ANAT2341 Lab 6: Difference between revisions
mNo edit summary |
No edit summary |
||
| (50 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
PRACTICAL CLASS PROGRAM: | |||
* Weekly Quiz + revision (15 minutes) | |||
* Practical class activities (45 minutes) | |||
* Guest Lecture by A/Prof Kirsty Walters | |||
* Practical Class Revision (15 minutes) | |||
PRACTICAL CLASS ACTIVITIES | |||
* Virtual embryo dissections | |||
* SmartSparrow module | |||
* Specimens of human birth abnormalities | |||
LEARNING OBJECTIVES: | |||
* Understand development of the reproductive system. | |||
* Understand development of the renal system. | |||
* Understand the developmental basis of abnormalities associated with placental development and the reproductive and urinary systems. | |||
GUEST LECTURER - Dr Kirsty Walters | |||
{| | |||
| width=185px valign=top| [[File:Kirsty Walters.jpg|180px]] | |||
[https://research.unsw.edu.au/people/dr-kirsty-walters Dr Kirsty Walters] | |||
| Dr Kirsty Walters is a Senior Lecturer in Women’s and Children’s Health at the University of New South Wales (UNSW), Sydney, specialising in the field of female reproduction and ovarian function. | |||
Background: | |||
Dr Walters was awarded her PhD in 2005 from Edinburgh University, Scotland, and was then recruited by the ANZAC Research Institute (ARI) to undertake a post-doctoral position investigating the role androgens play in regulating female reproduction and physiology. In February 2016 the UNSW recruited her to head up the Ovarian Biology Laboratory based in the Wallace Wurth, which forms part of the world-leading biomedical precinct at UNSW. | |||
Research interests: | |||
Dr Walters’ research involves using customized genetic mouse models in combination with clinical samples and trials to dissect out the fundamental mechanisms regulating female reproduction and polycystic ovary syndrome (PCOS). In particular, her research has focused on understanding the role androgens play in regulating female fertility and PCOS. Findings from this research will identify therapeutic targets for improved treatment of female infertility and the wide range of health issues associated with PCOS, including obesity, insulin resistance, type 2 diabetes and cardiovascular risk. | |||
|} | |} | ||
'''An Introduction to Polycystic Ovary Syndrome (PCOS)''' | |||
<html5media width="600" height="400">https://www.youtube.com/embed/NhyYZCBq5A8</html5media> | |||
'''Polycystic Ovary Syndrome Mouse Model''' | |||
{{#pmid:28320971}} | |||
{{ | |||
{| | |||
| | | [[File:Polycystic ovary syndrome mouse model.jpg|600px]] | ||
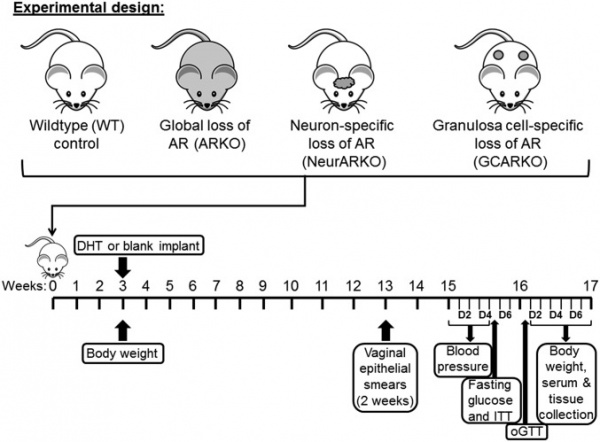
| [[File: | | '''Experimental design''' | ||
| | <br> | ||
For this study, PCOS was induced in wild-type, global, neuron-specific, and granulosa cell-specific androgen receptor knockout mice by s.c. inserting dihydrotestosterone implants in the mice for 3 mo. Control mice were implanted with blank implants. | |||
<br><br> | |||
Body weight, estrous cycling, blood pressure, fasting glucose, oral glucose tolerance, and insulin tolerance were assessed before collection of serum and tissues at 16 wk of age. | |||
<br><br> | |||
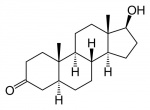
[[File:Dihydrotestosterone.jpg|thumb|150px|link=Genital - Male Development|alt=Dihydrotestosterone structure cartoon|link=Genital - Male Development|Dihydrotestosterone]] | |||
| | |||
'''Dihydrotestosterone''' - The hormonally active form of testosterone (male sex hormone) produced by enzyme (5-alpha reductase) conversion. In the male embryo, this can occur in the genital skin which then supports external genital development. In the adult, this conversion occurs in a number of different tissues. A known treatment for prostate cancer include 5-alpha reductase inhibitors. | |||
|} | |} | ||
'''Role of Androgens in the Ovary''' | |||
{{#pmid:28687450}} | |||
:"It has been well established for decades that androgens, namely {{testosterone}} (T) plays an important role in female reproductive physiology as the precursor for oestradiol (E2). However, in the last decade a direct role for androgens, acting via the androgen receptor (AR), in female reproductive function has been confirmed. Deciphering the specific roles of androgens in ovarian function has been hindered as complete androgen resistant females cannot be generated by natural breeding. In addition, androgens can be converted into estrogens which has caused confusion when interpreting findings from pharmacological studies, as observed effects could have been mediated via the AR or estrogen receptor. The creation and analysis of genetic {{mouse}} models with global and cell-specific disruption of the Ar gene, the sole mediator of pure androgenic action, has now allowed the elucidation of a role for AR-mediated androgen actions in the regulation of normal and pathological ovarian function. This review aims to summarize findings from clinical, animal, pharmacological and novel genetic AR mouse models to provide an understanding of the important roles androgens play in the {{ovary}}, as well as providing insights into the human implications of these roles." | |||
