| Embryology - 7 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Axolotls (Ambystoma mexicanum) are the larval form of the Mexican Salamander amphibian and are an animal model used in limb regeneration studies. Axolotls take about 12 months to reach sexual maturity, males release spermatophore into the water and the female may take them up, eventually laying around 200-600 eggs on plants. Egg development takes two weeks, the tadpole-like young remain attached to the plants for a further two weeks. Loss or amputation of the axolotl limb leads to the regeneration of the lost limb from trunk tissue, thereby repeating a developmental sequence as a repair process.
The sequence of axolotl embryonic developmental stages was characterised in the late 1980's.[1]
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Axolotl Development | Axolotl Embryology | Axolotl Limb Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Developmental Stages
The following stage information is based upon published staging data.[1]
| Stage | Time (hours) | Description |
| 1 | 0 | Freshly laid egg in jellycoat. |
| 2 | First appearance of the first cleavage furrow and the animal pole. | |
| 3 | 2.5 | Four cells. |
| 4 | 4 | Eight cells. |
| 5-6 | 5.5 | 16 cells. |
| 6 | 6.5 - 7 | 32 cells |
| 7 | 8 - 9 | 64 cells |
| 8 | 16 | Early blastula (fall of mitotic index in animal blastomeres). |
| 9 | 21 | Late blastula, surface is smooth. |
| 10 | 26 | Early gastrula I, first sign of dorsal blastopore lip. |
| 11 | 38 | Middle gastrula II, Blastopore covers three quadrants. Lateral lips are formed; ventral lip is marked only by pigment accumulation. Yolk plug reaches its maximum diameter. |
| 12 | 47 | Late gastrula II, Blastopore has an oval or circular shape. |
| 14 | 58 | Early neurula II: Neural plate is broad. Neural folds are outlined and begin to rise above the surface in the head region. Embryo is slightly elongated. |
| 15-16 | 59 - 63 | Early neurula III and middle neurula: Neural plate is shield-shaped and becomes sunken; neural folds are raised and bound all regions of the neural plate. |
| 17 | 64 | Late Neurula I: Neural folds are higher; especially in the head region. Further narrowing and deepening of the neural plate occur both in the head and in the spinal regions. Hyomandibular furrow limiting the mandibular arch is slightly outlined. The segmentation of the mesodermal material begins. There are two pairs of somites. |
| 18 | 66 | Late neurula II: Neural plate is deeply sunken. Neural folds are closing and are especially high in the head region where three slight bulges corresponding to fore-, mid- and hindbrain vesicles are outlined. The neural folds of the spinal region are almost in contact. Hyomandibular furrow is more marked. There are two pairs of somites. |
| 19 | 69 | Late neurula III: Neural folds are in contact throughout, but are not yet fused. Brain curvature is quite distinct in profile; fore-, mid- and hindbrain vesicles are also distinct. The swelling of optic vesicles is outlined (barely visible in these pictures because of unfortunate perspective). Hymandibular furrows are deeper. There are three pairs of somites. |
| 20 | 70 | Late Neurula IV: Späte Neurulation IV: Neural folds are fused in spinal region (or are starting this process in these pictures); in brain region, they are only in contact. Optical vesicles are destinct and becoming larger. Grooves in ectoderm appear at the level of the hindbrain. A very slight swelling marks the future gill region. Mandibular arch becomes prominent and four pairs of somites are present. |
| 22-23 | 73-74 | Neural folds are completely fused. The gill region and the pronephros are distinct; the tailbud is slightly outlined. Five to six pairs of somites are present. Primordium of the ear is outlined as a shallow depression in the ectoderm in the region above the future hyoid arch. The hyobranchial furrow appears, outlining the boundary between the hyoid arch and the first branchial arch. |
