| Embryology - 26 Feb 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The skeleton consists of bone developing from mesoderm, except within the head where neural crest also contributes connective tissues. Each tissue (cartilage, bone, and skeletal muscle) goes through many different mechanisms of differentiation.
The 2 key developmental processes are the initial "patterning" of bone location and then the overt "differentiation" of bone through the process of ossification. For details on specific bone differentiation in human development see Bone Development Timeline.
Bone is formed through a lengthy process involving ossification of a cartilage formed from mesenchyme. Two main forms of ossification occur in different bones, intramembranous ossification (eg skull) and endochondral ossification (eg limb long bones) ossification. Ossification continues postnatally, through puberty until mid 20's. Early ossification occurs at the ends of long bones. A study of 18,713 individuals identified the male/female age at attainment of peak bone mineral density (BMD).[1]
The two major parts of the human skeleton are the axial (80 bones in skull, vertebra, ribs, sternum) and appendicular (126 bones in limbs, shoulders, pelvis) skeletons.
Musculoskeletal and limb abnormalities are one of the largest groups of congenital abnormalities.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Bone Embryology | Bone Development | endochondral ossification | intramembranous ossification | osteoblast | osteoclast |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Adult Human Skeleton
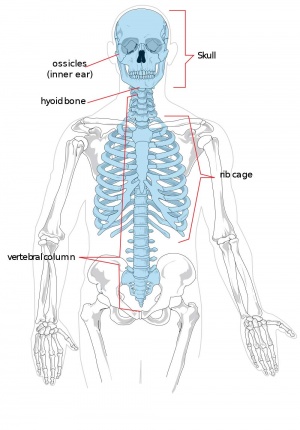
|

|
Textbooks
- The Developing Human: Clinically Oriented Embryology (8th Edition) by Keith L. Moore and T.V.N Persaud - Moore & Persaud Chapter 15 the skeletal system
- Larsen’s Human Embryology by GC. Schoenwolf, SB. Bleyl, PR. Brauer and PH. Francis-West - Chapter 11 Limb Dev (bone not well covered in this textbook)
- Before we Are Born (5th ed.) Moore and Persaud Chapter 16,17: p379-397, 399-405
- Essentials of Human Embryology Larson Chapter 11 p207-228
Objectives
- Identify the components of a somite and the adult derivatives of each component.
- Give examples of sites of (a) endochondral and (b) intramembranous ossification and to compare these two processes.
- Identify the general times (a) of formation of primary and (b) of formation of secondary ossification centres, and (c) of fusion of such centres with each other.
- Briefly summarise the development of the limbs.
- Describe the developmental abnormalities responsible for the following malformations: selected growth plate disorders; congenital dislocation of the hip; scoliosis; arthrogryposis; and limb reduction deformities.
Development Overview
Below is a very brief overview using simple figures of 3 aspects of early musculoskeletal development. More detailed overviews are shown on other notes pages Mesoderm and Somite, Vertebral Column, Limb in combination with serial sections and Carnegie images.