'''References''' | |||
{{#pmid:29635226}} | |||
{{ | {{#pmid:2977958}}1 | ||
{{#pmid:29503209}} | |||
{{#pmid:29365049}} | |||
{{ | {{#pmid:28687450}} | ||
'''Search PubMed:''' [https://www.ncbi.nlm.nih.gov/pubmed/?term=Walters%20KA%5BAuthor%5D&cauthor=true&cauthor_uid=28687450 Walters KA] | |||
{{ | <br> | ||
{{2018ANAT2341}} | |||
Latest revision as of 13:01, 11 June 2020
PRACTICAL CLASS PROGRAM:
- Weekly Quiz + revision (15 minutes)
- Practical class activities (45 minutes)
- Guest Lecture by A/Prof Kirsty Walters
- Practical Class Revision (15 minutes)
PRACTICAL CLASS ACTIVITIES
- Virtual embryo dissections
- SmartSparrow module
- Specimens of human birth abnormalities
LEARNING OBJECTIVES:
- Understand development of the reproductive system.
- Understand development of the renal system.
- Understand the developmental basis of abnormalities associated with placental development and the reproductive and urinary systems.
GUEST LECTURER - Dr Kirsty Walters
An Introduction to Polycystic Ovary Syndrome (PCOS)
<html5media width="600" height="400">https://www.youtube.com/embed/NhyYZCBq5A8</html5media>
Polycystic Ovary Syndrome Mouse Model Caldwell ASL, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ & Walters KA. (2017). Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc. Natl. Acad. Sci. U.S.A. , 114, E3334-E3343. PMID: 28320971 DOI.
Role of Androgens in the Ovary Walters KA & Handelsman DJ. (2017). Role of androgens in the ovary. Mol. Cell. Endocrinol. , , . PMID: 28687450 DOI.
- "It has been well established for decades that androgens, namely testosterone (T) plays an important role in female reproductive physiology as the precursor for oestradiol (E2). However, in the last decade a direct role for androgens, acting via the androgen receptor (AR), in female reproductive function has been confirmed. Deciphering the specific roles of androgens in ovarian function has been hindered as complete androgen resistant females cannot be generated by natural breeding. In addition, androgens can be converted into estrogens which has caused confusion when interpreting findings from pharmacological studies, as observed effects could have been mediated via the AR or estrogen receptor. The creation and analysis of genetic mouse models with global and cell-specific disruption of the Ar gene, the sole mediator of pure androgenic action, has now allowed the elucidation of a role for AR-mediated androgen actions in the regulation of normal and pathological ovarian function. This review aims to summarize findings from clinical, animal, pharmacological and novel genetic AR mouse models to provide an understanding of the important roles androgens play in the ovary, as well as providing insights into the human implications of these roles."
References
Walters KA, Edwards MC, Tesic D, Caldwell ASL, Jimenez M, Smith JT & Handelsman DJ. (2018). The Role of Central Androgen Receptor Actions in Regulating the Hypothalamic-Pituitary-Ovarian Axis. Neuroendocrinology , 106, 389-400. PMID: 29635226 DOI.
Leloup-Hatey J, Baloche S & Jolivet-Jaudet G. (1988). [Changes in corticosterone and aldosterone concentrations in various tissues of Xenopus laevis tadpoles during the metamorphosis]. C. R. Seances Soc. Biol. Fil. , 182, 354-60. PMID: 2977958 1
Bertoldo MJ, Walters KA, Ledger WL, Gilchrist RB, Mermillod P & Locatelli Y. (2018). In-vitro regulation of primordial follicle activation: challenges for fertility preservation strategies. Reprod. Biomed. Online , , . PMID: 29503209 DOI.
Upton DH, Walters KA, McTavish KJ, Holt J, Handelsman DJ & Allan CM. (2018). Reproductive failure in mice expressing transgenic follicle-stimulating hormone is not caused by loss of oocyte quality. Biol. Reprod. , 98, 491-500. PMID: 29365049 DOI.
Walters KA & Handelsman DJ. (2017). Role of androgens in the ovary. Mol. Cell. Endocrinol. , , . PMID: 28687450 DOI.
Search PubMed: Walters KA