| 24 | 80 | Ear pit is outlined and becomes more distinct. The hyobranchial furrow lengthens ventrally. The prophenic swelling is clearly outlined, and both the pronephros itself and the beginning of the pronephric duct are clearly visible Eight or nine pairs of somites are visible. |
| 29 | Since first cleavage, up to 97 hours have passed (sorry, I missed some hours here... ;-) ). Ear pit becomes quite distinct. The gill region is clearly outlined. The pronephric duct is clearly visible along six somites at least. The primordium of the olfactory organ appears as a tubercle on the anterior part of the head; the tailbud is gradually enlarging in all stages. Up to 16 pairs of somites are present. | |
| 30-31 | 110 | The body of the embryo continues to straighten; the tailbud enlarges. Dorsal finfold begins at somite 14-12. A groove appears in the region of the lens primordium (visual system - i.e. eyes) The third branchial furrow becomes apparent in the dorsal part of the gill region. |
| 32-34 | 115 | The dorsal fin develops until it begins at somite 10. |
| 35 | 122 | From this stage on, the body axis from the hindbrain to the tail base are quite straight. Three external gills show ad nodules on the surface of the gill swelling. The lateral line reaches to the sixth somite. The dorsal fin begins at the fifth somite. The first chromatophores appear; heart pulsation begins. |
| 36-37 | 177 | Gills elongate and push venventroposteriorly. No limb buds are yet visible. |
| 38 | Filament sprouts appear as two nodules on each gill. The primordium of the operculum is visible as a fold upon the hyoid arch. Neither of the two rudiments of the perculum reaches the midline. The limb buds are slightly outlined. | |
| 39 | 220 | The first gills have two pairs of filament sprouts; the second and third have three pairs each. The gills cover the limb buds. Both rudiments of the operculum approach the midline. The angle of the mouth begins to show. |
| 40 | 240 | The gills are longer and the number of filaments increases (four pairs in the first gills, six or seven pairs on the second or third gills). The rudiments of the operculum join at the middline. The angles of the mouth are marked more distinctly and limb buds protrude slightly. |
| 41 | 265 | The gills contunie to elongate, the number of filaments increases and they become longer. The mouth is distinctly outlined. The second lateral line runs along the flank toward the limb bud and bypasses it on the ventral side. Hatching begins. |
| 42 | 296 | The gills extend far beyond the forelimb buds. The mouth is completely outlined, but is not broken through. |
| 43 | With galvanic and convulsive movements the larva breaks free of the jelly coat. The mouth either is already opened or will open within the next 24 - 72 hours. | |
| Data[1] | ||
Thyroid Hormone Effects
|
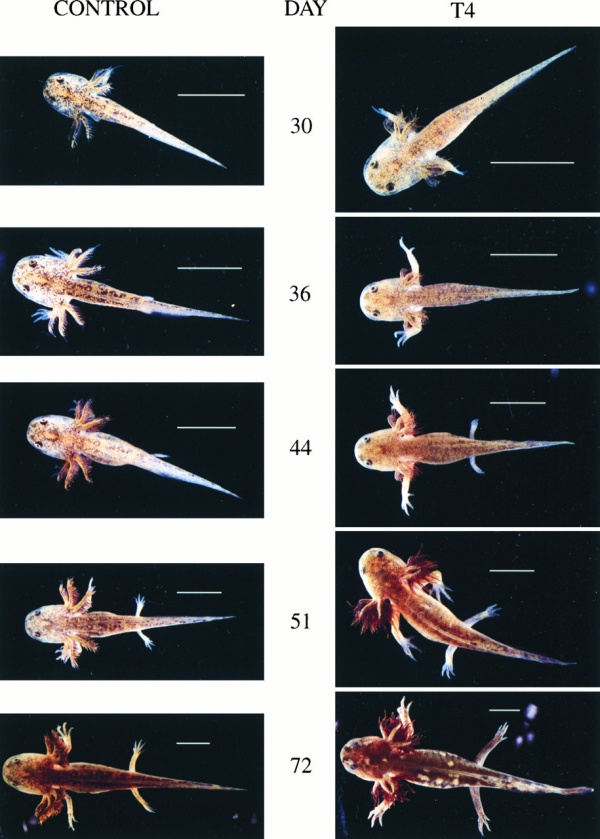
| The effect of thyroxine on the early larval development of the axolotl. The same control and 30 nM T4-treated (TH) sibling animals were photographed at the days postfertilization noted. T4 was added from day 14. (Bar = 1 cm.)[14] |
Limb Development
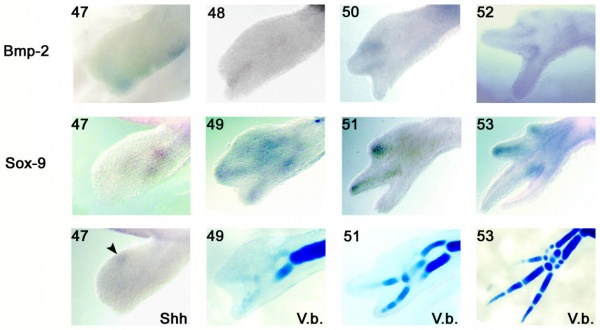
Axolotl developing limb Bmp2 and Sox9 expression and cartilage staining.[15]
Teeth Development
A study has used the axolotl as a model for tooth development.[16] Using transgenic axolotls and fate-mapping approaches they found "evidence of oral teeth derived from both the ectoderm and endoderm and, moreover, demonstrate teeth with a mixed ecto/endodermal origin. Despite the enamel epithelia having a different embryonic source, oral teeth in the axolotl display striking developmental uniformities and are otherwise identical. This suggests a dominant role for the neural crest mesenchyme over epithelia in tooth initiation and, from an evolutionary point of view, that an essential factor in teeth evolution was the odontogenic capacity of neural crest cells, regardless of possible 'outside-in' or 'inside-out' influx of the epithelium."
