Fertilization
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Fertilization is the fusion of haploid gametes, egg and sperm, to form the diploid zygote. Note though there can be subtle differences in the fertilization process which occurs naturally within the body or through reproductive technologies outside the body, the overall product in both cases is a diplod zygote. In fertilization research, after humans the mouse is the most studied species followed by domestic and farm animals. The process of fertilization involves components of, and signaling between, both sperm (spermatozoa) and egg (oocyte).
In addition to in vivo fertilization there are many new in vitro technologies related to human infertility (Assisted Reproductive Technology) and animal production somatic cell nuclear transfer (SCNT) to generate a zygote.
Note different spelling - USA spelling "fertilization", Australian spelling "fertilisation".
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Fertilization | In vitro Fertilization | Polar Body |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Objectives
- Understand the mechanisms of gamete formation.
- Understand the mechanisms of cell division.
- Describe the differences between mitosis and meiosis.
- Understand the mechanisms of fertilization, both in vivo and in vitro.
- Describe the cleavage of the zygote.
- Have a preliminary understanding of the role and process in male sex determination and X inactivation.
- Understand the abnormalities that occur during this period of development.
Movies
|
|
|
|
| |||||||||||||||
|
|
|
|
Fertilization Preparation
Prior to the fertilization process commencing both the gametes oocyte (egg) and spermatozoa (sperm) require completion of a number of biological processes.
- Oocyte meiosis - completes Meiosis 1 and commences Meiosis 2 (arrests at Metaphase II).
- Spermatozoa Capacitation - following release (ejaculation) and mixing with other glandular secretions, activates motility and acrosome preparation.
- Migration - both oocyte and spermatozoa.
- oocyte ovulation and release with associated cells, from ovary into fimbria then into uterine tube (oviduct, uterine horn, fallopian tube) and epithelial cilia mediated movement.
- spermatozoa ejaculation, deposited in vagina, movement of tail to "swim" in uterine secretions through cervix, uterine body and into uterine tube, have approximately 24-72h to fertilize oocyte.
Endocrinology - Diagram of the comparative anatomy of the male and female reproductive tracts
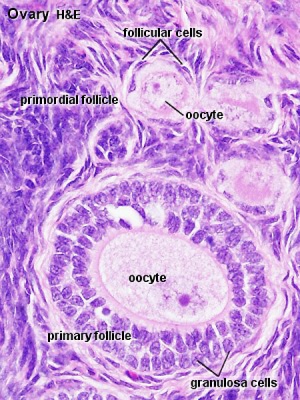
Oogenesis
- Process of oogonia mature into oocytes (ova, ovum, egg)
- all oogonia form primary oocytes before birth, therefore a maturation of preexisting cells in the female gonad, ovary
- humans usually only 1 ovum released every menstrual cycle (IVF- superovulation)
- oocyte and its surrounding cells = follicle
- primary -> secondary -> ovulation releases
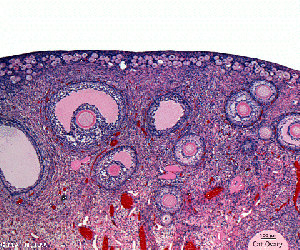
Ovary- Histology - whole transverse section (cortex, medulla)
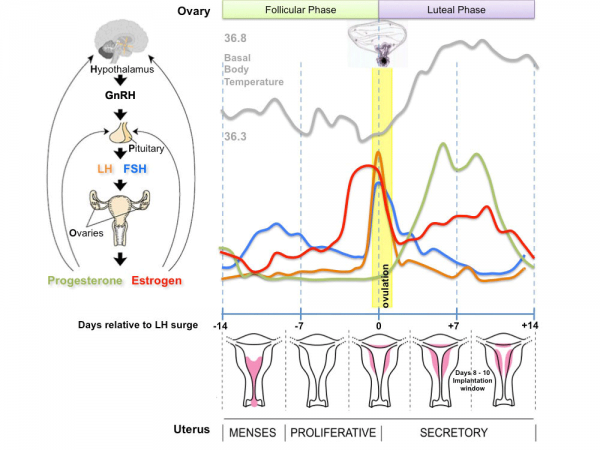
Menstrual Cycle
- Primary Oocyte - arrested at early Meiosis 1
- diploid: 22 chromosome pairs + 1 pair X chromosomes (46, XX)
- autosomes and sex chromosome
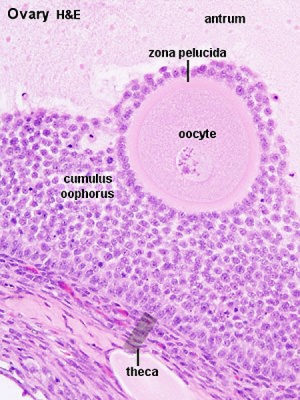