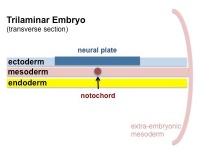
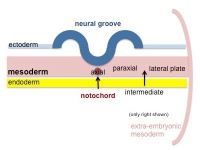
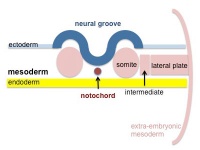
Mesoderm Development
|
|
|
|
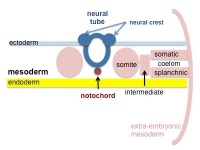
| Cells migrate through the primitive streak to form mesodermal layer. Extraembryonic mesoderm lies adjacent to the trilaminar embryo totally enclosing the amnion, yolk sac and forming the connecting stalk. | Paraxial mesoderm accumulates under the neural plate with thinner mesoderm laterally. This forms 2 thickened streaks running the length of the embryonic disc along the rostrocaudal axis. In humans, during the 3rd week, this mesoderm begins to segment. The neural plate folds to form a neural groove and folds. | Segmentation of the paraxial mesoderm into somites continues caudally at 1 somite/90minutes and a cavity (intraembryonic coelom) forms in the lateral plate mesoderm separating somatic and splanchnic mesoderm. Note intraembryonic coelomic cavity communicates with extraembryonic coelom through portals (holes) initially on lateral margin of embryonic disc. | Somites continue to form. The neural groove fuses dorsally to form a tube at the level of the 4th somite and "zips up cranially and caudally and the neural crest migrates into the mesoderm. |
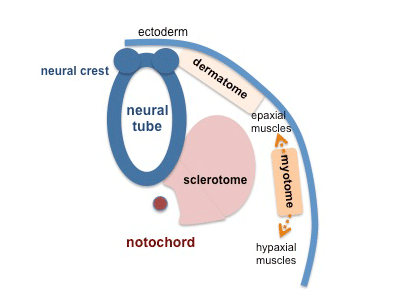
Limb Development
|
Ossification Centres
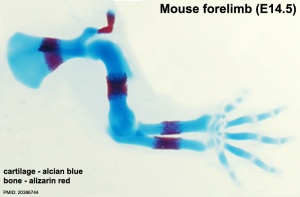
Primary Ossification
- Primary ossification centres are the first sites of bone formation and where cartilage has begun to degenerate. In long bones, this lis generally located mid-diaphysis (shaft). In other bones (e.g. base of skull) these are the initial locations of bone formation.
Secondary Ossification
- Secondary ossification centres develop in the cartilage epiphysis of the long bones.
- No medulary cavity forms in a secondary ossification center.
- Appears late in fetal development.
- Used as a marker for term development if a secondary ossification centre present in either: head of femur, head of tibia, of head of humerus.
- The last secondary centre to appear is the clavical medial epiphysis, that does not develop until 18 or 20 years.
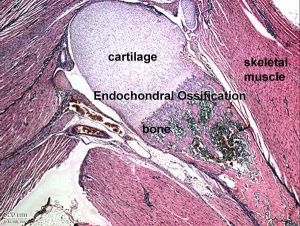
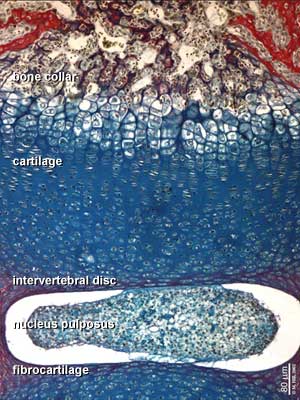
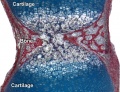