Additional Images
Historic Images
References
- ↑ 1.0 1.1 1.2 Bordzilovskaya NP, Dettlaf TA, Duhan ST, Malacinski GM: Developmental-stage series of axolotl embryos. In Developmental Biology of the Axolotl. Edited by: Armstrong JB, Malacinski GM. New York: Oxford University Press; 1989:201-219.
- ↑ Wells KM, Kelley K, Baumel M, Vieira WA & McCusker CD. (2021). Neural control of growth and size in the axolotl limb regenerate. Elife , 10, . PMID: 34779399 DOI.
- ↑ Vincent E, Villiard E, Sader F, Dhakal S, Kwok BH & Roy S. (2020). BMP signaling is essential for sustaining proximo-distal progression in regenerating axolotl limbs. Development , 147, . PMID: 32665245 DOI.
- ↑ Crowner A, Khatri S, Blichmann D & Voss SR. (2019). Rediscovering the Axolotl as a Model for Thyroid Hormone Dependent Development. Front Endocrinol (Lausanne) , 10, 237. PMID: 31031711 DOI.
- ↑ Crawford-Young SJ, Dittapongpitch S, Gordon R & Harrington KIS. (2018). Acquisition and reconstruction of 4D surfaces of axolotl embryos with the flipping stage robotic microscope. BioSystems , 173, 214-220. PMID: 30554603 DOI.
- ↑ Nowoshilow S, Schloissnig S, Fei JF, Dahl A, Pang AWC, Pippel M, Winkler S, Hastie AR, Young G, Roscito JG, Falcon F, Knapp D, Powell S, Cruz A, Cao H, Habermann B, Hiller M, Tanaka EM & Myers EW. (2018). The axolotl genome and the evolution of key tissue formation regulators. Nature , 554, 50-55. PMID: 29364872 DOI.
- ↑ Sanchez E, Küpfer E, Goedbloed DJ, Nolte AW, Lüddecke T, Schulz S, Vences M & Steinfartz S. (2018). Morphological and transcriptomic analyses reveal three discrete primary stages of postembryonic development in the common fire salamander, Salamandra salamandra. J. Exp. Zool. B Mol. Dev. Evol. , , . PMID: 29504232 DOI.
- ↑ Sefton EM, Piekarski N & Hanken J. (2015). Dual embryonic origin and patterning of the pharyngeal skeleton in the axolotl (Ambystoma mexicanum). Evol. Dev. , 17, 175-84. PMID: 25963195 DOI.
- ↑ Chatfield J, O'Reilly MA, Bachvarova RF, Ferjentsik Z, Redwood C, Walmsley M, Patient R, Loose M & Johnson AD. (2014). Stochastic specification of primordial germ cells from mesoderm precursors in axolotl embryos. Development , 141, 2429-40. PMID: 24917499 DOI.
- ↑ Lee J & Gardiner DM. (2012). Regeneration of limb joints in the axolotl (Ambystoma mexicanum). PLoS ONE , 7, e50615. PMID: 23185640 DOI.
- ↑ Caulet S, Pelczar H & Andéol Y. (2010). Multiple sequences and factors are involved in stability/degradation of Awnt-1, Awnt-5A and Awnt-5B mRNAs during axolotl development. Dev. Growth Differ. , 52, 209-22. PMID: 20151991 DOI.
- ↑ Hutchison C, Pilote M & Roy S. (2007). The axolotl limb: a model for bone development, regeneration and fracture healing. Bone , 40, 45-56. PMID: 16920050 DOI.
- ↑ Lévesque M, Guimond JC, Pilote M, Leclerc S, Moldovan F & Roy S. (2005). Expression of heat-shock protein 70 during limb development and regeneration in the axolotl. Dev. Dyn. , 233, 1525-34. PMID: 15965983 DOI.
- ↑ Brown DD. (1997). The role of thyroid hormone in zebrafish and axolotl development. Proc. Natl. Acad. Sci. U.S.A. , 94, 13011-6. PMID: 9371791
- ↑ Guimond JC, Lévesque M, Michaud PL, Berdugo J, Finnson K, Philip A & Roy S. (2010). BMP-2 functions independently of SHH signaling and triggers cell condensation and apoptosis in regenerating axolotl limbs. BMC Dev. Biol. , 10, 15. PMID: 20152028 DOI.
- ↑ Soukup V, Epperlein HH, Horácek I & Cerny R. (2008). Dual epithelial origin of vertebrate oral teeth. Nature , 455, 795-8. PMID: 18794902 DOI.