- Oogenesis- pre-antral then antral follicle (Graafian follicle is mature antral follicle released)
- Secondary oocyte
- 1 Day before ovulation completes (stim by LH) Meiosis 1
- haploid: 22 chromosomes + 1 X chromosome (23, X)
- nondisjunction- abnormal chromosome segregation
- begins Meiosis 2 and arrests at metaphase
- note no interphase replication of DNA, only fertilization will complete Meiosis 2
Ovulation (HPG Axis)
- Hypothalmus releases gonadotropin releasing hormone (GRH, luteinizing hormone–releasing hormone, LHRH) -> Pituitary releases follicle stimulating hormone (FSH) and lutenizing hormone (LH) -> ovary follicle development and ovulation.
- release of the secondary oocyte and formation of corpus luteum
- secondary oocyte encased in zona pellucida and corona radiata
- Ovulation associated with follicle rupture and ampulla movement.
Zona Pellucida
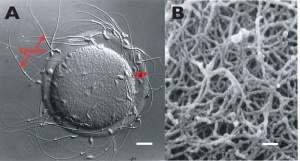
zona pellucida glycoprotein shell ZP1, ZP2, ZP3, ZP4 (mouse only ZP1-3)
- mechanical protection of egg
- involved in the fertilization process
- sperm binding
- adhesion of sperm to egg
- acrosome reaction
- releases enzymes to locally breakdown
- cortical granules modify to block of polyspermy
- altered to prevent more than 1 sperm penetrating
- mechanical protection of zygote, blastomeres, morula and blastocyst
- role in development of the blastocyst
Corona Radiata
- granulosa cells and extracellular matrix
- protective and nutritional role for cells during transport
- cells are also lost during transport along oviduct
Spermatogenesis
- process of spermatagonia mature into spermatozoa (sperm)
- continuously throughout life occurs in the seminiferous tubules in the male gonad- testis (plural testes)
- at puberty spermatagonia activate and proliferate (mitosis)
- primary spermatocyte -> secondary spermatocyte-> spermatid->sperm
- Seminiferous Tubule is site of maturation involving meiosis and spermiogenesis
- Spermatogenesis- Meiosis
- meiosis is reductive cell division
- 1 spermatagonia (diploid) 46, XY (also written 44+XY) = 4 sperm (haploid); 23, X 23, X 23, Y 23, Y
Spermiogenesis
- morphological (shape) change from round spermatids to elongated sperm
- loose cytoplasm
- Transform golgi apparatus into acrosome (in head)
- Organize microtubules for motility (in tail, flagellum)
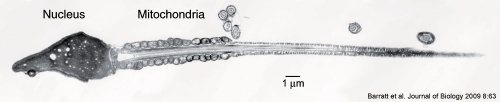
- Segregate mitochondria for energy (in tail)
- Links: spermatozoa
Ejaculate
- Human Ejaculate
- By volume <10 % sperm and accessory glands contribute majority of volume (60 % seminal vesicle, 10 % bulbourethral, 30 % prostate)
- 3.5 ml, 200-600 million sperm.
- Capacitation is the removal of glycoprotein coat and seminal proteins and alteration of sperm mitochondria.
- Ovulation-inducing factor identified in several species.[9]
- Infertility can be due to Oligospermia, Azoospermia, Immotile Cilia Syndrome
- Oligospermia (Low Sperm Count) - less than 20 million sperm after 72 hour abstinence from sex
- Azoospermia (Absent Sperm) - blockage of duct network
- Immotile Cilia Syndrome - lack of sperm motility
- Links: spermatozoa | prostate
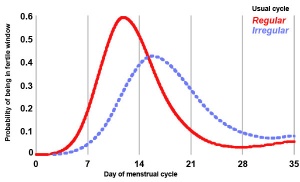
Fertility Window
Clinical guidelines have typically identified the "fertile window" between days 10 and 17 within the typical 28 day menstrual cycle.
