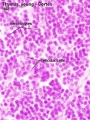
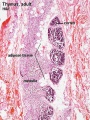
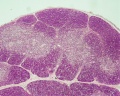
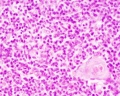
Thymus Development
| Embryology - 5 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The thymus has a key role in the development of an effective immune system as well as an endocrine function. In the adult thymus, specialised microenvironments allow the production of self-tolerant T cells from immature precursors.
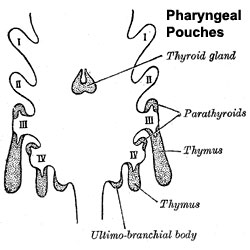
The thymus has two origins for the lymphoid thymocytes and the thymic epithelial cells. The thymic epithelium begins as two flask-shape endodermal diverticula that form from only the third pharyngeal pouch[1] and extend lateralward and backward into the surrounding mesoderm and neural crest-derived mesenchyme (capsule, perivascular)[2][3] in front of the ventral aorta. (More? Neural Crest Development) The immune system T cells are essential for responses against infections and much research concerns the postnatal development of T cells within the thymus.
History - Stieda in 1881[4] was the first to observe that the thymus gland originated from a visceral (pharyngeal) pouch (third pharyngeal pouch endoderm). More recently there was discussion of possible surface ectoderm (third pharyngeal cleft ectoderm) contribution, but mouse tracing studies have discounted this contribution.[5]
The mature thymus epithelium has two main cell types: cortical thymic epithelial (cTECs) and medullary thymic epithelial cells (mTECs) or stromal cells. These thymic stromal cells provide signals for T cell differentiation.
- Cortex - early stages of T cell development, commitment to positive selection.
- Medulla - central tolerance, negative selection and T regulatory cell induction.
The thymic medulla phagocytes negatively selects auto-reactive CD4+ and CD8+ thymocytes, eliminate T cells bearing autoreactive T cell antigen receptors (TCRs). This process to prevent autoimmunity is also known as "negative selection", see adult thymus T cell elimination movie.
| Immune Links: immune | blood | spleen | thymus | lymphatic | lymph node | Antibody | Med Lecture - Lymphatic Structure | Med Practical | Immune Movies | vaccination | bacterial infection | Abnormalities | Category:Immune | ||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Thymus Embryology | thymic epithelial cells | lymphoid thymocytes | Hassall's Corpuscles |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Development Overview
The thymus and parathyroid are derived from 3rd pharyngeal pouches.
Development is a series of epithelial/mesenchymal inductive interactions between neural crest-derived arch mesenchyme and pouch endoderm. There is also the possibility that the surface ectoderm of 3rd pharyngeal clefts participates in thymus development.
Thymic epithelial cells (TECs) are derived from the endoderm of the third pharyngeal pouch.
Hassall's corpuscles (bodies) form between 6 and 10 lunar months in humans. They appear after lymphopoiesis has been established and the cortex, medulla and the cortico-medullary junction are able to select of T lymphocytes undergoing progressive maturation. (Text modified from Bodey and Kaiser, 1997)
Experimental studies have shown that a neural crest contribution is also required during early thymic organogenesis.
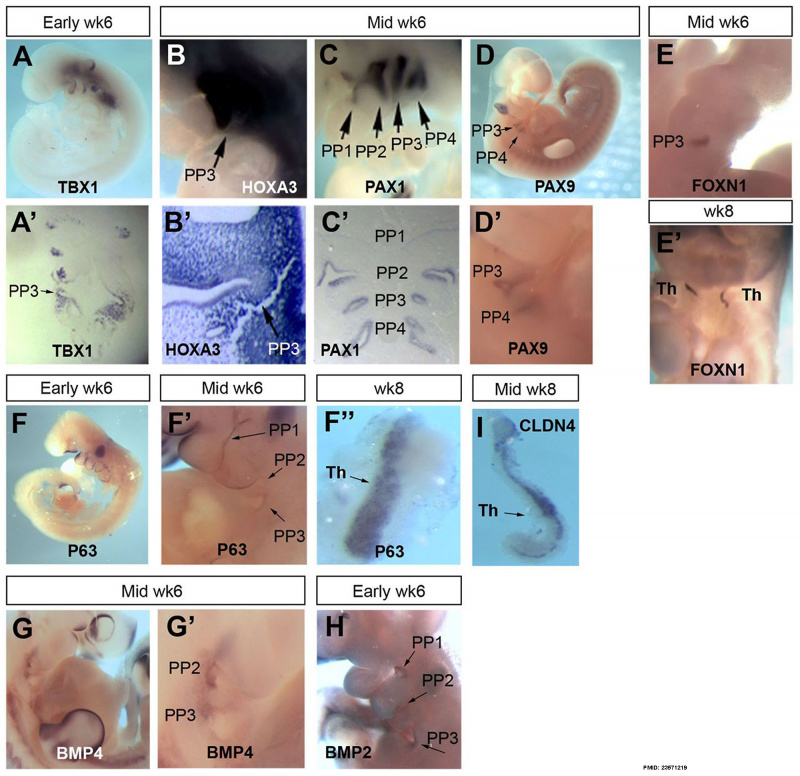
Week 6
| Human thymus week 6[1] |
|---|
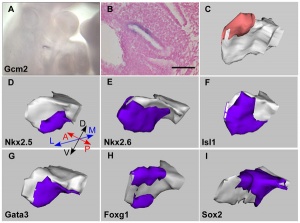
| Expression profile of known regulators of mouse thymus organogenesis in human thymus development.
Images show whole or sectioned human embryos or microdissected thymic primordia after in situ hybridization with the probes indicated. Ages of embryos or dissected thymus lobes are as shown. (A-D′) TBX1, HOXA3 and PAX1 images show side view of whole embryos (top) and coronal sections (bottom). PAX9 images show side view of whole embryo; lower panel shows detail of upper panel. (E,E′) FOXN1 images showing ventral view of whole embryos. (F-F″) P63 images showing side view of whole embryos (left and middle) and dissected thymic lobe. (G-H) BMP4 and BMP2 images showing side view of whole embryos. (I) CLDN4 image showing dissected thymic lobe. Images are representative of at least two independent analyses. Legend: PP1, first PP; PP2, second PP; PP3, third PP; PP4, fourth PP; Th, thymus; Wk, week. Embryos - mid- to late week 6 (17). |
Week 8
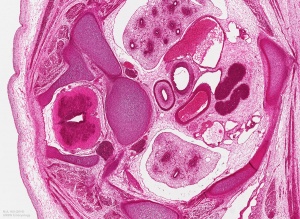
Late embryonic thymus development.
|
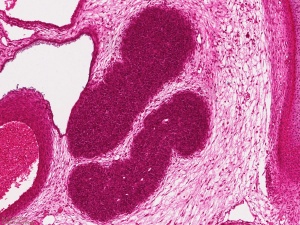
|
| Image shows general position of the developing thymus in entire embryo cross-section. The developing thymus is shown in the midline, located behind the sternum (right) and in front of the oesophagus and trachea. | Selected high power image of thymus from complete cross-section above. |
- Links: Carnegie stage 22
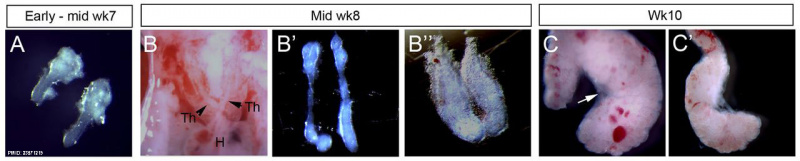
Week 7 to 10
Human thymus week 7-10[1]
| Figure Legend |
|---|
| Expression profile of known regulators of mouse thymus organogenesis in human thymus development.
