| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
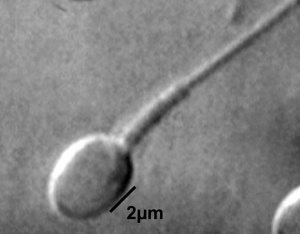
This page introduces spermatogenesis the development of spermatozoa, the male haploid gamete cell. In humans at puberty, spermatozoa are produced by spermatogonia meiosis in the seminiferous tubules of the testis (male gonad). A second process of spermiogenesis leads to change in cellular organisation and shape before release into the central lumen of the seminiferous tubule. This overall process has been variously divided into specific identifiable stages in different species: 6 in human, 12 in mouse, and 14 in rat. Structurally, the seminiferous tubule epithelium is divided into a basal and an apical (adluminal) compartment by the blood–testis barrier (BTB). (More? Testis Development).
A second unique feature of this process is that during mitosis and meiosis the dividing cells remain connected by cytoplasmic bridges as the cells do not complete cytokinesis. This cellular organization is described as a syncytium, only ending with release into the central lumen of the seminiferous tubule, when the cell cytoplasm is discarded. Retinoic acid has been shown to be a key regulator of the development process. (More? Retinoic acid)
- In a healthy adult human male it takes about 48 days from meiosis to produce a mature spermatozoa, and he produces somewhere between 45 to 207 million spermatozoa per day, or about 1 to 2,000 every second. (More? Statistics)
| Anton van Leeuwenhoek (1632 – 1723), was a Dutch scientist who developed the early compound microscope. In 1677 on examination of his own ejaculate under the microscope, he identified tiny “animalcules” he found wriggling inside. He submitted this new observation to the Royal Society London, with the following caveat: “If your Lordship should consider that these observations may disgust or scandalise the learned, I earnestly beg your Lordship to regard them as private and to publish or destroy them as your Lordship sees fit.” Royal Society London 1677. |
Genital Links: genital | Lecture - Medicine | Lecture - Science | Lecture Movie | Medicine - Practical | primordial germ cell | meiosis | endocrine gonad | Genital Movies | genital abnormalities | Assisted Reproductive Technology | puberty | Category:Genital
