Tongue Development
| Embryology - 9 May 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The tongue's embryonic orgin is derived from all pharyngeal arches contributing different components. As the tongue (Latin, lingua; Greek, glossa) develops "inside" the floor of the oral cavity, it is not readily visible in the external views of the embryonic (Carnegie) stages of development. Tongue muscle cells originate from somites mesoderm, while muscles of mastication derive from the unsegmented somitomeres. This current page gives a brief overview of early tongue development.
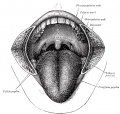
The dorsal tongue is covered by a stratified squamous epithelium, with numerous papillae and taste buds. There are also 8 to 12 circumvallate papillae arranged in an inverted V-shape towards the base of the tongue. These notes cover development of the muscular tongue, not the sense of taste.
| Taste Links: Introduction | Student project | Tongue Development | Category:Taste | ||
|
gastrointestinal tract | head | Category:Tongue
| Historic Embryology - Tongue |
|---|
| 1902 Tongue | 1917 von Ebner's glands | 1921 Tongue |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Tongue Development | Tongue Embryology |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
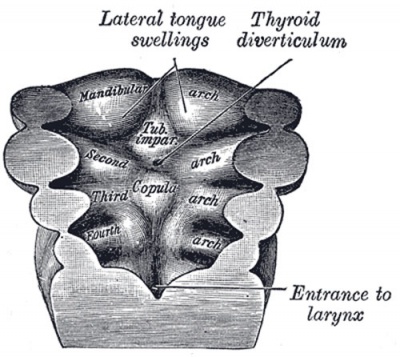
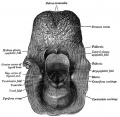
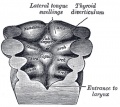
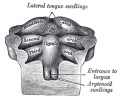
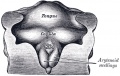
Pharyngeal Arch Contributions
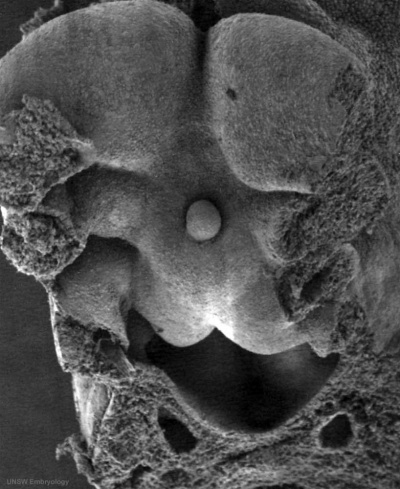
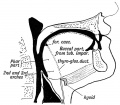
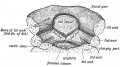
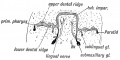
The tongue has contributions from all pharyngeal arches which changes with time. The tongue initially begins as swelling rostral to foramen cecum, the median tongue bud.
|
Animation shows the sequence of development of the tongue. The different colours represents the relative contribution from each pharyngeal arch.
|

|
|
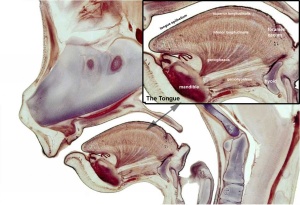
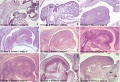
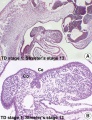
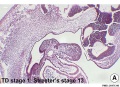
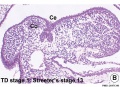
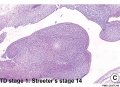
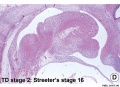
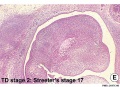
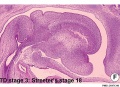
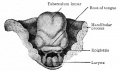
Embryonic
Human embryonic tongue development (mid-sagittal section)[9]
Week 4
- Links: Carnegie stage 13 | Week 4
Week 8
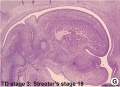
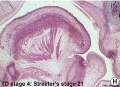
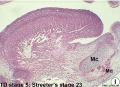
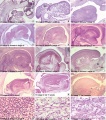
These histology images are from the Carnegie Stage 22 human embryo.