Images show whole or sectioned human embryos or microdissected thymic primordia after in situ hybridization with the probes indicated. Ages of embryos or dissected thymus lobes are as shown. (A-D′) TBX1, HOXA3 and PAX1 images show side view of whole embryos (top) and coronal sections (bottom). PAX9 images show side view of whole embryo; lower panel shows detail of upper panel. (E,E′) FOXN1 images showing ventral view of whole embryos. (F-F″) P63 images showing side view of whole embryos (left and middle) and dissected thymic lobe. (G-H) BMP4 and BMP2 images showing side view of whole embryos. (I) CLDN4 image showing dissected thymic lobe. Images are representative of at least two independent analyses. Legend: PP1, first PP; PP2, second PP; PP3, third PP; PP4, fourth PP; Th, thymus; Wk, week. Embryos - mid- to late week 6 (17) (Text modified from original figure legend) |
Fetal

|
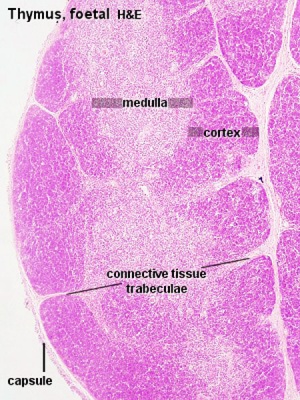
|
| Fetal thymus anatomy | Fetal thymus histology]] |
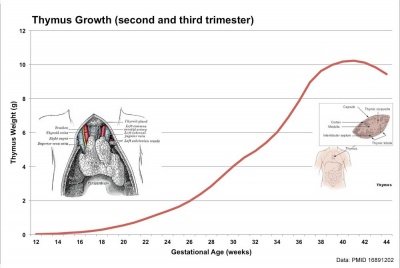
Human Fetal Thymus Weight Growth |
Overall Size Changes with age
|
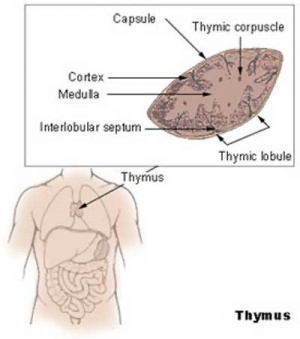
Thymus Anatomy
- Superior mediastinum, anterior to heart
- Bilobed lymphoepithelial organ
- Contains reticular cells but no fibers
- Stem lymphocytes
- proliferate and differentiate
- forms long-lived T- lymphocytes
Mouse Model
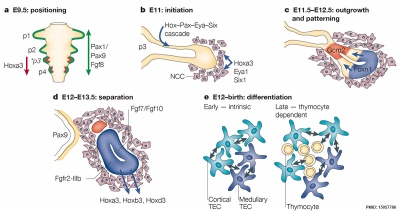
These two figure and descriptions are from a review.[17]
| Structure and function | Mouse model thymus development | |
|---|---|---|
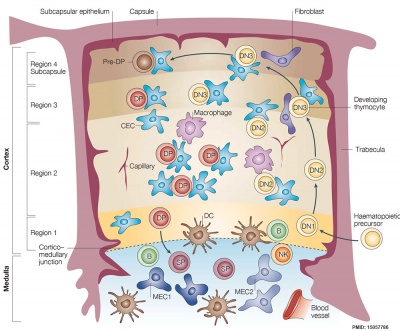
|
|
|
| Cartoon shows the thymus histological regions (cortex and medulla) each contains several different thymic epithelial cell (TEC) subtypes. | Cartoon gives embryonic timing, key events and genes for prenatal mouse thymus development. |
Mouse Thymus Gene Expression Pattern
Data from review.[18]
- Hoxa3 (homeobox A3) - Early E9.5–E10.5, third cleft surface ectoderm.
- Pax1 (paired box gene 1) Early E9.5–E10.5, all pharyngeal pouch endoderm. Late, progressively restricted to a minor population of cells in the adult cortex
- Pax9 (paired box gene 9) Early E9.5–E10.5, all pharyngeal pouch endoderm.
- Eya1 (eyes absent 1 homologue ) - Early E9.5–E10.5, all pharyngeal pouch endoderm, cleft ectoderm and neural crest cell mesenchyme organogenesis. Late N.D.
- Foxn1 (forkhead box N1) - Early E11.25, the thymus domain of third pouch (high level of expression); hair follicles and the epidermis from about E14.5. Late all thymic epithelial cells.
- Links: Mouse Development | Homeobox | FGF | Fox | Pax
Thymus Cell Types
T cell maturation requires interaction with thymic epithelial cells (TECs) in different regions of the thymus lobule
- cortex - (cortical thymic epithelial cells, cTECs)
- medulla - (medullary thymic epithelial cells, mTECs).
- Thymus Histology: Fetal Thymus overview | Fetal Thymus Medulla | Fetal Thymus Cortex | Adult Thymus | unlabeled fetal overview | unlabeled fetal medulla |unlabeled fetal thymic corpuscle |unlabeled fetal cortex | unlabeled adult overview | Category:Thymus | Immune System Development
T Cells
T Cells (T lymphocytes) undergo maturation within the thymus.
- T cell progenitors enter the thymus at the cortex/medulla border via post–capillary venules
- migrate toward the capsule in response to chemokine signalling.
- In the cortex, thymocytes undergo positive selection by cTECs then migrate to the medulla
- In the medulla, thymocytes are screened for reactivity to tissue-restricted self antigens expressed by mTECs.
- Mature T cells exit the thymus via blood or lymphatic vessels in response to a sphingosine-1-phosphate (S1P) gradient.
- cortex and medulla - more numerous (denser) in cortex
- majority of them developing T-lymphocytes (= thymic lymphocytes or thymocytes)
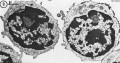
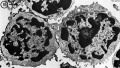
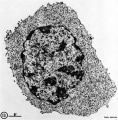
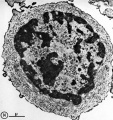
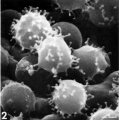
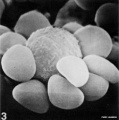
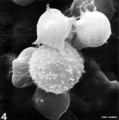
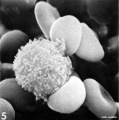
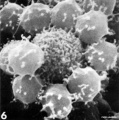
The following images are electron micrographs of T Lymphocyte (T cell) at different stages.