Data from a large USA NIEHS - Early Pregnancy Study (1982-86) identified the timing of the “fertile window” within a range of different menstrual cycles.[10]
- fertile window occurred during a broad range of days in the menstrual cycle.
- between days 6 and 21 women had at minimum a 10% probability of being in their fertile window.
- women cannot predict a sporadic late ovulation; 4 - 6% of women whose cycles had not yet resumed were potentially fertile in the fifth week of their cycle.
- only about 30% of women is the fertile window entirely within the days of the menstrual cycle identified by clinical guidelines (between days 10 and 17)
- most women reach their fertile window earlier and others much later.
- women should be advised that the timing of their fertile window can be highly unpredictable, even if their cycles are usually regular.
- Links: menstrual cycle
Fertilization Site
- Fertilization usually occurs in first 1/3 of oviduct
- Fertilization can also occur outside oviduct, associated with In Vitro Fertilization (IVF, GIFT, ZIFT...) and ectopic pregnancy
- The majority of fertilized eggs do not go on to form an embryo
Fertilization - Spermatozoa
- Sperm Binding - zona pellucida protein ZP2 acts as receptor for sperm
- Acrosome Reaction - exyocytosis of acrosome contents (Calcium mediated) MBoC - Figure 20-31. The acrosome reaction that occurs when a mammalian sperm fertilizes an egg
- enzymes to digest the zona pellucida
- exposes sperm surface proteins to bind ZP2
- Membrane Fusion - between sperm and egg, allows sperm nuclei passage into egg cytoplasm
- Contact between spermatozoa and oocyte egg coat (zona pellucida [ZP]) glycoproteins triggers increases in intracellular calcium ion (iCa2+) concentration in spermatozoa[11]
- CATSPER channels on the distal portion of sperm (the principal piece) are required for the ZP-induced iCa2+ increases
- iCa2+ increase starts from the spermatozoa tail and propagates toward the head
- Store depletion-activated Ca2+ entry is thought to mediate the sustained phase
Fertilization - Oocyte
- Membrane Depolarization - in non-mammalian species, caused by sperm membrane fusion, acts as a primary block to polyspermy.
- Cortical Reaction - IP3 pathway elevates intracellular Calcium, exocytosis of cortical granules MBoC - Figure 20-32. How the cortical reaction in a mouse egg is thought to prevent additional sperm from entering the egg
- enzyme alters ZP2 so it will no longer bind sperm plasma membrane
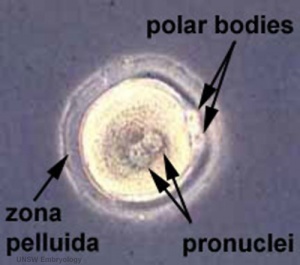
- Meiosis 2 - completion of 2nd meiotic division
- forms second polar body (a third polar body may be formed by meiotic division of the first polar body)
Spermatozoa - Oocyte Interaction
Membrane Fusion
Spermatozoa and oocyte fusion in the membrane adhesion area requires the presence of 3 membrane proteins (spermatozoa Izumo1; oocyte receptor Juno and Cd9).[12]
Izumo
The sperm-specific protein Izumo is named for a Japanese shrine dedicated to marriage and is essential for sperm-egg plasma membrane binding and fusion. It interacts with the spermatozoa folate receptor 4 (Folr4).
- Links: OMIM 609278
Juno
folate receptor 4 (Folr4)
Folate receptors are also known as the folic acid (FA) binding protein and bind of 5-methyltetrahydrofolate (5-MeTHF).
CD9
Oocyte cell surface protein acts as a receptor for pregnancy-specific glycoprotein 17 (Psg17).