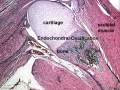
Endochondral Ossification
Most of the bony skeleton forms by this process, that replaces a developmental cartilage template with bone.
| Longitudinal views of endochondral bone formation in mouse limbs.[10] | |||||
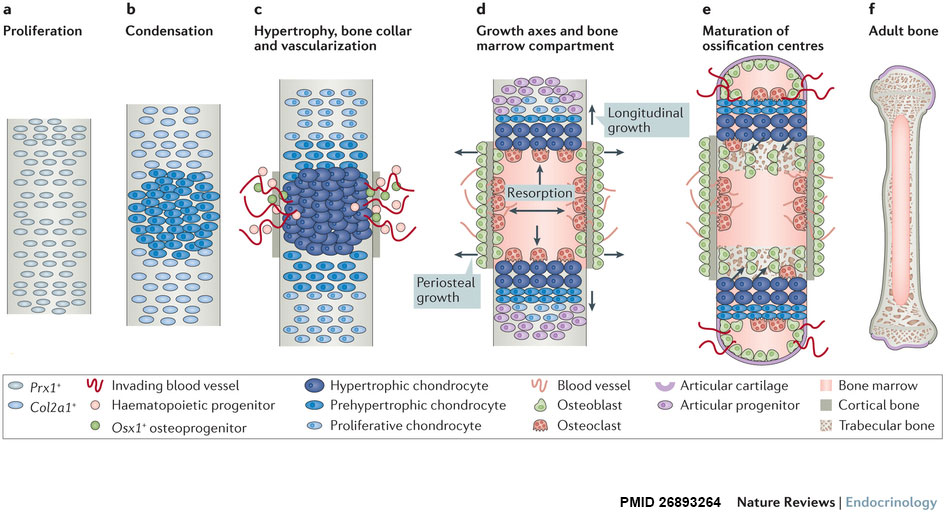
| |||||
| a - Prx1+ progenitors from lateral plate mesoderm proliferate to populate the emerging limb bud. | b - Cells nearest the centre undergo mesenchymal condensation, express Col2a1 as they enter a chondrogenic differentiation program, and deposit a cartilage template. | c to d - Differentiating cells upregulate Col10a1 as they become hypertrophic, which triggers local formation of a bone collar and vascularization of the cartilage template. Invading blood vessels deliver an influx of haematopoietic cells that give rise to osteoclasts which excavate the cartilage template, and Osx1+ osteoblast progenitors and other blood cell types that populate the newly formed marrow cavity. | d - A longitudinal growth axis is established when vascularization and osteoclast-mediated resorption bisect the presumptive skeletal element, producing two growth plates with opposing directionality. A perpendicular growth axis is driven by periosteal osteoblasts and allows the bone to grow in width. | e - Within the remodelled cartilage template, bone-forming osteoblasts are derived from Osx1+ cells arriving with the invading vasculature, as well as hypertrophic Col10a1+ chondroctyes that transdifferentiate as they exit the growth plate into the marrow cavity. As bones grow in length and width, a second wave of vascularization forms the secondary ossification centres. | f - Mature endochondral bone. |
See also Bone Histology
- Links: Blue Histology - endochondral | Dev Biology - endochondral ossification | endochondral ossification animation
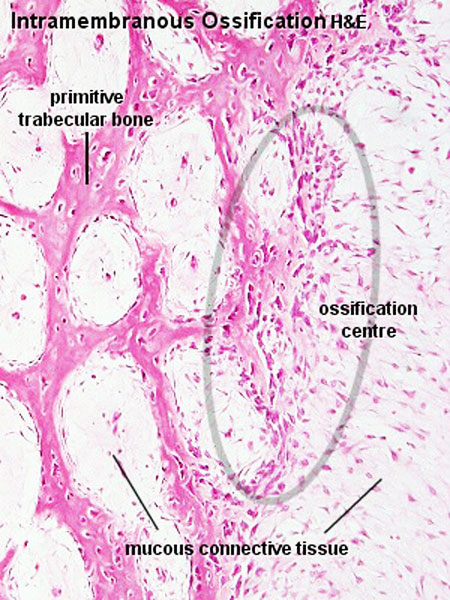
Intramembranous Ossification
The process of intramembranous ossification (desmal ossification) occurs with mesenchyme directly ossifying into bone without a pre-existing cartilage template. Vascularised regions of mesenchymal cells[11] proliferate and differentiate into initially pre-osteoblasts and then osteoblasts, occurs in parts of the skull and the clavicle.