- Lymphocyte EM Images: T and B Lymphocytes 1 TEM | T and B Lymphocytes 2 TEM | T Lymphocyte SEM | B lymphocyte 1 TEM | B lymphocyte 2 TEM | B lymphocyte 3 TEM | Plasma Cell TEM | T2 Lymphocyte 1 TEM | T2 Lymphocyte 2 TEM | lymphocyte rosettes | T lymphocyte 1 | T lymphocyte 2 | T lymphocyte 3 | T lymphocyte 4 | T lymphocyte 5 | T lymphocyte 6 | B lymphocyte | B lymphocytes TEM | Immune System Development | Blood
Thymic Epithelial Cells
Thymic epithelial cells (TECs) Reticuloendothelial cells, reticular cells.
T cell maturation occurs through interaction with thymic epithelial cells (TECs) in different microenvironment and regions of the thymus lobule
- cortex - (cortical thymic epithelial cells, cTECs)
- medulla - (medullary thymic epithelial cells, mTECs)
These cells also ensheathe cortical capillaries, form an epitheloid layer (form blood-thymus barrier)
- abundant, eosinophilic, large, ovoid and light nucleus 1-2 nucleoli
Thymus Macrophages
These Macrophages (phagocytes) are derived from the same lineage as monocytes in the bone marrow.
- located in cortex and medulla
- histologically difficult to distinguish from reticular cells in (Stain - Haematoxylin Eosin)
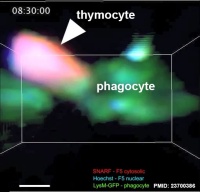
| <html5media height="530" width="490">File:Mouse adult thymus 01.mp4</html5media> | Movie shows how dying thymocytes are efficiently cleared by phagocytes.[19]
Examples of cell death in situ during negative selection. Purified CD4+CD8+ F5 thymocytes labeled with SNARF and Hoechst were introduced into LysM-GFP thymic slices then treated with 1 nM specific peptide for 30 min. The incubation was continued for the indicated times and the slices were imaged by time-lapse two-photon microscopy. The arrowheads point to dy|}ing thymocytes. Scale bars are 5 µm.
|
Blood-Thymus Barrier
The T-lymphocytes in the thymus cortex are separated from cortical capillaries by the "blood-thymus barrier". This is a functional and selective barrier first identified in the 1960's.[21] Venules located at the cortico-medullary junction are "leaky" for blood antigens, while the capillaries draining the cortex are largely impermeable.
The barrier is structurally formed by both epithelioreticular (reticular) cells and macrophages surrounding the cortical blood vessels.
Barrier layers: capillary endothelium - endothelial basal lamina - perivascular CT sheath - basal lamina of epithelioreticular cells - epithelioreticular cell sheath
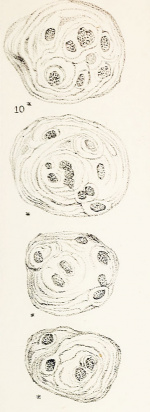
Hassall’s Corpuscles
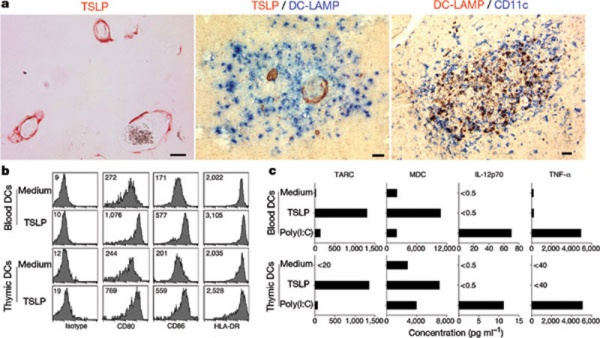
Hassall's corpuscles (Hassall's bodies, thymic corpuscles) are epithelial reticular cells located in the thymic medulla. Several "suggested" functions include removal of apoptotic thymocytes and the maturation of developing thymocytes. A study has shown that these regions instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus.[22]
These features are named after Arthur Hill Hassall (1817-1894) a British physician and chemist who first identified these regions in 1849.[23]
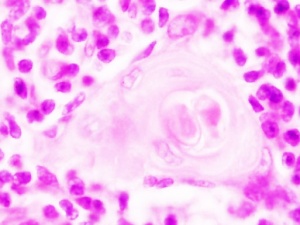
Hassall’s Corpuscle Fetal Thymus (GA week 20)
Human Development
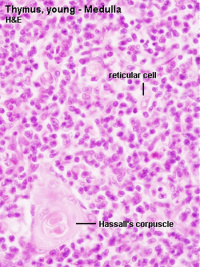
Fetal thymus showing Hassall's corpuscles |
|
Function
Hassall's corpuscles express thymic stromal lymphopoietin (TSLP), suggesting that Hassall's corpuscles have a critical role in dendritic-cell-mediated secondary positive selection of medium-to-high affinity self-reactive T cells, leading to the generation of CD4(+)CD25(+) regulatory T cells within the thymus.[22]
Thymic stromal lymphopoietin is an epithelial cell-derived cytokine expressed in several tissues (skin, gut, lungs, and thymus) that signals through a TSLP receptor (TSLPR). This receptor is a heterodimer of the IL-7 receptor alpha chain and the TSLPR chain.