- Links: OMIM 143030
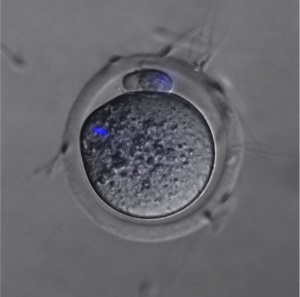
Ovastacin
| (Astacin-Like Metalloendopeptidase, ASTL) An oocyte enzyme (zinc-dependent metalloprotease) involved in altering zona pellucida structure, by cleaving zona pellucida glycoprotein (ZP2), following fertilisation.[13] This change in zona pellucida structure is one of the blockers to polyspermy. | 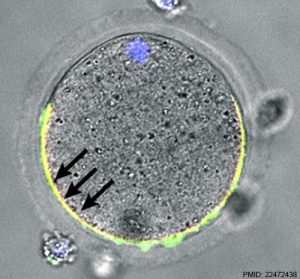 Mouse oocyte cortical granule (green) ovastacin (red) staining.[13] |
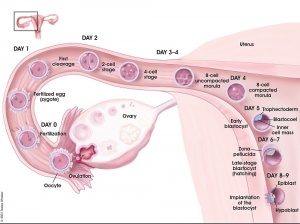
Formation of the Zygote
- Pronuclei - Male and Female haploid nuclei approach each other and nuclear membranes break down
- chromosomal pairing, DNA replicates, first mitotic division
- Spermatozoa contributes - centriole which organizes mitotic spindle
- Oocyte contributes - mitochondria (maternally inherited)
Mitochondria
- Mitochondria of the spermatozoa are specifically destroyed in early development by proteolysis (mouse 4 to 8 cell transition).[14]
- Metaphase II oocytes in rats have an average mitochondrial DNA (mtDNA) copy number of 147,600 (+/-3000) that only increases at the 8-cell stage.[15]
Sex Determination
- based upon whether an X or Y carrying sperm has fertilized the egg, should be 1.0 sex ratio.
- actually 1.05, 105 males for every 100 females, some studies show more males 2+ days after ovulation.
- cell totipotent (equivalent to a stem cell, can form any tissue of the body)
Men - Y Chromosome
- Y Chromosome carries Sry gene, protein product activates pathway for male gonad (covered in genital development)
Women - X Chromosome
- Gene dosage, one {ChrX}} chromosome in each female embryo cell has to be inactivated
- process is apparently random and therefore 50% of cells have father's X, 50% have mother's X
- Note that because men only have 1 X chromosome, if abnormal, this leads to X-linked diseases more common in male that female where bothe X's need to be abnormal.
Fertilization Protein Changes
A recent study in mice has shown that after fertilization the maternal proteins present in the original oocyte are quickly degraded by the zygote stage. MII oocytes have 185,643 different peptides while zygotes contain only 85,369 peptides.[16]
Protein Expression Classified by Molecular Functions
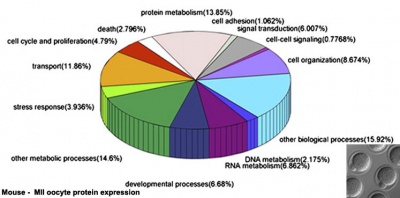
|
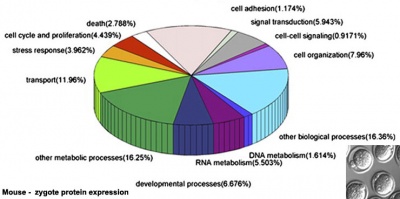
|
Abnormalities
The abnormalities listed below relate to genetic abnormalities resulting in infertility or occurring during fertilisation. There are of course many additional genetic abnormalities inherited and introduced by the recombined maternal and paternal genomes, other than trisomy 21 these will not be covered here. Note the sex different genes responsible shown for the examples of male and female infertility.