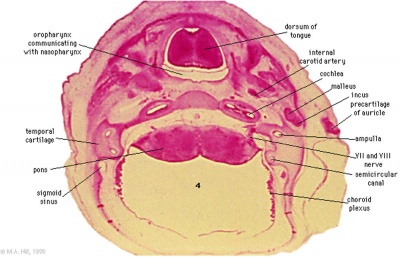
|
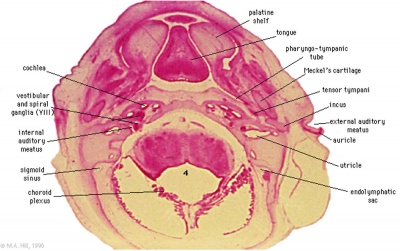
|
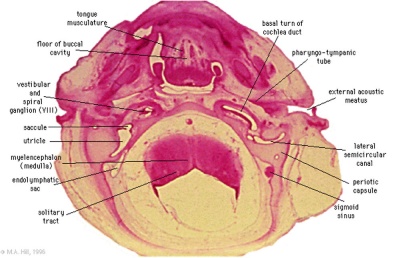
|
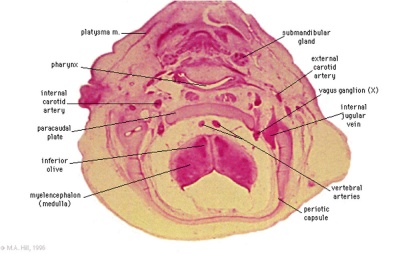
|
Carnegie Stage 23 oral cavity floor
| Carnegie Stage 23 oral cavity floor | |
|---|---|
|
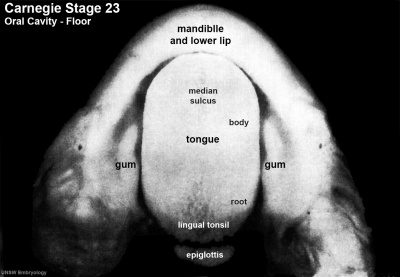
|
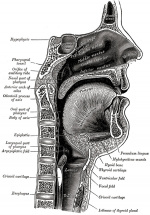
Fetal
Week 10
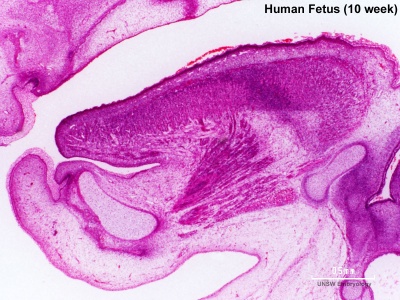
|
Section shows detail of the tongue muscular structure, formed from occipital somites.
The genioglossus (fan-shaped muscle) lies between the tongue and mandible and is innervated by the hypoglossal nerve (CN XII). Poking out your tongue is a test of this nerve. Note the tongue connective tissue and vasculature are derived from neural crest. |
:Links: Week 10 | week 10 - sagittal section
Week 12 - 24
The following measurements of fetal tongue circumference show linear growth during the second trimester and are based upon multiple (2-10) separate ultrasound measurements.[10]
| Fertilisation Age (weeks) |
Gestational Age GA (weeks) |
Mean Circumference (mm) |
|---|---|---|
| 12 | 14 | 28 |
| 13 | 15 | 33 |
| 14 | 16 | 36 |
| 15 | 17 | 37 |
| 16 | 18 | 43 |
| 17 | 19 | 48 |
| 18 | 20 | 51 |
| 19 | 21 | 55 |
| 20 | 22 | 58 |
| 21 | 23 | 62 |
| 22 | 24 | 64 |
| 23 | 25 | 70 |
| 24 | 26 | 73 |
| Table data[10] Tongue circumference measured by transvaginal ultrasonography between 14 and 17 GA weeks, and by abdominal ultrasound between 18 and 26 GA weeks of gestation. Links: tongue | fetal | ultrasound | ||
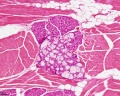
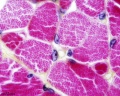
Tongue Muscles
- Tongue muscles originate from the somites.
- Masticatory muscles (MM) originate from the somitomeres. These muscles develop late and are not complete even at birth.
- Tongue muscles develop before masticatory muscles and complete by birth.
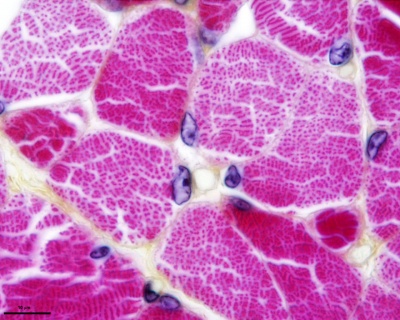
Developing muscle fibers within the tongue. Note the multinucleated appearance of each muscle fiber and their overall organization. Muscle goes through the same developmental changes as other skeletal muscle.