Disease Association
There has been one report showing changes in Hassall's bodies morphology associated with congenital heart defects.[24]
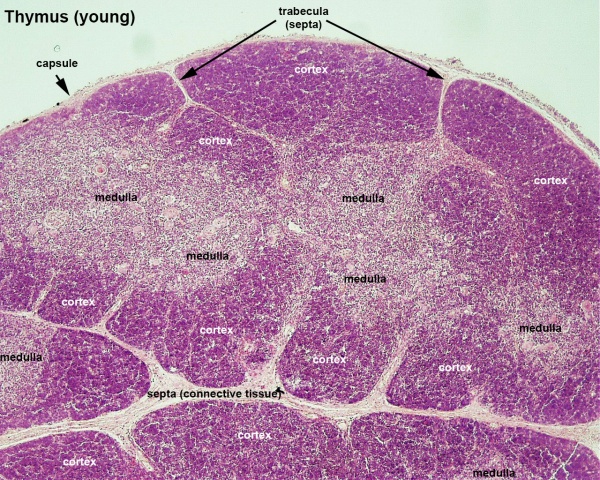
Fetal/Young Thymus
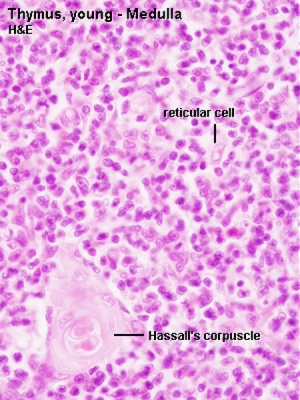
|
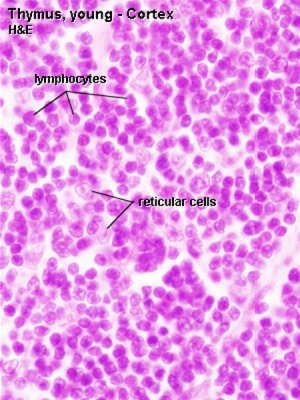
|
| Young medulla | Young cortex |
Thymic corpuscle
Hassall’s corpuscle - Mass of concentric epithelioreticular cells
Thymus Involution
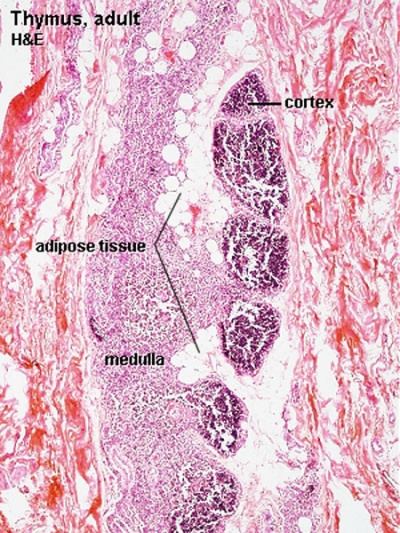
Adult Thymus Histology |
A postnatal process defined as a decrease in the size, weight and activity of the gland with advancing age.
There has been a review of the time course of this process in humans and regression leads to a decline in naїve T cells generated, modifying the composition of the peripheral T cells pool, and altering their phenotype and function.[25]
|
Histology
- Thymus Histology: Fetal Thymus overview | Fetal Thymus Medulla | Fetal Thymus Cortex | Adult Thymus | unlabeled fetal overview | unlabeled fetal medulla |unlabeled fetal thymic corpuscle |unlabeled fetal cortex | unlabeled adult overview | Category:Thymus | Immune System Development
The developing fetal thymus shown below is from a 20 week gestational age (GA), 18 week post-fertilization age, or second trimester stage of development.
Molecular Development
Most molecular data based upon mouse development timing and expression.

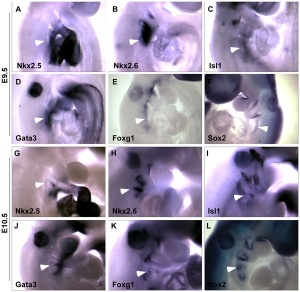
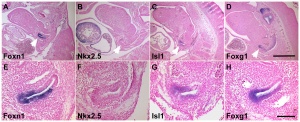
Data from mouse review.[18]
- Hoxa3 (homeobox A3) - Early E9.5 to E10.5, third cleft surface ectoderm.
- Pax1 (paired box gene 1) Early E9.5 to E10.5, all pharyngeal pouch endoderm. Late, progressively restricted to a minor population of cells in the adult cortex
- Pax9 (paired box gene 9) Early E9.5 to E10.5, all pharyngeal pouch endoderm.
- Eya1 (eyes absent 1 homologue ) - Early [[:Category:Mouse E9.5|E9.5 to E10.5, all pharyngeal pouch endoderm, cleft ectoderm and neural crest cell mesenchyme organogenesis. Late N.D.
- Foxn1 (forkhead box N1) - Early E11.25, the thymus domain of third pouch (high level of expression); hair follicles and the epidermis from about E14.5. Late all thymic epithelial cells.
Foxn1
The transcription factor FOXN1 is the master regulator of thyme epithelial cell (TEC) differentiation and function.[27]
Foxg1 and Isl1
Transcription factors that appear to have a role in early thymic epithelial cell (TEC) differentiation.
Cited2
Cited2 deletion in the mouse is embryonic lethal with cardiovascular malformations, adrenal agenesis, cranial ganglia fusion, exencephaly, and left-right patterning defects.[28]
- "Examination of Lmo4-deficient embryos revealed partially penetrant cardiovascular malformations and hypoplastic thymus. Examination of Lmo4;Cited2 compound mutants indicated that there is a genetic interaction between Cited2 and Lmo4 in control of thymus development. Our data suggest that this may occur, in part, through control of expression of a common target gene, Tbx1, which is necessary for normal thymus development."
Eva and Six
Both Eva and Six have been implicated in thymus development.[29]
- Eya - human homolog of the Drosophila 'eyes absent' (Eya) gene.
- Six - vertebrate genes which are homologs of the Drosophila 'sine oculis' (so) gene.
Growth Factors
Adult thymus functions are regulated by a range of factors i, including a range of peptides:
- ghrelin
- leptin
- nerve growth factor
- interleukins
References
- ↑ 1.0 1.1 1.2 1.3 Farley AM, Morris LX, Vroegindeweij E, Depreter ML, Vaidya H, Stenhouse FH, Tomlinson SR, Anderson RA, Cupedo T, Cornelissen JJ & Blackburn CC. (2013). Dynamics of thymus organogenesis and colonization in early human development. Development , 140, 2015-26. PMID: 23571219 DOI.
- ↑ Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D & Coles M. (2008). Contribution of neural crest-derived cells in the embryonic and adult thymus. J. Immunol. , 180, 3183-9. PMID: 18292542
- ↑ Müller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M & Rodewald HR. (2008). Neural crest origin of perivascular mesenchyme in the adult thymus. J. Immunol. , 180, 5344-51. PMID: 18390716
- ↑ Stieda L (1881) Untersuchungen über die Entwickelung der Glandular Thymus, Glandular Thyreoidea, und Glandular carotidica. Leipzig, Engelmann p38.
- ↑ Gordon J, Wilson VA, Blair NF, Sheridan J, Farley A, Wilson L, Manley NR & Blackburn CC. (2004). Functional evidence for a single endodermal origin for the thymic epithelium. Nat. Immunol. , 5, 546-53. PMID: 15098031 DOI.
- ↑ 6.0 6.1 6.2 6.3 Wei Q & Condie BG. (2011). A focused in situ hybridization screen identifies candidate transcriptional regulators of thymic epithelial cell development and function. PLoS ONE , 6, e26795. PMID: 22087235 DOI.
- ↑ Gordon J. (2018). Hox genes in the pharyngeal region: how Hoxa3 controls early embryonic development of the pharyngeal organs. Int. J. Dev. Biol. , 62, 775-783. PMID: 30604847 DOI.
- ↑ García-León MJ, Fuentes P, de la Pompa JL & Toribio ML. (2018). Dynamic regulation of NOTCH1 activation and Notch ligand expression in human thymus development. Development , 145, . PMID: 30042180 DOI.
- ↑ Ayran Fidan P, Kaymaz FF & Dagdeviren A. (2019). Implications for thymus growth in childhood: histogenesis of cortex and medulla. Anat Sci Int , 94, 111-118. PMID: 30155680 DOI.