The vascularising endothelial cells promotes mesenchymal intramembranous ossification by BMP signaling pathway activated through Yap/Taz transcriptional activity.[12]
See also Bone Histology
- Links:
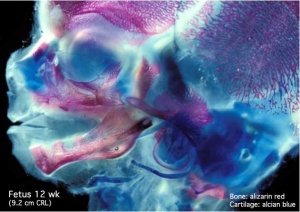
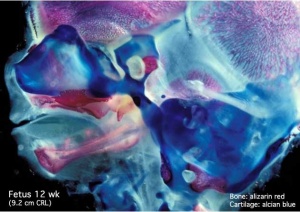
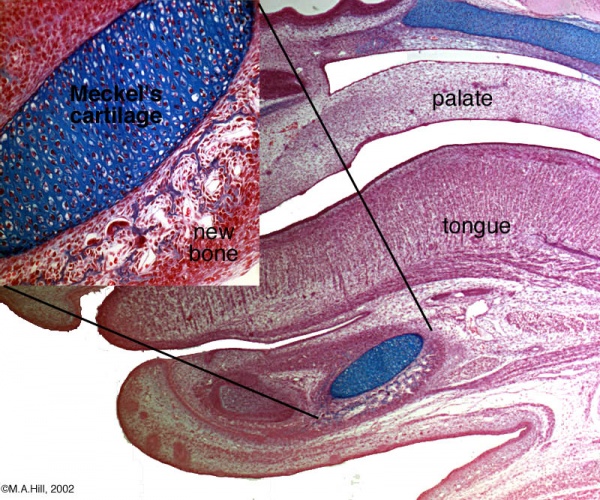
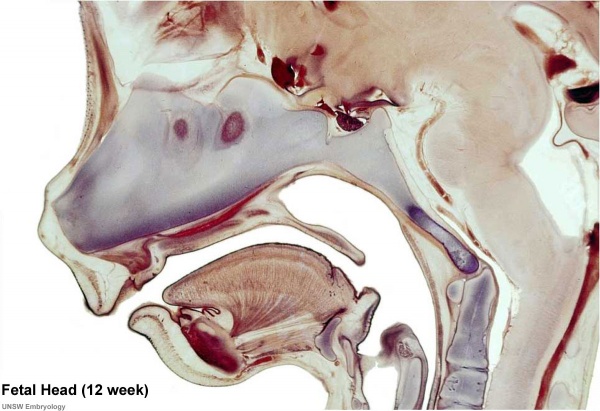
Human Fetal Head (12 week)
Bone Mineral Density
Bone mineral density (BMD) is measured postnatally by an X-ray test and is generally used to detect bone loss associated with osteoporosis. The density of the total hip is a predictor for a hip fracture, while the lumbar spine is the site for monitoring the effect of clinical treatment.
A study of 18,713 individuals, has identified the postnatal male/female age at attainment of peak bone mineral density (BMD).[1]
| Age (years) of Peak Bone Mineral Density (BMD) | |||
|---|---|---|---|
| femoral neck | total hip | lumbar spine | |
| Male | 20.5 | 21.2 | 23 .6 |
| Female | 18.7 | 19.0 | 20.1 |
| Table based on data from[1] {More? bone) | |||
There are two main identified forms of osteoporosis:
- Primary osteoporosis - related to bone loss from ageing.
- Secondary osteoporosis - results from specific conditions that may be reversible.
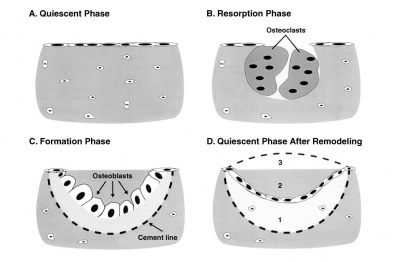
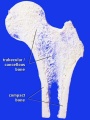
Bone Structure
Terminology
|

|
Compact bone
- (dense) no spaces or hollows in the bone matrix visible to the eye.
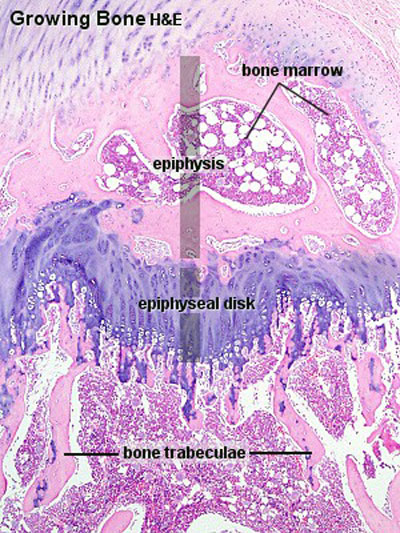
- forms the thick-walled tube of the shaft (or diaphysis) of long bones, which surrounds the marrow cavity (or medullary cavity). A thin layer of compact bone also covers the epiphyses of long bones.
Trabecular bone
- (cancellous or spongy bone) consists of delicate bars (spicules) and sheets of bone, trabeculae
- branch and intersect to form a sponge-like network
- ends of long bones (or epiphyses) consist mainly of trabecular bone.
Periosteum
The embryonic origin of this layer is still controversial. The connective tissue coating covering the surface of bone, except at the articular surfaces, consisting of two distinct main layers with sub-layers.[13]
|
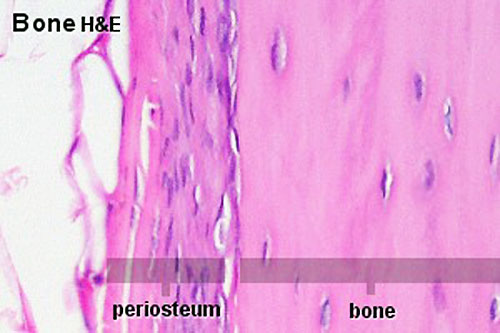
|
Endosteum
Connective tissue lining inner surface of bone.