Male Infertility Genes
| Gene abbreviation | Name | Gene Location | Online Mendelian Inheritance in Man (OMIM) |
HUGO Gene Nomenclature Committee (HGNC) |
GeneCards (GCID) | Diagnosis |
|---|---|---|---|---|---|---|
| AURKC | Aurora kinase C | 19q13.43 | 603495 | 11391 | GC19P057230 | Macrozoospermia |
| CATSPER1 | Cation channel sperm-associated 1 | 11q13.1 | 606389 | 17116 | GC11M066034 | Asthenozoospermia |
| CFTR | Cystic fibrosis transmembrane conductance regulator | 7q31.2 | 602421 | 1884 | GC07P117465 | Obstructive azoospermia |
| DNAH1 | Dynein axonemal heavy chain 1 | 3p21.1 | 603332 | 2940 | GC03P052350 | Asthenozoospermia |
| DPY19L2 | Dpy-19-like 2 gene | 12q14.2 | 613893 | 19414 | GC12M063558 | Globozoospermia |
| GALNTL5 | Polypeptide N-acetylgalactosaminyltransferase-like 5 | 7q36.1 | 615133 | 21725 | GC07P151956 | Asthenozoospermia |
| MAGEB4 | MAGE family member B4 | Xp21.2 | 300153 | 6811 | GC0XP030260 | Azoospermia |
| NANOS1 | Nanos C2HC-type zinc finger 1 | 10q26.11 | 608226 | 23044 | GC10P119029 | Azoospermia |
| NR0B1 | Nuclear receptor subfamily 0 group B member 1 | Xp21.2 | 300473 | 7960 | GC0XM030322 | Azoospermia |
| NR5A1 | Nuclear receptor subfamily 5 group A member 1 | 9q33.3 | 184757 | 7983 | GC09M124481 | Azoospermia |
| SOHLH1 | Spermatogenesis and oogenesis-specific basic helix–loop–helix 1 | 9q34.3 | 610224 | 27845 | C09M135693 | Azoospermia |
| vSPATA16 | Spermatogenesis-associated 16 | 3q26.31 | 609856 | 29935 | GC03M172889 | Globozoospermia |
| SYCE1 | Synaptonemal complex central element protein 1 | 10q26.3 | 611486 | 28852 | GC10M133553 | Azoospermia |
| TAF4B | TATA-box binding protein-associated factor 4b | 18q11.2 | 601689 | 11538 | GC18P026225 | Azoospermia |
| TEX11 | Testis expressed 11 | Xq13.1 | 300311 | 11733 | GC0XM070528 | Azoospermia |
| TEX15 | Testis expressed 15, meiosis and synapsis associated | 8p12 | 605795 | 11738 | GC08M030808 | Azoospermia |
| WT1 | Wilms tumour 1 | 8p12 | 607102 | 12796 | GC11M032365 | Azoospermia |
| ZMYND15 | Zinc-finger MYND-type containing 15 | 17p13.2 | 614312 | 20997 | GC17P004740 | Azoospermia |
| Table data source[17] (table 1) Links: fertilization | spermatozoa | testis | Male Infertility Genes | Female Infertility Genes | oocyte | ovary | Genetic Abnormalities | ART Asthenozoospermia - (asthenospermia) term for reduced spermatozoa motility. Azoospermia - term for no spermatozoa located in the ejaculate. Globozoospermia - term for spermatozoa with a round head and no acrosome. | ||||||
Female Infertility Genes
| Gene abbreviation | Name | Gene Location | Online Mendelian Inheritance in Man (OMIM) |
HUGO Gene Nomenclature Committee (HGNC) |
GeneCards (GCID) | Diagnosis |
|---|---|---|---|---|---|---|
| BMP15 | Bone morphogenetic protein 15 | Xp11.22 | 300247 | 1068 | GC0XP050910 | Primary ovarian insufficiency |
| CLPP | Caseinolytic mitochondrial matrix peptidase proteolytic subunit | 19p13.3 | 601119 | 2084 | GC19P006369 | Primary ovarian insufficiency |
| EIF2B2 | Eukaryotic translation initiation factor 2B subunit beta | 14q24.3 | 606454 | 3258 | GC14P075002 | Primary ovarian insufficiency |
| FIGLA | Folliculogenesis-specific BHLH transcription factor | 2p13.3 | 608697 | 24669 | GC02M070741 | Primary ovarian insufficiency |
| FMR1 | Fragile X mental retardation 1 | Xq27.3 | 309550 | 3775 | GC0XP147912 | Primary ovarian insufficiency |