See also: Embryonic and postnatal development of masticatory and tongue muscles.[11]
- Links: Skeletal Muscle Histology
Tongue Innervation
The tongue is innervated by a range of cranial nerve connections related to muscles, oral mucosa, taste buds, and minor salivary glands.
- trigeminal nerve (CN V) - lingual branch
- facial nerve (CN VII) - chorda tympani branch
- glossopharyngeal nerve (CN IX)
- hypoglossal nerve (CN XII) - motor components of innervated muscles.
The hypoglossal nerve (CN CN XII) provides the motor innervation of the intrinsic and extrinsic tongue muscles allowing protrusion, retrusion, and changes in the shape of the tongue. Motor units within the hypoglossal motor system can be categorized as predominantly fast fatigue resistant.[12]
The human tongue innervation has been recently analysed histologically and described as extremely dense and complex.[13] The structure of the motor endplate junctions (neuromuscular junctions) was found to be of the multiple en grappe (grapelike cluster) form. The transverse muscle group that comprises the core of the tongue was found to have the most complex innervation. The pattern of innervation of the human tongue also has specializations not found in other mammalian tongues, this allows for fine motor control of tongue shape.
The pathway of the hypoglossal nerve can be imaged using magnetic imaging (MRI) while computer tomography (CT) can show the bony anatomy of the neurovascular foramina of the skull base. Clinically, the nerve pathway can be divided into three regions: intra-axial, cisternal, skull base and extracranial segments.[14]
- Links: Cranial Nerve Development
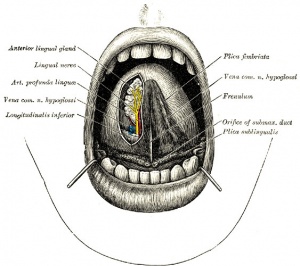
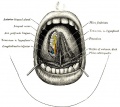
Lingual Frenulum
Frenulum is a general term for a small fold of integument (skin) or mucous membrane that limits the movements of an organ or part. There are several anatomical frenula associated with the genital system, while the lingual frenulum is associated with the inferior side of the tongue.
The lingual frenulum length (short) and position of insertion (anterior) can lead to speech disorders and may affect postnatal feeding.[15] Interestingly, it is the prevalence of pain in mothers breastfeeding infants with ankyloglossia that presents many problems in breastfeeding.[16]
Children with a frenulum length of more than 2 cm do not show these speech problems. Ankyloglossia (tongue-tie) is the general clinical term for the short frenulum which limits the range of movement of the tongue, there is still no accurate classification for this condition.[17] Frenotomy, frenectomy, and frenuloplasty are the main surgical treatment options to release or remove an ankyloglossia.
Histology
Abnormalities
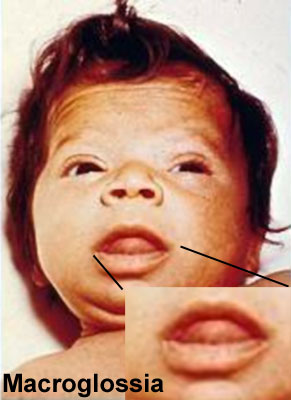
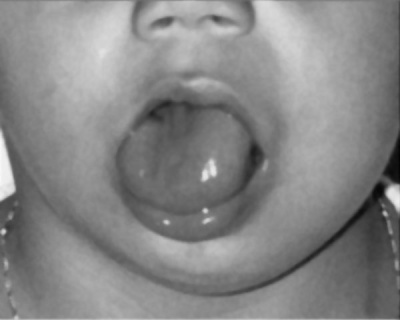
Macroglossia
Term means an abnormally large tongue. Macroglossia is more common than microglossia and can be associated with a number of genetic abnormalities including: trisomy 21 (Down syndrome), acromegaly, Beckwith-Wiedemann syndrome, mucopolysaccharidoses and primary amyloidosis. There is also an association with congenital hypothyroidism and diabetes.
Macroglossia associated with Beckwith-Wiedemann syndrome.
- Links: Trisomy 21 | Medlineplus - Macroglossia
Microglossia
Term means an abnormally small tongue. A recent study has identified cranial neural crest fibroblasts non-canonical transforming growth factor β (TGFβ) regulation of FGF and BMP signalling can cause similar tongue muscle developmental defects.[5]
Ankyloglossia
Ankyloglossia (tongue-tie) is the general clinical term for the short lingual frenulum (less than 2 cm), that limits the range of movement of the tongue, prevalence ranges between 4.2% and 10.7%. This is associated with speech development disorders and has been suggested as also associated with feeding disorders. There is still no accurate classification for this condition.[17] Frenotomy, frenectomy, and frenuloplasty are the main surgical treatment options to release or remove an ankyloglossia, though there is still discussion about surgical intervention.