Bone Growth
- Appositional growth occurs at either the periosteum (outer surface), or the endosteum (inner surface).
- Osteoblasts secrete osteoid, a pre-bone material composed mainly of type I collagen that becomes mineralized.
- Early bone matrix deposited in development and during repair is woven rather than lamellar in appearance and structure.
- In development, there are 2 distinct types of bone formation (intramembranous and endochondral)
Bone Cells
Osteoblasts
- derive from osteogenic stem cells the osteoprogenitor cells that differentiate to form pre-osteoblast then osteoblasts maturing to an osteocyte
- osteoprogenitor cells - "resting cell" line the inner and outer surfaces of bone
Osteocytes
- mature bone-forming cells embedded in lacunae within the bone matrix
- osteoblasts and osteocytes - secrete organic matrix of bone (osteoid), converted into osteocytes when become embedded in matrix (which calcifies soon after deposition)
Osteoclasts
Recently, Heterogeneous nuclear ribonucleoprotein K (hnRNPK), a DNA/RNA-binding protein, has been shown to be essential for the formation of osteoclast.PubmedParser error: Invalid PMID, please check. (PMID: 31538344PMID31538344)
- bone-resorbing multinucleated macrophage-like cells
- originate by fusion of monocytes or macrophages, Blood macrophage precursor, Attach to bone matrix
- seal a small segment of extracellular space (between plasma membrane and bone surface), HCl and lysosomes secreted into this space by osteoclasts dissolves calcium phosphate crystals (give bone rigidity and strength)
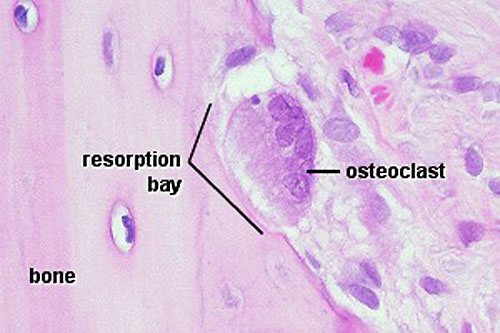
- Resorptive bay - (Howship's lacuna) shallow bay lying directly under an osteoclast.
- do not mistake for megakaryocytes, found in bone marrow not associated with bone matrix.
- megakaryocytes are also multi-niucleated and form platelets
- Links: OMIM - hnRNPK
Osteoclastogenesis
- Formation of mature osteoclasts involved in bone resorption - the osteoblasts regulate this process through the production of RANKL (Receptor Activator for Nuclear Factor κ B Ligand) which is found on the cell surface of osteoblasts.
- RANKL is a key player in rheumatoid arthritis.
- Osteoclast origin - fusion of monocytes or macrophages, Blood macrophage precursor
- Attach to bone matrix - very large cells containing 15-20 nucleii.
- Lysosomes - released into space between ruffled border and bone matrix, enzymes break down collagen fibres, resorption bays or Howship's lacunae
Hippo
Hippo signaling pathway has recently been identified as a regulator of osteoclast formation (see review[14]
- Hippo signaling pathway regulatory molecules - RASSF2, NF2, MST1/2, SAV1, LATS1/2, MOB1, YAP, and TAZ.
- osteoclast differentiation - upon activation, MST and LAST, transcriptional co-activators YAP and TAZ bind to the members of the TEA domain (TEAD) family transcription factors
- regulate expression of downstream target genes connective tissue growth factor (CTGF/CCN2) and cysteine-rich protein 61 (CYR61/CCN1).
- RANKL-mediated signaling cascades including NF-κB, MAPKs, AP1, and NFATc1, Hippo-signaling molecules such as YAP/TAZ/TEAD complex, RASSF2, MST2, and Ajuba could also potentially modulate osteoclast differentiation and function.
