Vision - Lens Development
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The lens or crystalline lens or aquula (Latin, aquula = a little stream) has a key role in focussing light (with the cornea) upon the neural retina. The lens embryonic origin is from surface ectoderm of the sensory placodes that form in the head region (More? Placodes).
The lens focusses by refracting light as it passes through the biconvex lens, which can be altered in shape (accommodation) by surrounding ciliary muscles. These ciliary muscles are activated (contracted) by parasympathetic innervation from the ciliary ganglion itself innervated by the oculomotor nerve (Cranial Nerve CN III).
The lens has recently been shown in the chicken model to not be required for specification of the iris and ciliary body.[1]
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Lens Embryology |
Development Overview
surface ectoderm -> lens placode -> lens pit -> lens vesicle -> lens fibres -> lens capsule and embryonic/fetal nucleus.
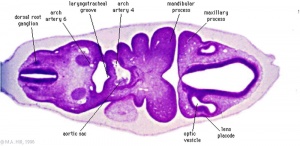
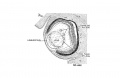
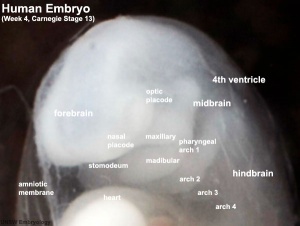
Week 4
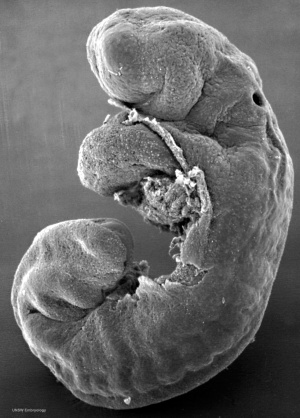
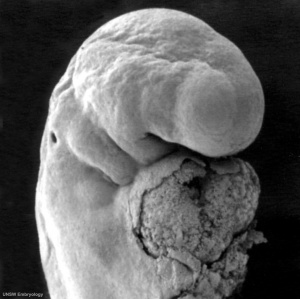
Human Embryo Carnegie stage 11 optic pit
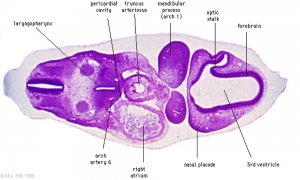
Week 5
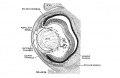
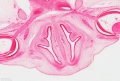
Week 8
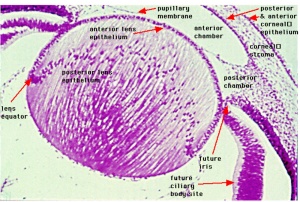
- Human Embryo Lens (week 7-8)
Reference[6]
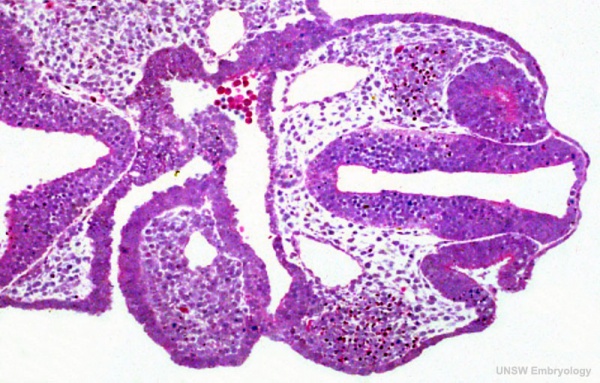
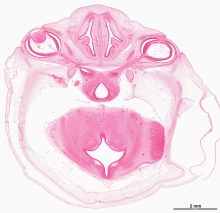
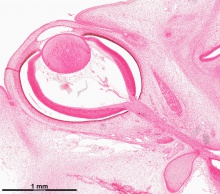
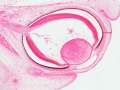
The images below link to virtual slides of the human developing eye at Carnegie stage 22. Click on the image to open or select specific regions from the regions of interest links.
