancreatic (Amy2) human amylase loci in prenatal and postnatal development. J. Med. Genet. , 13, 96-102. PMID: 933119
Journals
- Pancreas The official journal of the American Pancreatic Association and the Japan Pancreas Society
- Pancreatology Official Journal of the International Association of Pancreatology (IAP); European Pancreatic Club (EPC)and 16 other societies and study groups.
- Journal of the Pancreas electronic journal of pancreatology
Online Textbooks
Endocrinology: An Integrated Approach Nussey, S.S. and Whitehead, S.A. Oxford, UK: BIOS Scientific Publishers, Ltd; 2001. table of Contents
NIH Genes & Disease Chapter 41 - Endocrine
Pathophysiology of the Endocrine System The Endocrine Pancreas
Developmental Biology (6th ed) Gilbert, Scott F. Sunderland (MA): Sinauer Associates, Inc.; c2000.
Molecular Biology of the Cell (4th Edn) Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter. New York: Garland Publishing; 2002. table 15-1. Some Hormone-induced Cell Responses Mediated by Cyclic AMP
Health Services/Technology Assessment Text (HSTAT) Bethesda (MD): National Library of Medicine (US), 2003 Oct.
Search NLM Online Textbooks- "pancreas development" : Endocrinology | Molecular Biology of the Cell | The Cell- A molecular Approach
Search Bookshelf Pancreas Development
Search Pubmed
Search Pubmed Now: Pancreas Development | Exocrine Pancreas Development
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 17) Embryology Gastrointestinal Tract - Pancreas Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Gastrointestinal_Tract_-_Pancreas_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G