A short lingual frenulum is also associated with a number of genetic syndromes such as: ROR2-Related Robinow Syndrome, Dystrophic Epidermolysis Bullosa, Oral-Facial-Digital Syndrome Type I, Opitz Syndrome (X-Linked Opitz G/BBB Syndrome) and Van der Woude syndrome.
- Links: Medline Plus - Tongue tie | ROR2-Related Robinow Syndrome | Dystrophic Epidermolysis Bullosa | Oral-Facial-Digital Syndrome Type I | X-Linked Opitz G/BBB Syndrome
Additional Images
Historic
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Human Embryology and Morphology. Keith, A. (1902) London: Edward Arnold.
Anatomy of the Human Gray, H. (1918) Philadelphia: Lea & Febiger.
Text-Book of Embryology Bailey, F.R. and Miller, A.M. (1921). New York: William Wood and Co. - Tongue Development
References
- ↑ Okuhara S, Birjandi AA, Adel Al-Lami H, Sagai T, Amano T, Shiroishi T, Xavier GM, Liu KJ, Cobourne MT & Iseki S. (2019). Temporospatial sonic hedgehog signalling is essential for neural crest-dependent patterning of the intrinsic tongue musculature. Development , 146, . PMID: 31719045 DOI.
- ↑ Castillo-Azofeifa D, Seidel K, Gross L, Golden EJ, Jacquez B, Klein OD & Barlow LA. (2018). SOX2 regulation by hedgehog signaling controls adult lingual epithelium homeostasis. Development , 145, . PMID: 29945863 DOI.
- ↑ Cobourne MT, Iseki S, Birjandi AA, Adel Al-Lami H, Thauvin-Robinet C, Xavier GM & Liu KJ. (2018). How to make a tongue: Cellular and molecular regulation of muscle and connective tissue formation during mammalian tongue development. Semin. Cell Dev. Biol. , , . PMID: 29784581 DOI.
- ↑ Zhu XJ, Yuan X, Wang M, Fang Y, Liu Y, Zhang X, Yang X, Li Y, Li J, Li F, Dai ZM, Qiu M, Zhang Z & Zhang Z. (2017). A Wnt/Notch/Pax7 signaling network supports tissue integrity in tongue development. J. Biol. Chem. , 292, 9409-9419. PMID: 28438836 DOI.
- ↑ 5.0 5.1 Iwata J, Suzuki A, Pelikan RC, Ho TV & Chai Y. (2013). Noncanonical transforming growth factor β (TGFβ) signaling in cranial neural crest cells causes tongue muscle developmental defects. J. Biol. Chem. , 288, 29760-70. PMID: 23950180 DOI.
- ↑ Song Z, Liu C, Iwata J, Gu S, Suzuki A, Sun C, He W, Shu R, Li L, Chai Y & Chen Y. (2013). Mice with Tak1 deficiency in neural crest lineage exhibit cleft palate associated with abnormal tongue development. J. Biol. Chem. , 288, 10440-50. PMID: 23460641 DOI.
- ↑ Aoyama K, Yamane A, Suga T, Suzuki E, Fukui T & Nakamura Y. (2011). Bone morphogenetic protein-2 functions as a negative regulator in the differentiation of myoblasts, but not as an inducer for the formations of cartilage and bone in mouse embryonic tongue. BMC Dev. Biol. , 11, 44. PMID: 21736745 DOI.
- ↑ Kim JY, Lee MJ, Cho KW, Lee JM, Kim YJ, Kim JY, Jung HI, Cho JY, Cho SW & Jung HS. (2009). Shh and ROCK1 modulate the dynamic epithelial morphogenesis in circumvallate papilla development. Dev. Biol. , 325, 273-80. PMID: 19014928 DOI.