- ↑ Kernfeld EM, Genga RMJ, Neherin K, Magaletta ME, Xu P & Maehr R. (2018). A Single-Cell Transcriptomic Atlas of Thymus Organogenesis Resolves Cell Types and Developmental Maturation. Immunity , 48, 1258-1270.e6. PMID: 29884461 DOI.
- ↑ Hansen VL & Miller RD. (2017). On the prenatal initiation of T cell development in the opossum Monodelphis domestica. J. Anat. , 230, 596-600. PMID: 28052333 DOI.
- ↑ Vaidya HJ, Briones Leon A & Blackburn CC. (2016). FOXN1 in thymus organogenesis and development. Eur. J. Immunol. , 46, 1826-37. PMID: 27378598 DOI.
- ↑ Diemert A, Hartwig I, Pagenkemper M, Mehnert R, Hansen G, Tolosa E, Hecher K & Arck P. (2016). Fetal thymus size in human pregnancies reveals inverse association with regulatory T cell frequencies in cord blood. J. Reprod. Immunol. , 113, 76-82. PMID: 26851722 DOI.
- ↑ Chojnowski JL, Masuda K, Trau HA, Thomas K, Capecchi M & Manley NR. (2014). Multiple roles for HOXA3 in regulating thymus and parathyroid differentiation and morphogenesis in mouse. Development , 141, 3697-708. PMID: 25249461 DOI.
- ↑ Ge Q & Zhao Y. (2013). Evolution of thymus organogenesis. Dev. Comp. Immunol. , 39, 85-90. PMID: 22266420 DOI.
- ↑ Neves H, Dupin E, Parreira L & Le Douarin NM. (2012). Modulation of Bmp4 signalling in the epithelial-mesenchymal interactions that take place in early thymus and parathyroid development in avian embryos. Dev. Biol. , 361, 208-19. PMID: 22057081 DOI.
- ↑ Gessner JE. (1972). "Bell's" palsy. Md State Med J , 21, 82-3. PMID: 5057786
- ↑ 18.0 18.1 Blackburn CC & Manley NR. (2004). Developing a new paradigm for thymus organogenesis. Nat. Rev. Immunol. , 4, 278-89. PMID: 15057786 DOI.
- ↑ Dzhagalov IL, Chen KG, Herzmark P & Robey EA. (2013). Elimination of self-reactive T cells in the thymus: a timeline for negative selection. PLoS Biol. , 11, e1001566. PMID: 23700386 DOI.
- ↑ Raviola E & Karnovsky MJ. (1972). Evidence for a blood-thymus barrier using electron-opaque tracers. J. Exp. Med. , 136, 466-98. PMID: 4115129
- ↑ Ribatti D. (2015). The discovery of the blood-thymus barrier. Immunol. Lett. , 168, 325-8. PMID: 26522647 DOI.
- ↑ 22.0 22.1 Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W & Liu YJ. (2005). Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature , 436, 1181-5. PMID: 16121185 DOI.
- ↑ Hassall AH. The microscopic anatomy of the human body, in health and disease. (1849) Samuel Hurley, Fleet Street, London.
- ↑ Varga I, Pospisilova V, Jablonska V, Sisovsky V, Galfiova P, Polak S & Adamkov M. (2010). Thymic Hassall's bodies of children with congenital heart defects. Bratisl Lek Listy , 111, 552-7. PMID: 21125801
- ↑ Rezzani R, Nardo L, Favero G, Peroni M & Rodella LF. (2014). Thymus and aging: morphological, radiological, and functional overview. Age (Dordr) , 36, 313-51. PMID: 23877171 DOI.
- ↑ Appay V, Sauce D & Prelog M. (2010). The role of the thymus in immunosenescence: lessons from the study of thymectomized individuals. Aging (Albany NY) , 2, 78-81. PMID: 20354268 DOI.
- ↑ O'Neill KE, Bredenkamp N, Tischner C, Vaidya HJ, Stenhouse FH, Peddie CD, Nowell CS, Gaskell T & Blackburn CC. (2016). Foxn1 Is Dynamically Regulated in Thymic Epithelial Cells during Embryogenesis and at the Onset of Thymic Involution. PLoS ONE , 11, e0151666. PMID: 26983083 DOI.
- ↑ Michell AC, Bragança J, Broadbent C, Joyce B, Franklyn A, Schneider JE, Bhattacharya S & Bamforth SD. (2010). A novel role for transcription factor Lmo4 in thymus development through genetic interaction with Cited2. Dev. Dyn. , 239, 1988-94. PMID: 20549734 DOI.
- ↑ Zou D, Silvius D, Davenport J, Grifone R, Maire P & Xu PX. (2006). Patterning of the third pharyngeal pouch into thymus/parathyroid by Six and Eya1. Dev. Biol. , 293, 499-512. PMID: 16530750 DOI.
Reviews
Thapa P & Farber DL. (2019). The Role of the Thymus in the Immune Response. Thorac Surg Clin , 29, 123-131. PMID: 30927993 DOI.
Vaidya HJ, Briones Leon A & Blackburn CC. (2016). FOXN1 in thymus organogenesis and development. Eur. J. Immunol. , 46, 1826-37. PMID: 27378598 DOI.
Boehm T & Swann JB. (2013). Thymus involution and regeneration: two sides of the same coin?. Nat. Rev. Immunol. , 13, 831-8. PMID: 24052146 DOI.
Gordon J & Manley NR. (2011). Mechanisms of thymus organogenesis and morphogenesis. Development , 138, 3865-78. PMID: 21862553 DOI.
Anderson G, Jenkinson EJ & Rodewald HR. (2009). A roadmap for thymic epithelial cell development. Eur. J. Immunol. , 39, 1694-9. PMID: 19582736 DOI.
Boehm T. (2008). Thymus development and function. Curr. Opin. Immunol. , 20, 178-84. PMID: 18403191 DOI.
Rodewald HR. (2008). Thymus organogenesis. Annu. Rev. Immunol. , 26, 355-88. PMID: 18304000 DOI.
Nowell CS, Farley AM & Blackburn CC. (2007). Thymus organogenesis and development of the thymic stroma. Methods Mol. Biol. , 380, 125-62. PMID: 17876091 DOI.
Savino W. (2006). The thymus is a common target organ in infectious diseases. PLoS Pathog. , 2, e62. PMID: 16846255 DOI.
Holländer G, Gill J, Zuklys S, Iwanami N, Liu C & Takahama Y. (2006). Cellular and molecular events during early thymus development. Immunol. Rev. , 209, 28-46. PMID: 16448532 DOI.