- Links: Hippo
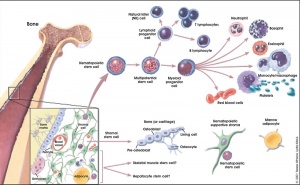
Bone Marrow
- red marrow - mainly haematopoietic (myeloid) tissue, newborn has all red marrow
- yellow marrow - mainly fat cells, found in diaphysis region of long bones
- stromal cells - all other support cells not involved in haematopoiesis
- Links: blood
Marrow stroma components:
- osteoblasts - enclose the marrow compartment in bone tissue.
- endothelial and smooth muscle cells - organized into a complex vascular network composed of arterioles, capillaries, sinusoids, and a large central vein.
- nerves - sensory and sympathetic nerve fibres, glia, and perineural cells that innervate the marrow compartment to form a neural network.
- adipocytes - support metabolic functions of the bone marrow.
- stromal cells - support haematopoiesis and retain skeletal potential.
Bone Matrix
The bone matrix has 2 major components.
- Organic portion composed of mainly collagen Type 1 (about 95%) and amorphous ground substance.
- Inorganic portion (50% dry weight of the matrix) composed of hydroxyapatite crystals, calcium, phosphorus, bicarbonate, nitrate, Mg, K, Na.
- storage calcium and phosphate
- regulate blood calcium levels
Haversian Systems
- also called osteons
- Volkmann's canals - interconnect Haversian systems
Lamellae
- concentric - surrounding each Haversian System
- interstitial - bony plates that fill in between the haversian systems.
- circumferential - layers of bone that underlie the periosteum and endosteum
Cells
- osteocytes extending cytoplasmic processes into canaliculi
- Additional Histology images: low | medium | high
Molecular
The transcription factors Runx2 and Runx3 are essential for chondrocyte maturation, while Runx2 and Osterix are essential for osteoblast differentiation.
Osterix
Osterix (OSX) encodes a transcription factor containing three Cys2-His2 zinc-finger DNA-binding domains at its C terminus that has been shown to be essential for bone formation.
Abnormalities
Osteogenesis Imperfecta
Osteogenesis Imperfecta (OI, brittle bone disease) originally described as a collagen 1 gene mutation, but can have several different genetic causes and can be classified into eight different types (I-VIII).[16]
- COL1A1 and COL1A2 mutations
- CRTAP and LEPRE1 mutations, in severe/lethal and recessively inherited osteogenesis imperfecta
References
- ↑ 1.0 1.1 1.2 Xue S, Kemal O, Lu M, Lix LM, Leslie WD & Yang S. (2020). Age at attainment of peak bone mineral density and its associated factors: The National Health and Nutrition Examination Survey 2005-2014. Bone , 131, 115163. PMID: 31760214 DOI.
- ↑ Funato N. (2020). New Insights Into Cranial Synchondrosis Development: A Mini Review. Front Cell Dev Biol , 8, 706. PMID: 32850826 DOI.
- ↑ Kegelman CD, Nijsure MP, Moharrer Y, Pearson HB, Dawahare JH, Jordan KM, Qin L & Boerckel JD. (2020). YAP and TAZ promote periosteal osteoblast precursor expansion and differentiation for fracture repair. J. Bone Miner. Res. , , . PMID: 32835424 DOI.
- ↑ Wang L, Huang J, Moore DC, Song Y, Ehrlich MG & Yang W. (2019). SHP2 regulates intramembranous ossification by modifying the TGFβ and BMP2 signaling pathway. Bone , 120, 327-335. PMID: 30471432 DOI.
- ↑ Jha S, Laucis N, Kim L, Malayeri A, Dasgupta A, Papadakis GZ, Karantanas A, Torres M & Bhattacharyya T. (2018). CT analysis of anatomical distribution of melorheostosis challenges the sclerotome hypothesis. Bone , 117, 31-36. PMID: 30218789 DOI.
- ↑ Langen UH, Pitulescu ME, Kim JM, Enriquez-Gasca R, Sivaraj KK, Kusumbe AP, Singh A, Di Russo J, Bixel MG, Zhou B, Sorokin L, Vaquerizas JM & Adams RH. (2017). Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat. Cell Biol. , 19, 189-201. PMID: 28218908 DOI.