| FOXL2 | Forkhead box L2 | 3q22.3 | 605597 | 1092 | GC03M138944 | Primary ovarian insufficiency |
| FSHR | Follicle stimulating hormone receptor | 2p16.3 | 136435 | 3969 | GC02M048866 | Primary ovarian insufficiency |
| GALT | Galactose-1-phosphate uridylyltransferase | 9p13.3 | 606999 | 4135 | GC09P034636 | Primary ovarian insufficiency |
| GFD9 | Growth differentiation factor 9 | 5q31.1 | 601918 | 4224 | GC05M132861 | Primary ovarian insufficiency |
| HARS2 | Histidyl-TRNA synthetase 2, mitochondrial | 5q31.3 | 600783 | 4817 | GC05P141975 | Primary ovarian insufficiency |
| HFM1 | HFM1, ATP-dependent DNA helicase homolog | 1p22.2 | 615684 | 20193 | GC01M091260 | Primary ovarian insufficiency |
| HSD17B4 | Hydroxysteroid 17-beta dehydrogenase 4 | 5q23.1 | 601860 | 5213 | GC05P119452 | Primary ovarian insufficiency |
| LARS2 | Leucyl-TRNA synthetase 2, mitochondrial | 3p21.31 | 604544 | 17095 | GC03P045405 | Primary ovarian insufficiency |
| LHCGR | Luteinizing hormone/choriogonadotropin receptor | 2p16.3 | 152790 | 6585 | GC02M048647 | Primary ovarian insufficiency |
| LHX8 | LIM homeobox 8 | 1p31.1 | 604425 | 28838 | GC01P075128 | Primary ovarian insufficiency |
| MCM8 | Minichromosome maintenance 8 homologous recombination repair factor | 20p12.3 | 608187 | 16147 | GC20P005926 | Primary ovarian insufficiency |
| MCM9 | Minichromosome maintenance 9 homologous recombination repair factor | 6q22.31 | 610098 | 21484 | GC06M118813 | Primary ovarian insufficiency |
| NOBOX | NOBOX oogenesis homeobox | 7q35 | 610934 | 22448 | GC07M144397 | Primary ovarian insufficiency |
| NOG | Noggin | 17q22 | 602991 | 7866 | GC17P056593 | Primary ovarian insufficiency |
| PMM2 | Phosphomannomutase 2 | 16p13.2 | 601785 | 9115 | GC16P008788 | Primary ovarian insufficiency |
| POLG | DNA polymerase gamma, catalytic subunit | 15q26.1 | 174763 | 9179 | GC15M089316 | Primary ovarian insufficiency |
| REC8 | REC8 meiotic recombination protein | 14q12 | 608193 | 16879 | GC14P024171 | Primary ovarian insufficiency |
| SMC1B | Structural maintenance of chromosomes 1B | 22q13.31 | 608685 | 11112 | GC22M045344 | Primary ovarian insufficiency |
| SOHLH1 | Spermatogenesis and oogenesis-specific basic helix–loop–helix 1 | 9q34.3 | 610224 | 27845 | GC09M135693 | Primary ovarian insufficiency |
| STAG3 | Stromal antigen 3 | 7q22.1 | {{Chr 608489 | 11356 | GC07P100177 | Primary ovarian insufficiency |
| SYCE1 | Synaptonemal Complex Central Element Protein 1 | 10q26.3 | 611486 | 28852 | GC10M133553 | Primary ovarian insufficiency |
| TLE6 | Transducin-like enhancer of split 6 | 19p13.3 | 612399 | 30788 | GC19P002976 | Embryonic lethalithy |
| TUBB8 | Tubulin beta 8 Class VIII | 10p15.3 | 616768 | 20773 | GC10M000048 | Oocyte maturation arrest |
| TWNK | Twinkle MtDNA helicase | 10q24.31 | 606075 | 1160 | GC10P100991 | Primary ovarian insufficiency |
| Table data source[17] (table 1) Links: fertilization | oocyte | ovary | | Female Infertility Genes | spermatozoa | testis | Male Infertility Genes | Genetic Abnormalities | ART Primary ovarian insufficiency - depletion or dysfunction of ovarian follicles with cessation of menses before age 40 years. | ||||||
Complete Hydatidiform Mole
- Chromosomal genetic material from the oocyte (ovum, egg) is lost, by an unknown process.