Boehm T, Bleul CC & Schorpp M. (2003). Genetic dissection of thymus development in mouse and zebrafish. Immunol. Rev. , 195, 15-27. PMID: 12969307
Sen J. (2001). Signal transduction in thymus development. Cell. Mol. Biol. (Noisy-le-grand) , 47, 197-215. PMID: 11292256
Bodey B & Kaiser HE. (1997). Development of Hassall's bodies of the thymus in humans and other vertebrates (especially mammals) under physiological and pathological conditions: immunocytochemical, electronmicroscopic and in vitro observations. In Vivo , 11, 61-85. PMID: 9067775
Articles
Albano F, Vecchio E, Renna M, Iaccino E, Mimmi S, Caiazza C, Arcucci A, Avagliano A, Pagliara V, Donato G, Palmieri C, Mallardo M, Quinto I & Fiume G. (2019). Insights into Thymus Development and Viral Thymic Infections. Viruses , 11, . PMID: 31505755 DOI.
Kondo K, Ohigashi I & Takahama Y. (2018). Thymus machinery for T-cell selection. Int. Immunol. , , . PMID: 30476234 DOI.
Calder AE, Hince MN, Dudakov JA, Chidgey AP & Boyd RL. (2011). Thymic involution: where endocrinology meets immunology. Neuroimmunomodulation , 18, 281-9. PMID: 21952680 DOI.
Cromi A, Ghezzi F, Raffaelli R, Bergamini V, Siesto G & Bolis P. (2009). Ultrasonographic measurement of thymus size in IUGR fetuses: a marker of the fetal immunoendocrine response to malnutrition. Ultrasound Obstet Gynecol , 33, 421-6. PMID: 19306477 DOI.
Itoi M, Tsukamoto N, Yoshida H & Amagai T. (2007). Mesenchymal cells are required for functional development of thymic epithelial cells. Int. Immunol. , 19, 953-64. PMID: 17625108 DOI.
Zalel Y, Gamzu R, Mashiach S & Achiron R. (2002). The development of the fetal thymus: an in utero sonographic evaluation. Prenat. Diagn. , 22, 114-7. PMID: 11857615
Search PubMed: Thymus Development | Thymus Embryology
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Sudler, MT. The Development of the Nose and of the Pharynx and its Derivatives in Man. (1902) Amer. J. Anat 1:391–416. Thymus Gland
Terms
- adenoid - (Greek " +-oeides = in form of) in the form of a gland, glandular; the pharyngeal tonsil.
- afferent lymph - vessel carrying lymph towards a node containing antigen-presenting cells, antigen, effector and memory T cells, and regulatory T cells.
- acquired immune deficiency syndrome - (AIDS) note this is now better described as "advanced HIV disease", decrease in the number of CD4 T cells. (More? Immunobiology - AIDS)
- anastomose - joining of two tubes or structures together.
- Antibody mediated immunity - the immune function of plasma cells (active B lymphocytes) secreting antibody which binds antigen.
- antibodies - mammals have five classes (IgA, IgD, IgE, IgG, and IgM)
- antigen - any substance that is recognised by the immune system and stimulates antibody production.
- appendix - is a gut-associated lymphoid tissue (GALT) located at the beginning of the colon. The anatomy is as a finger-like structure that arises from the cecum. The length (2.5-13 cm) is longer in both infants and children and also has more abundant lymphatic tissue in early life. The wall structure is similar to the small intestine (though with no villi), nor plicae circularis. Lymph nodules surround the lumen of the gastrointestinal tract and extend from the mucosa into the submucosa.
- B cell - (B-cell, B lymphocyte) historically named after a structure called the bursa of Fabricius in birds, a source of antibody-producing lymphocytes. These immune cells develop in the bone marrow. (More? Electron micrographs of nonactivate and activated lymphocytes)
- blood - liquid connective tissue containing cells of the lymphatic system see Cardiovascular terms
- B lymphocyte - (B cell, B-cell)
- BALT - (Bronchus Associated Lymphoid Tissue) immune tissue associated with the respiratory tract.
- band cell - (band neutrophil or stab cell) immature neutrophil seen in bone marrow smear, a cell undergoing granulopoiesis, derived from a metamyelocyte, and leading to a mature granulocyte. Also occasionally seen in circulating blood.
- bone marrow sinusoid - endothelial cells and no supporting cells vascular space supplied by arteriole and capillary vessels, interconnected by inter-sinusoidal capillaries, spanning throughout the bone marrow. Radially distributed around the draining central sinus (about 100 µm in diameter). Bone marrow sinusoids are unique and are not comparable with regular veins.
- cecum - (caecum, Latin, caecus = "blind") within the gastrointestinal tract a pouch that connects the ileum with the ascending colon of the large intestine.
- cell - has a specific cell biology definition, but is often used instead of "lymphocyte" when describing B and T cells.
- cell-mediated immunity - the immune function of T lymphocytes. (More? Immunobiology - T Cell-Mediated Immunity)
- central tolerance - in thymus mediated by cortical epithelial cells, medullary epithelial cells and thymic dendritic cells, involves deletion of self reactive thymocytes (T cell) (see [https://www.ncbi.nlm.nih.gov/pubmed/30476234 PMID30476234).
- "clockface" - a term used to describe the appearance of plasma cell nuclei due to the clumping of the chromatin at the nucleus periphery. More clearly seen in tissue plasma cells that the bone marrow smear, where they are sometimes confused with the basophilic erythroblasts. Image - plasma cell
- CD - (cluster of differentiation) identifies immunological surface markers on cells. Positive (+) generally means that the substance is expressed/identified, while negative (-) means that it is missing/not identified.
- CD4+ - (T helper cells) refers to T lymphocytes that express CD4 (cluster of differentiation 4, a glycoprotein of the immunoglobulin superfamily) on their surface, associated with helper/inducer function. These cells can be infected by human immunodeficiency virus (HIV).
- CD4/CD8 ratio - clinical measurement of different immune cell types (ratios between 1.5 to 2.5 are considered normal). Viral infections such as HIV, cytomegalovirus, Epstein-Barr virus, and influenza virus, associated with an inversion of the ratio.
- CD8+ - (cytotoxic T cells) refers to T lymphocytes that express CD8 (glycoprotein of the immunoglobulin superfamily) on their surface, associated with cytotoxic/suppressor activity.
- "clockface" - a term used to describe the appearance of plasma cell nuclei due to the clumping of the chromatin at the nucleus periphery. More clearly seen in tissue plasma cells that the bone marrow smear, where they are sometimes confused with the basophilic erythroblasts.
- cords of Billroth - spleen cellular columns located in red pulp. surrounded by splenic sinusoids. Cords contain reticular cells, macrophages, lymphocytes, plasma cells and erythrocytes.
- cortex - outer layer, used in association with medulla (innner layer or core) a general description that can be applied to describing an organ with a layered structure.
- cortical Thymic Epithelial Cell - (cTEC, types I - IV) support and antigen presenting cells located in the cortex regions of the thymus required for positive and negative selection of maturing T cells. See also medullary epithelial cell.