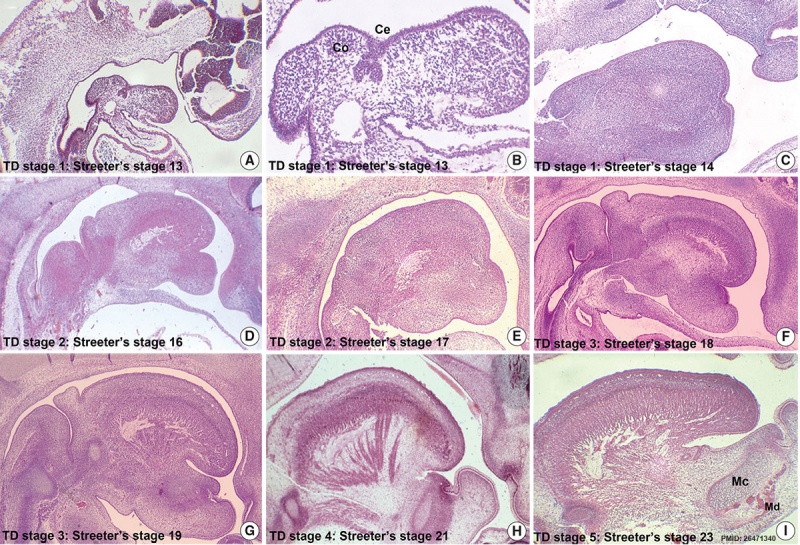
- ↑ Hong SJ, Cha BG, Kim YS, Lee SK & Chi JG. (2015). Tongue Growth during Prenatal Development in Korean Fetuses and Embryos. J Pathol Transl Med , 49, 497-510. PMID: 26471340 DOI.
- ↑ 10.0 10.1 Achiron R, Ben Arie A, Gabbay U, Mashiach S, Rotstein Z & Lipitz S. (1997). Development of the fetal tongue between 14 and 26 weeks of gestation: in utero ultrasonographic measurements. Ultrasound Obstet Gynecol , 9, 39-41. PMID: 9060129 DOI.
- ↑ Yamane A. (2005). Embryonic and postnatal development of masticatory and tongue muscles. Cell Tissue Res. , 322, 183-9. PMID: 16041600 DOI.
- ↑ Smith JC, Goldberg SJ & Shall MS. (2005). Phenotype and contractile properties of mammalian tongue muscles innervated by the hypoglossal nerve. Respir Physiol Neurobiol , 147, 253-62. PMID: 16087149 DOI.
- ↑ Mu L & Sanders I. (2010). Human tongue neuroanatomy: Nerve supply and motor endplates. Clin Anat , 23, 777-91. PMID: 20607833 DOI.
- ↑ Alves P. (2010). Imaging the hypoglossal nerve. Eur J Radiol , 74, 368-77. PMID: 20347541 DOI.
- ↑ Queiroz Marchesan I. (2004). Lingual frenulum: classification and speech interference. Int J Orofacial Myology , 30, 31-8. PMID: 15832860
- ↑ Segal LM, Stephenson R, Dawes M & Feldman P. (2007). Prevalence, diagnosis, and treatment of ankyloglossia: methodologic review. Can Fam Physician , 53, 1027-33. PMID: 17872781
- ↑ 17.0 17.1 Suter VG & Bornstein MM. (2009). Ankyloglossia: facts and myths in diagnosis and treatment. J. Periodontol. , 80, 1204-19. PMID: 19656020 DOI.
Reviews
Cobourne MT, Iseki S, Birjandi AA, Adel Al-Lami H, Thauvin-Robinet C, Xavier GM & Liu KJ. (2018). How to make a tongue: Cellular and molecular regulation of muscle and connective tissue formation during mammalian tongue development. Semin. Cell Dev. Biol. , , . PMID: 29784581 DOI.
Adameyko I & Fried K. (2016). The Nervous System Orchestrates and Integrates Craniofacial Development: A Review. Front Physiol , 7, 49. PMID: 26924989 DOI.
Parada C, Han D & Chai Y. (2012). Molecular and cellular regulatory mechanisms of tongue myogenesis. J. Dent. Res. , 91, 528-35. PMID: 22219210 DOI.
Articles
Michailovici I, Eigler T & Tzahor E. (2015). Craniofacial Muscle Development. Curr. Top. Dev. Biol. , 115, 3-30. PMID: 26589919 DOI.
Noden DM & Francis-West P. (2006). The differentiation and morphogenesis of craniofacial muscles. Dev. Dyn. , 235, 1194-218. PMID: 16502415 DOI.
Achiron R, Ben Arie A, Gabbay U, Mashiach S, Rotstein Z & Lipitz S. (1997). Development of the fetal tongue between 14 and 26 weeks of gestation: in utero ultrasonographic measurements. Ultrasound Obstet Gynecol , 9, 39-41. PMID: 9060129 DOI.
Search PubMed
Search PubMed: Tongue Development | Macroglossia | Microglossia
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Clinical Methods The Tongue
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, May 9) Embryology Tongue Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Tongue_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G