- ↑ Lin YC, Roffler SR, Yan YT & Yang RB. (2015). Disruption of Scube2 Impairs Endochondral Bone Formation. J. Bone Miner. Res. , 30, 1255-67. PMID: 25639508 DOI.
- ↑ Walzer SM, Cetin E, Grübl-Barabas R, Sulzbacher I, Rueger B, Girsch W, Toegel S, Windhager R & Fischer MB. (2014). Vascularization of primary and secondary ossification centres in the human growth plate. BMC Dev. Biol. , 14, 36. PMID: 25164565 DOI.
- ↑ Galli A, Robay D, Osterwalder M, Bao X, Bénazet JD, Tariq M, Paro R, Mackem S & Zeller R. (2010). Distinct roles of Hand2 in initiating polarity and posterior Shh expression during the onset of mouse limb bud development. PLoS Genet. , 6, e1000901. PMID: 20386744 DOI.
- ↑ Salazar VS, Gamer LW & Rosen V. (2016). BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol , 12, 203-21. PMID: 26893264 DOI.
- ↑ Percival CJ & Richtsmeier JT. (2013). Angiogenesis and intramembranous osteogenesis. Dev. Dyn. , 242, 909-22. PMID: 23737393 DOI.
- ↑ Uemura M, Nagasawa A & Terai K. (2016). Yap/Taz transcriptional activity in endothelial cells promotes intramembranous ossification via the BMP pathway. Sci Rep , 6, 27473. PMID: 27273480 DOI.
- ↑ Dwek JR. (2010). The periosteum: what is it, where is it, and what mimics it in its absence?. Skeletal Radiol. , 39, 319-23. PMID: 20049593 DOI.
- ↑ Yang W, Han W, Qin A, Wang Z, Xu J & Qian Y. (2018). The emerging role of Hippo signaling pathway in regulating osteoclast formation. J. Cell. Physiol. , 233, 4606-4617. PMID: 29219182 DOI.
- ↑ Xu W, Chen Q, Liu C, Chen J, Xiong F & Wu B. (2017). A novel, complex RUNX2 gene mutation causes cleidocranial dysplasia. BMC Med. Genet. , 18, 13. PMID: 28173761 DOI.
- ↑ Shapiro JR & Sponsellor PD. (2009). Osteogenesis imperfecta: questions and answers. Curr. Opin. Pediatr. , 21, 709-16. PMID: 19907330 DOI.
Reviews
32850793
Katsimbri P. (2017). The biology of normal bone remodelling. Eur J Cancer Care (Engl) , 26, . PMID: 28786518 DOI.
Berendsen AD & Olsen BR. (2015). Bone development. Bone , 80, 14-18. PMID: 26453494 DOI.
Takigawa M. (2013). CCN2: a master regulator of the genesis of bone and cartilage. J Cell Commun Signal , 7, 191-201. PMID: 23794334 DOI.
Long F & Ornitz DM. (2013). Development of the endochondral skeleton. Cold Spring Harb Perspect Biol , 5, a008334. PMID: 23284041 DOI.
Dwek JR. (2010). The periosteum: what is it, where is it, and what mimics it in its absence?. Skeletal Radiol. , 39, 319-23. PMID: 20049593 DOI.
Yang Y. (2009). Skeletal morphogenesis during embryonic development. Crit. Rev. Eukaryot. Gene Expr. , 19, 197-218. PMID: 19883365
Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS & Mirams M. (2008). Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. , 40, 46-62. PMID: 17659995 DOI.
Articles
Journals
- BONE is an interdisciplinary forum for the rapid publication of original articles and reviews on basic, translational, and clinical aspects of bone and mineral metabolism.
Search PubMed
Search Pubmed: Bone Development | developmental ossification | endochondral ossification | intramembranous ossification
Additional Images
Terms
| Bone Terms | ||
|---|---|---|
Bone Development
| ||
|
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Virtual Slidebox of Histology (USA) Skeletal system
- e-radiography Ossification
- UWA Blue Histology bone
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, February 26) Embryology Musculoskeletal System - Bone Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Musculoskeletal_System_-_Bone_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G