| ||||
|
Medicine Practical | fertilization | Category:Spermatozoa
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Spermatozoa Development | Spermatogonia Development | Spermatogonia Stem Cells | Spermatozoa Meiosis | Primary Spermatocyte | Secondary Spermatocyte | Spermatid | Spermatogenesis | Spermiogenesis | Sertoli Cell Development | Spermatozoa Chemotaxis | Acrosome |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Spermatozoa Movies
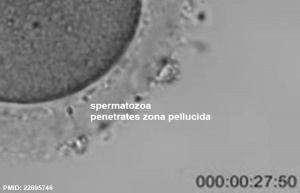
See also Week 1 movies.
|
|
| |||||||||||||
|
|
|
|
- Links: Week 1 movies | Movies
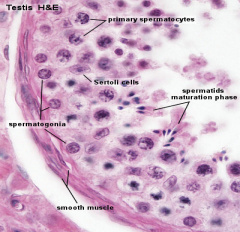
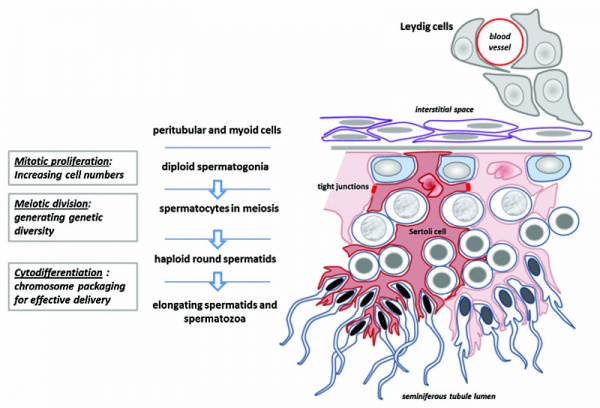
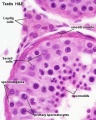
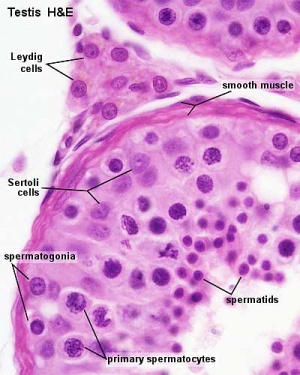
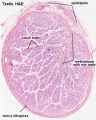
Seminiferous Tubule
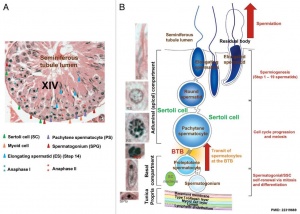
Seminiferous tubule cartoon[13]
- Spermatogonia - are the first cells of spermatogenesis
- Primary spermatocyte - large, enter the prophase of the first meiotic division
- Secondary spermatocytes - small, complete the second meiotic division
- Spermatid - immature spermatozoa
- Spermatozoa - differentiated gamete
- Spermatozoa development: primordial germ cell - spermatogonia - primary spermatocyte - Template:Secondary spermatocyte - spermatid - spermatozoa
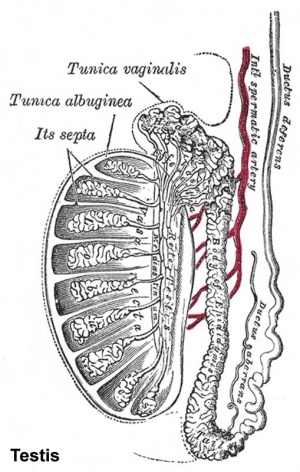
- Links: Testis Histology | testis
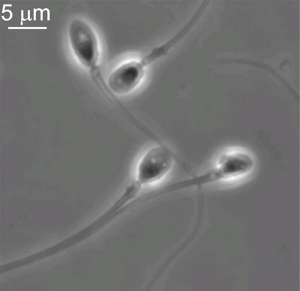
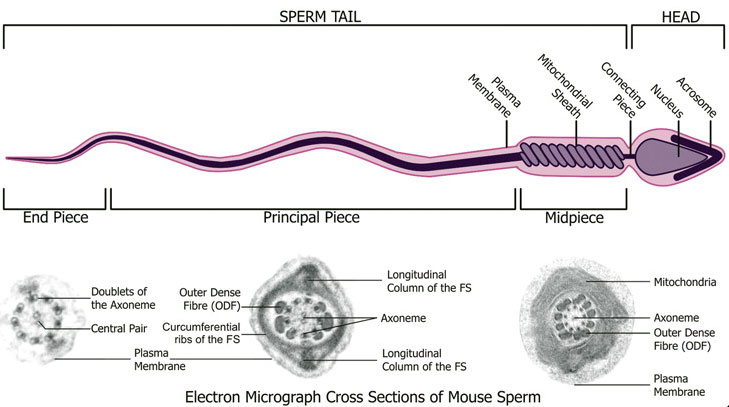
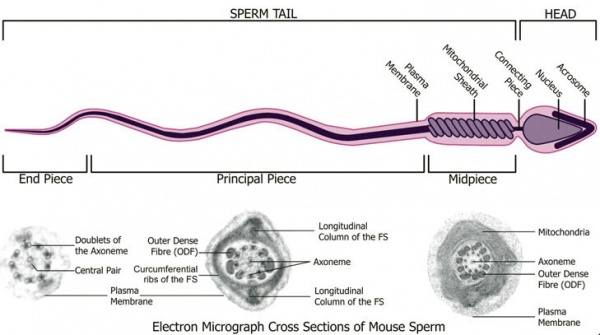
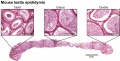
Spermatozoa Structure
Spermatozoa (mouse) cross-sections of tail (EM) and diagram[14]
Other main cell types seen in the histological sections
- sertoli cells- support cells seen within the seminiferous tubule
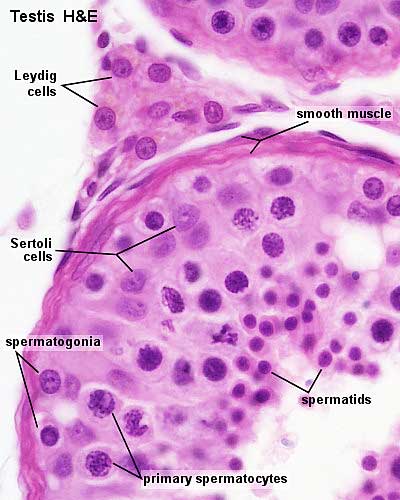
- Interstitial cells or Leydig cells - produce hormone
- Smooth muscle - surround seminiferous tubule and contribute to contraction of the tubule
Human Spermatozoa Development
- Spermatogenesis process of spermatagonia mature into spermatazoa (sperm).