Reviews
Desnitskiy AG. (2018). Cell cycles during early steps of amphibian embryogenesis: A review. BioSystems , 173, 100-103. PMID: 30240720 DOI.
Payzin-Dogru D & Whited JL. (2018). An integrative framework for salamander and mouse limb regeneration. Int. J. Dev. Biol. , 62, 393-402. PMID: 29943379 DOI.
Nowoshilow S, Schloissnig S, Fei JF, Dahl A, Pang AWC, Pippel M, Winkler S, Hastie AR, Young G, Roscito JG, Falcon F, Knapp D, Powell S, Cruz A, Cao H, Habermann B, Hiller M, Tanaka EM & Myers EW. (2018). The axolotl genome and the evolution of key tissue formation regulators. Nature , 554, 50-55. PMID: 29364872 DOI.
Maden M. (2008). Axolotl/newt. Methods Mol. Biol. , 461, 467-80. PMID: 19030817 DOI.
Roy S & Gatien S. (2008). Regeneration in axolotls: a model to aim for!. Exp. Gerontol. , 43, 968-73. PMID: 18814845 DOI.
Frost-Mason SK & Mason KA. (1996). What insights into vertebrate pigmentation has the axolotl model system provided?. Int. J. Dev. Biol. , 40, 685-93. PMID: 8877441
Epperlein HH, Löfberg J & Olsson L. (1996). Neural crest cell migration and pigment pattern formation in urodele amphibians. Int. J. Dev. Biol. , 40, 229-38. PMID: 8735933
Epperlein HH & Löfberg J. (1993). The development of the neural crest in amphibians. Ann. Anat. , 175, 483-99. PMID: 8297037 DOI.
Articles
Brooks GC, Gorman TA, Jiao Y & Haas CA. (2020). Reconciling larval and adult sampling methods to model growth across life-stages. PLoS ONE , 15, e0237737. PMID: 32822355 DOI.
Fei JF, Lou WP, Knapp D, Murawala P, Gerber T, Taniguchi Y, Nowoshilow S, Khattak S & Tanaka EM. (2018). Application and optimization of CRISPR-Cas9-mediated genome engineering in axolotl (Ambystoma mexicanum). Nat Protoc , 13, 2908-2943. PMID: 30429597 DOI.
Nowoshilow S, Schloissnig S, Fei JF, Dahl A, Pang AWC, Pippel M, Winkler S, Hastie AR, Young G, Roscito JG, Falcon F, Knapp D, Powell S, Cruz A, Cao H, Habermann B, Hiller M, Tanaka EM & Myers EW. (2018). The axolotl genome and the evolution of key tissue formation regulators. Nature , 554, 50-55. PMID: 29364872 DOI.
Simon HG. (2012). Salamanders and fish can regenerate lost structures--why can't we?. BMC Biol. , 10, 15. PMID: 22369645 DOI.
Caulet S, Pelczar H & Andéol Y. (2010). Multiple sequences and factors are involved in stability/degradation of Awnt-1, Awnt-5A and Awnt-5B mRNAs during axolotl development. Dev. Growth Differ. , 52, 209-22. PMID: 20151991 DOI.
Hutchison C, Pilote M & Roy S. (2007). The axolotl limb: a model for bone development, regeneration and fracture healing. Bone , 40, 45-56. PMID: 16920050 DOI.
Lévesque M, Guimond JC, Pilote M, Leclerc S, Moldovan F & Roy S. (2005). Expression of heat-shock protein 70 during limb development and regeneration in the axolotl. Dev. Dyn. , 233, 1525-34. PMID: 15965983 DOI.
Nye HL, Cameron JA, Chernoff EA & Stocum DL. (2003). Extending the table of stages of normal development of the axolotl: limb development. Dev. Dyn. , 226, 555-60. PMID: 12619140 DOI.
Meuler DC & Malacinski GM. (1985). An analysis of protein synthesis patterns during early embryogenesis of the urodele--Ambystoma mexicanum. J Embryol Exp Morphol , 89, 71-92. PMID: 4093754
Signoret J. (1980). Evidence of the first genetic activity required in axolotl development. Results Probl Cell Differ , 11, 71-4. PMID: 7444204
Search Pubmed
Search Pubmed: axolotl development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Axolotl - Developmental stages
- Axolotl Org - Developmental stages
- Oxford University Press Developmental Biology of the Axolotl, John B. Armstrong and George M. Malacinski
- [www.axolotl-omics.org Axolotl-omics] website that allows rapid ortholog searches, and access to genome and transcriptomic resources.
| Animal Development: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | goat | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | pig | platypus | rabbit | rat | salamander | sea squirt | sea urchin | sheep | worm | zebrafish | life cycles | development timetable | development models | K12 |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 7) Embryology Axolotl Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Axolotl_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G