|
|
Virtual Slide - Regions of Interest |
Links: Carnegie stage 22 | Embryo Virtual Slides
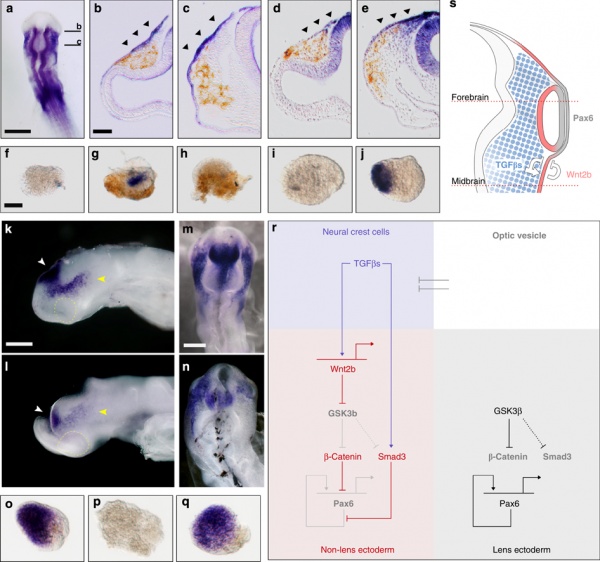
Molecular Signaling
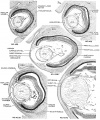
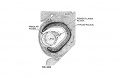
Wnt mediates lens repression by neural crest cells and Transforming growth factor-β[7] (open image for full description)
- Links: Lens Development | Neural Crest Development | Wnt | Lens repression by neural crest cells | Proposed model how NCCs organize the eye | molecular model to explain TGF-β- and Wnt-mediated lens restriction
References
- ↑ Dias da Silva MR, Tiffin N, Mima T, Mikawa T & Hyer J. (2007). FGF-mediated induction of ciliary body tissue in the chick eye. Dev. Biol. , 304, 272-85. PMID: 17275804 DOI.
- ↑ Cvekl A & Ashery-Padan R. (2014). The cellular and molecular mechanisms of vertebrate lens development. Development , 141, 4432-47. PMID: 25406393 DOI.
- ↑ Cantù C, Zimmerli D, Hausmann G, Valenta T, Moor A, Aguet M & Basler K. (2014). Pax6-dependent, but β-catenin-independent, function of Bcl9 proteins in mouse lens development. Genes Dev. , 28, 1879-84. PMID: 25184676 DOI.
- ↑ Augusteyn RC. (2010). On the growth and internal structure of the human lens. Exp. Eye Res. , 90, 643-54. PMID: 20171212 DOI.
- ↑ Burgess D, Zhang Y, Siefker E, Vaca R, Kuracha MR, Reneker L, Overbeek PA & Govindarajan V. (2010). Activated Ras alters lens and corneal development through induction of distinct downstream targets. BMC Dev. Biol. , 10, 13. PMID: 20105280 DOI.
- ↑ Streeter GL. Developmental Horizons In Human Embryos Description Or Age Groups XIX, XX, XXI, XXII, And XXIII, Being The Fifth Issue Of A Survey Of The Carnegie Collection. (1957) Carnegie Instn. Wash. Publ. 611, Contrib. Embryol., 36: 167-196.
- ↑ Grocott T, Johnson S, Bailey AP, Streit A. Neural crest cells organize the eye via TGF-β and canonical Wnt signalling. Nat Commun. 2011 Apr;2:265. PMID21468017 | Nat Commun.
Reviews
Articles
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
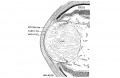
- Human Embryo Lens (week 7-8)
Reference[1]
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Vision - Lens Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Vision_-_Lens_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
- ↑ Streeter GL. Developmental Horizons In Human Embryos Description Or Age Groups XIX, XX, XXI, XXII, And XXIII, Being The Fifth Issue Of A Survey Of The Carnegie Collection. (1957) Carnegie Instn. Wash. Publ. 611, Contrib. Embryol., 36: 167-196.