- Fertilization then occurs with one or two spermatozoa and an androgenic (from the male only) conceptus (fertilized oocyte) is formed.
- The embryo (fetus, baby) does not develop at all but the placenta does grow.
- Links: hydatidiform mole
Partial Hydatidiform Mole
Three sets of chromosomes instead of the usual two and this is called triploidy.
- chromosomal (genetic) material from the oocyte (ovum, egg) is retained and the egg is fertilized by one or two spermatozoa.
- with partial mole there are maternal chromosomes and there is a fetus.
- the three sets of chromosomes means the fetus is always grossly abnormal and will not survive.
- Links: hydatidiform mole
Parthenogenesis
(Greek, parthenos = virgin, genesis = birth) The development of an unfertilized oocyte (no spermatozoa). Other than mammals, many different species (plants, insects, reptiles) can develop from unfertilized eggs. An embryo so formed without sperm contribution. Blocking of parthenogenesis in mammals appears to be related to genomic imprinting. Abnormal parthenogenic processes can occur in mammals, and more recently a parthenogenic mouse has been made in the laboratory.
Trisomy 21
Trisomy 21 (Down's or Down syndrome) is caused by nondisjunction of chromosome 21 in a parent who is chromosomally normal and is one of the most common chromosomal aneuploidy abnormalities in liveborn children. The frequency of trisomy 21 in the population is approximately 1 in 650 to 1,000 live births, in Australia between 1991-97 there were 2,358 Trisomy 21 infants. There are other less frequently occurring trisomies (Trisomy 18, Trisomy 13 and Trisomy X).
- Links: Trisomy 21
References
- ↑ Que EL, Duncan FE, Bayer AR, Philips SJ, Roth EW, Bleher R, Gleber SC, Vogt S, Woodruff TK & O'Halloran TV. (2017). Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr Biol (Camb) , 9, 135-144. PMID: 28102396 DOI.
- ↑ Uñates DR, Guidobaldi HA, Gatica LV, Cubilla MA, Teves ME, Moreno A & Giojalas LC. (2014). Versatile action of picomolar gradients of progesterone on different sperm subpopulations. PLoS ONE , 9, e91181. PMID: 24614230 DOI.
- ↑ Bianchi E, Doe B, Goulding D & Wright GJ. (2014). Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature , 508, 483-7. PMID: 24739963 DOI.
- ↑ Yamauchi Y, Shaman JA & Ward WS. (2011). Non-genetic contributions of the sperm nucleus to embryonic development. Asian J. Androl. , 13, 31-5. PMID: 20953203 DOI.
- ↑ Jégou A, Ziyyat A, Barraud-Lange V, Perez E, Wolf JP, Pincet F & Gourier C. (2011). CD9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization. Proc. Natl. Acad. Sci. U.S.A. , 108, 10946-51. PMID: 21690351 DOI.
- ↑ Gahlay G, Gauthier L, Baibakov B, Epifano O & Dean J. (2010). Gamete recognition in mice depends on the cleavage status of an egg's zona pellucida protein. Science , 329, 216-9. PMID: 20616279 DOI.
- ↑ Wassarman PM. (2008). Zona pellucida glycoproteins. J. Biol. Chem. , 283, 24285-9. PMID: 18539589 DOI.
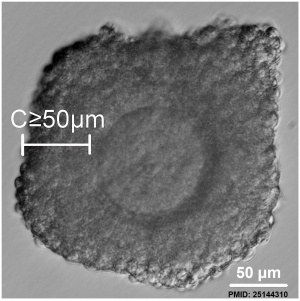
- ↑ Zhou HX, Ma YZ, Liu YL, Chen Y, Zhou CJ, Wu SN, Shen JP & Liang CG. (2014). Assessment of mouse germinal vesicle stage oocyte quality by evaluating the cumulus layer, zona pellucida, and perivitelline space. PLoS ONE , 9, e105812. PMID: 25144310 DOI.