- crypt - (tonsil crypt) tonsil squamous epithelium infold, with intraepithelial passages containing non-epithelial cells. Functions include: intimate contact between immune response effector cells, facilitate transport of antigens, synthesise secretory components, and contain a pool of immunoglobulins. PMID 7559106
- dendritic cell - (DC, antigen-presenting cell, APC) cells that present antigens and induce a primary immune response in resting naïve T lymphocytes. Originate from the same common progenitor as monocytes (PMID 20193011). In 2011 Ralph M. Steinman received half the Nobel Prize half of the award to to Ralph M. Steinman for his discovery of the dendritic cell and its role in adaptive immunity.
- Effector cells - the immune functioning (active) B and T lymphocytes.
- Efferent lymph - vessel carrying lymph away from a node.
- fibroblastic reticular cell - (FRC) specialized myofibroblasts that form the structural mesenchymal network "sponge" within lymphoid tissue that regulate immune cell migration, activation, and survival. Immune T cells, B cells, dendritic cells (DCs), plasma cells and macrophages move and interact.
- follicular dendritic cell - (FDC) in B cell follicles of secondary lymphoid organs, cells interspersed within the stromal cell network function: Primary - help B cells to cluster. Secondary - in GC long-term retention of intact antigen and support B cell survival.
- GALT - Gut Associated Lymphatic Tissue consisting of Peyer’s patches, isolated lymphoid follicles and mesenteric lymph nodes.
- germinal centre - (GC) centre of B cell follicles of secondary lymphoid organs, where antigen-activated B-cell clones expand and undergo immunoglobulin gene hypermutation and selection.
- haemopoiesis (hemopoiesis) formation of blood cells.
- Hassall's corpuscles - (Hassall's body, thymic corpuscle) Epithelial reticular cells located in the thymic medulla. Named after Arthur Hill Hassall (1817-1894) a British physician and chemist.
- high endothelial venule - (HEV) the specialised post-capillary venous region that enables blood lymphocytes and pre-dendritic cells to enter a lymph node. The endothelial cells express ligands that bind lymphocytes, aiding their adhesion and subsequent transmigration into the lymph node. With inflammation, monocytes and NK cells can also enter here.
- humoral immune response - production of antibody by plasma cells derived from B lymphocytes (B cells).
- IEL - Intraepithelial Lymphocyte are T lymphocytes located in the gastrointestinal tract epithelium. Natural IELs (previously ‘type b’ IELs) acquire activated phenotype during development in the thymus in the presence of self antigens. Induced IELs (previously ‘type a’ IELs) progeny of conventional T cells activated post-thymically in response to peripheral antigens.
- IgA - the main class of antibody released at mucosal surfaces and in secretions (saliva, tears, milk, and respiratory and intestinal secretions) and the most abundantly produced antibody (70%). PMID 22566964
- IgD - the immunoglobulin B cell starts to produce as a cell-surface molecule after leaving the bone marrow.
- IgE - bind Fc receptors (surface of mast cells in tissues and basophils in the blood) release of potent pro inflammatory molecules mediators of allergic reactions.
- IgG - the major class of immunoglobulin in the blood.
- IgM - the first class of antibody made by a developing B cell, which may switch to making other classes of antibody.
- immunodeficiency - when one or more components of the immune system is defective. (More? Immunobiology - immunodeficiency)
- immunoglobulin - (antibody, Ab) protein produced by plasma cells.
- immunosenescence - in ageing and disease, refers to a weaker immune responses producing a progressive deterioration and increased susceptibility to infectious diseases, neoplasia, and autoimmune diseases.
- innate lymphoid cells - (ILCs) subset of lymphocytes that lack antigen-specific receptors, are located in peripheral tissues and abundant at barrier surfaces, decrease in number with age. PMID 29924974
- intraepithelial lymphocyte (IEL) immune cells residing in the gastrointestinal tract epithelium. image - Intraepithelial lymphocyte differentiation
- involution - in the thymus refers to the replacement, mainly in the cortex, of cells by adipose tissue. (More? PubMed- thymus involution) | Cancer Medicine - Thymomas and Thymic Tumors)
- Kupffer cells - stellate macrophage cells located in the liver sinusoids, named after Karl Wilhelm von Kupffer (1829 - 1902) a German anatomist who originally identified these cells. (More? Liver Development)
- lacteal - term used to describe the lymphatic vessels of the small intestine.
- lamina propria - a layer of loose connective tissue found underneath an epithelium, together with the epithelium described as mucosa.
- Langerhans cell - (LC, dendritic cell) Antigen-presenting immune cell found mainly in the basal/suprabasal layers of adult skin and mucosa. Cells lie in the basal/suprabasal layers of stratified epidermal and mucosal tissues. First in the innate antiviral immune defines and can migrate to lymph nodes and induce a T cell–mediated adaptive immune response. (More? Integumentary | Immune System Development)
- Leukocyte - (Greek, lukos = clear, white) white blood cell.
- lingual - related to the tongue, as in lingual tonsil, forms part of Waldeyer’s ring.
- lymph node - connective tissue encapsulated lymphoid organ (1mm - 2cm in size), positioned in the pathway of lymph vessels. (More? Lymph Node Development)
- lymphangion - the functional unit of a lymph vessel that lies between two semilunar (half moon-shaped) valves.
- lymphangiogenesis - formation of new lymph vessels from pre-existing lymphatic structures. During embryogenesis and in adult tissues as reaction to inflammation or injury.
- M cell - (microfold cell) found in the follicle-associated epithelium of the Peyer's patch. Function to transport gut lumen organisms and particles to immune cells across the epithelial barrier.
- macrophage - a large highly motile white blood cell which engulfs foreign material (bacteria etc) and both degenerating cells and cell fragments. Differentiates from a monocyte and found in many different tissues and locations. Current theory suggests tissue macrophage is also derived from resident stem cell population in many tissues. More? Immunobiology - Defects in phagocytic cells are associated with persistence of bacterial infection)
- MALT - Mucosa Associated Lymphoid Tissue.
- medulla - inner layer or core, used in association with cortex (outer layer) a general description that can be applied to describing an organ with a layered structure.
- medullary Thymic Epithelial Cell - (mTEC, types I-VII) support and antigen presenting cells located in the medullary regions of the thymus, required for central tolerance (negative selection) of maturing T cells (PMID 11375064). See also cortical thymic epithelial cell.
- Memory Cell - effector T cell (lymphocyte)
- mesenteric lymph nodes - Part of GALT as well as being involved in gut-draining. image - mesenteric lymph nodes
- Mononuclear Phagocytic System - (MPS, Lymphoreticular System, Reticuloendothelial System, RES) Consists of circulating monocytes in the peripheral blood and non-circulating (fixed) tissue macrophages (MΦ) located in tissues and organs.
- NAVL - (naval) mnemonic to remember the neurovascular bundle components Nerve Artery Vein Lymph found travelling together within organs and tissues.
- negative selection - T cells bearing autoreactive T cell antigen receptors (TCRs) are eliminated during their development in the thymus, protects against autoimmunity.
- normoblast - seen in bone marrow smear, a developing erythroblast (red blood cell) that still retains a nucleus.
- nude mice - (nu/nu) mice which are congenitally hairless and athymic, therefore they do not reject tissue and tumor grafts.