- ↑ Ratto MH, Leduc YA, Valderrama XP, van Straaten KE, Delbaere LT, Pierson RA & Adams GP. (2012). The nerve of ovulation-inducing factor in semen. Proc. Natl. Acad. Sci. U.S.A. , 109, 15042-7. PMID: 22908303 DOI.
- ↑ Wilcox AJ, Dunson D & Baird DD. (2000). The timing of the "fertile window" in the menstrual cycle: day specific estimates from a prospective study. BMJ , 321, 1259-62. PMID: 11082086
- ↑ Xia J & Ren D. (2009). Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol. Reprod. , 80, 1092-8. PMID: 19211808 DOI.
- ↑ Chalbi M, Barraud-Lange V, Ravaux B, Howan K, Rodriguez N, Soule P, Ndzoudi A, Boucheix C, Rubinstein E, Wolf JP, Ziyyat A, Perez E, Pincet F & Gourier C. (2014). Binding of sperm protein Izumo1 and its egg receptor Juno drives Cd9 accumulation in the intercellular contact area prior to fusion during mammalian fertilization. Development , 141, 3732-9. PMID: 25209248 DOI.
- ↑ 13.0 13.1 Burkart AD, Xiong B, Baibakov B, Jiménez-Movilla M & Dean J. (2012). Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J. Cell Biol. , 197, 37-44. PMID: 22472438 DOI.
- ↑ Cummins JM. (2000). Fertilization and elimination of the paternal mitochondrial genome. Hum. Reprod. , 15 Suppl 2, 92-101. PMID: 11041517
- ↑ Kameyama Y, Filion F, Yoo JG & Smith LC. (2007). Characterization of mitochondrial replication and transcription control during rat early development in vivo and in vitro. Reproduction , 133, 423-32. PMID: 17307910 DOI.
- ↑ Wang S, Kou Z, Jing Z, Zhang Y, Guo X, Dong M, Wilmut I & Gao S. (2010). Proteome of mouse oocytes at different developmental stages. Proc. Natl. Acad. Sci. U.S.A. , 107, 17639-44. PMID: 20876089 DOI.
- ↑ 17.0 17.1 Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I, Lundin K, Mertes H, Morris M, Pennings G, Sermon K, Spits C, Soini S, van Montfoort APA, Veiga A, Vermeesch JR, Viville S & Macek M. (2018). Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur. J. Hum. Genet. , 26, 12-33. PMID: 29199274 DOI.
Textbooks
- Human Embryology (2nd ed.) Larson Ch1 p1-32
- The Developing Human: Clinically Oriented Embryology (6th ed.) Moore and Persaud
- Before we Are Born (5th ed.) Moore and Persaud Ch 2 p14-33
- Essentials of Human Embryology Larson Ch1 p1-16
- Human Embryology Fitzgerald and Fitzgerald Ch2 p8-14
Search NCBI Bookshelf fertilization | fertilisation
Reviews
Bianchi E & Wright GJ. (2016). Sperm Meets Egg: The Genetics of Mammalian Fertilization. Annu. Rev. Genet. , 50, 93-111. PMID: 27617973 DOI.
Mou L & Xie N. (2017). Male infertility-related molecules involved in sperm-oocyte fusion. J. Reprod. Dev. , 63, 1-7. PMID: 27904014 DOI.
Articles
Suzuki B, Sugano Y, Ito J, Saito H, Niimura S & Yamashiro H. (2017). Location and expression of Juno in mice oocytes during maturation. JBRA Assist Reprod , 21, 321-326. PMID: 29124919 DOI.
Duncan FE, Que EL, Zhang N, Feinberg EC, O'Halloran TV & Woodruff TK. (2016). The zinc spark is an inorganic signature of human egg activation. Sci Rep , 6, 24737. PMID: 27113677 DOI.
Wilcox AJ, Weinberg CR & Baird DD. (1998). Post-ovulatory ageing of the human oocyte and embryo failure. Hum. Reprod. , 13, 394-7. PMID: 9557845
Search Pubmed
April 2010
- fertilization - All (51803) Review (5928) Free Full Text (11715)
Search Pubmed Now: fertilization | fertilisation | zona pellucida | zygote
Additional Images
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- PBS - First Human Eggs Fertilized in a Laboratory 1944.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Fertilization. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Fertilization
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G