- PALS - acronym for PeriArterial Lymphoid Sheath in the spleen white pulp.
- parenchyma - (Greek = enkeim "to pour in") cells forming the functional cells of an organ or tissue. These cells carry out the function of the organ at a cellular level, and are not the structural cells, connective tissue, extracellular matrix (stromal).
- periarterial lymphoid sheath - (PALS) in the spleen the white pulp that surrounds the central arteries. (T-lymphocytes,macrophages and plasma cells)
- pharyngeal pouch III - origin of endodermal component of the thymus (also formed from neural crest). Pharyngeal arches
- Plasma Cell - active B cell (lymphocyte) which is secreting antibody. Located in either bone marrow or peripheral lymphoid tissues, these cells have and increased cytoplasmic volume (due to increase rough endoplasmic reticulum) in comparison to the inactive (non-secreting) lymphocyte.
- primary follicle - follicle that does not contain germinal centre, secondary follicles do germinal centre.
- red pulp - spleen region, organized as cell cords (splenic cords, cords of Billroth) and vascular sinuses.
- regulatory T cells - (Tregs) maintain self tolerance and suppress pathological immune responses by control of immune response to non-self antigens.
- reticular fibres - reticular cells secrete this extracellular matrix protein, composed of type III collagen.
- right lymphatic duct - drains most of the right upper quadrant. See also thoracic duct.
- secondary follicle - contain germinal centre, primary follicle does not contain germinal centre.
- sentinel lymph node - the hypothetical first lymph node or group of nodes reached by metastasizing cancer cells from a primary tumour.
- sinus - a larger vessel or space usually curved that may contain air, blood, or lymph. e.g. splenic medullary sinus, lymph node medullary sinus, sub-capsular sinus, trabecular sinus.
- sinusoid - a tiny vessel with a tortuous path and many connections to similar vessels. e.g. hepatic and bone marrow sinusoids.
- splenic capillary sheaths - in spleen around capillary endothelium and consist of three main cell types: CD271+ stromal capillary sheath cells, CD68+CD163− macrophages and recirculating B-lymphocytes. Sheaths may; 1. allow interaction among sheath macrophages and B-lymphocytes, 2. attract recirculating B-lymphocytes from the open circulation of the red pulp to start migration into white pulp follicles. 30356180
- splenic sinusoids - enlarged splenic spaces located in red pulp and surrounding cords of Billroth.
- sphingosine-1-phosphate - (S1P) sphingolipid secreted into the extracellular space establishing a gradient acting through G protein-coupled receptors to attract lymphocytes out of lymphoid organs (lymph node, thymus, spleen) into the circulation.
- stroma - (Greek = "a cover, table-cloth, bedding") tissue forming the framework/support of an organ or tissue. That is the structural cells which form connective tissue and secrete extracellular matrix, rather than the functional cells (parenchymal). All organs can therefore be functionally divided into these 2 components, stromal/parenchymal.
- Subcapsular sinus (=marginal sinus) space lying under the connective tissue capsule which receives lymph from afferent lymphatic vessels.
- T cell - (T-cell, T lymphocyte) named after thymus, where they develop, the active cell is responsible for cell-mediated immunity (killer T cells and helper T cells). Cells express T-cell receptor on surface and directly kill virally or bacterially infected cells. These cells can themselves be infected by HIV. (More? Electron micrographs of nonactivate and activated lymphocytes)
- TEC - (Thymic Epithelial Cell) thymus support and antigen presenting cells further divided anatomically and functionally into medullary TEC (mTEC, types I-VII, for central tolerance) and cortical epithelial cell (cTEC, types I-IV, positive and negative selection) populations (see PMID 28800929 PMID 30308217).
- T cell activation - (T lymphocyte activation)The activation process begins with T-cells searching for and encountering antigen-bearing dendritic cells within lymph nodes.
- thoracic duct - (TD) largest and main lymphatic vessel, drains the lower body including the extremities and abdomen. Intra-thoracic tributaries include: intercostal, mediastinal, and bronchomediastinal trunks.
- Thymic corpuscle - (Hassall's corpuscle) a mass of concentric epithelioreticular cells found in the thymus. The number present and size tend to increase with thymus age. (see classical description of Hammar, J. A. 1903 Zur Histogenese und Involution der Thymusdriise. Anat. Anz., 27: 1909 Fiinfzig Jahre Thymusforschung. Ergebn. Anat. Entwickl-gesch. 19: 1-274.)
- thymic epitheliocytes - reticular cells located in the thymus cortex that ensheathe the cortical capillaries, creating and maintain the microenvironment necessary for the development of T-lymphocytes in the cortex.
- T helper cells - (helper T-cells) (Th cells, CD4+) refers to T lymphocytes that when mature express CD4 (glycoprotein of the immunoglobulin superfamily) on their surface.
- T lymphocyte - (T cell, T-cell) regulate cell-mediated immunity.
- thymus - an immune/endocrine (thymic hormone, thymosins) organ involved in the maturation (differentiation) of T lymphocytes (T-cells).
- tonsils - lymph nodules embedded in the mucus membranes located at the back of the mouth and top of the throat. The overlying epithelium helps identify the location.
- tonsillitis - a common bacterial infection of the palatine tonsils, occurring mostly in children and young adults and can also become recurrent tonsillitis.
- vermiform appendix - see appendix, anatomical region containing gut-associated lymphoid tissue located within the gastrointestinal tract at the beginning of the colon. The anatomy is as a finger-like structure that arises from the cecum. The length (2.5-13 cm) is longer in both infants and children and also has more abundant lymphatic tissue in early life. The wall structure is similar to the small intestine (though with no villi), nor plicae circularis. Lymph nodules surround the lumen of the gastrointestinal tract and extend from the mucosa into the submucosa.
- VDJ recombination - (variable, diversity and joining gene segments) genetic recombination event that occurs in immune cell maturation in primary lymphoid organs, B cells ((bone marrow) and T cells (thymus).
- Waldeyer’s ring - ring of lymphoid tissue in the pharyngeal wall: palatine tonsils, nasopharyngeal tonsil (adenoid) and lingual tonsil. First described in 1884 by von Waldeyer-Hartz.
- white pulp - (Malpighian follicles, Malpighian bodies of the spleen, white nodules, splenic lymphoid nodules) spleen lymphoid region, organized as lymphoid sheaths with both T-cell and B-cell compartments, around the branching arterial vessels (resembles lymph node structure).
| Other Terms Lists |
|---|
| Terms Lists: ART | Birth | Bone | Cardiovascular | Cell Division | Endocrine | Gastrointestinal | Genital | Genetic | Head | Hearing | Heart | Immune | Integumentary | Neonatal | Neural | Oocyte | Palate | Placenta | Radiation | Renal | Respiratory | Spermatozoa | Statistics | Tooth | Ultrasound | Vision | Historic | Drugs | Glossary |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- NIAID - Immune System
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 5) Embryology Thymus Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Thymus_Development
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G