- Continuously throughout life occurs in the seminiferous tubules in the male gonad- testis (plural testes).
- At puberty spermatagonia activate and proliferate (mitosis).
- about 48 days from entering meiosis until morphologically mature spermatozoa
- about 64 days to complete spermatogenesis, depending reproduction time of spermatogonia
- follicle stimulating hormone (FSH) - stimulates the spermatogenic epithelium
- luteinizing-hormone (LH) - stimulates testosterone production by Leydig cells
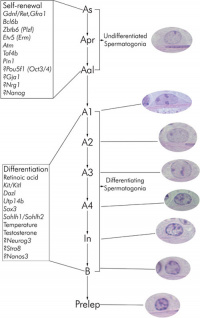
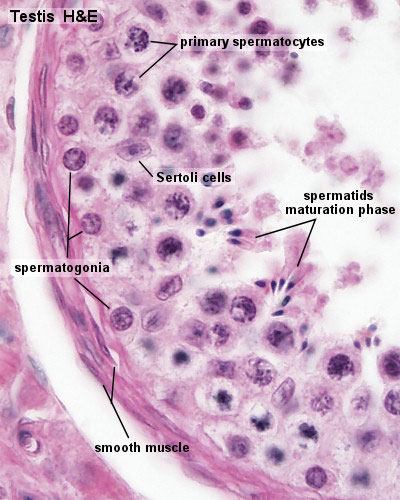
Spermatogonia
In humans at about 2 months of age, primordial germ cells (gonocytes) are replaced by adult dark (Ad) and pale (Ap) spermatogonia forming the spermatogonial stem cell (SSC) population that at puberty will commence differentiation into spermatozoa.
The spermatogonia are the diploid stem cell (spermatogonial stem cell, SSC) progenitor for spermatozoa development. They are located on the basal lamina around the periphery of the seminiferous tubule wall. See this recent spermatogonia review.[15]
In 1963 Clermont identified spermatogonia as Ap (pale) and Ad (dark) on basis of light microscope staining.[16]
- now also type B
- 60 years - Ap spermatogonia number decrease
- 80 years - Ad spermatogonia number decrease
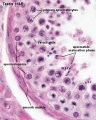
| Pre-puberty | Post-puberty |
|---|---|
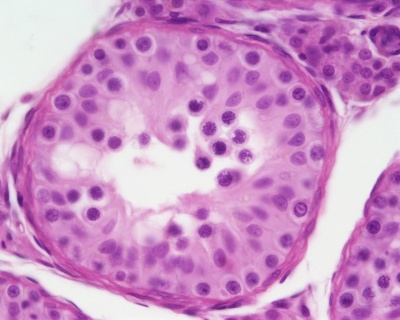
|
|
| Containing only spermatogonia and sertoli cells | Containing spermatogonia, sertoli cells and stages of spermatozoa cell meiosis |
|
Mouse spermatogonia have been shown to require a number of factors to regulate both their spermatogonial self-renewal and differentiation.Zhou Q & Griswold MD. (2008). Regulation of spermatogonia. , , . PMID: 20614596 DOI.
The mouse "As model" originally stated that the As spermatogonia are the SSCs. A more recent proposal suggests that only some of the As spermatogonia have the potential for long-term self-renewal, while others have a limited capacity, indicating the presence of a SSC hierarchy.[15] In the mouse testis, spermatogonial stem cells can also be identified by Id4 expression[17], a dominant-negative transcription factor containing a basic helix-loop-helix (bHLH) region. Id4 inhibits binding to DNA and transcriptional transactivation by hetero-dimerization with other bHLH proteins. |
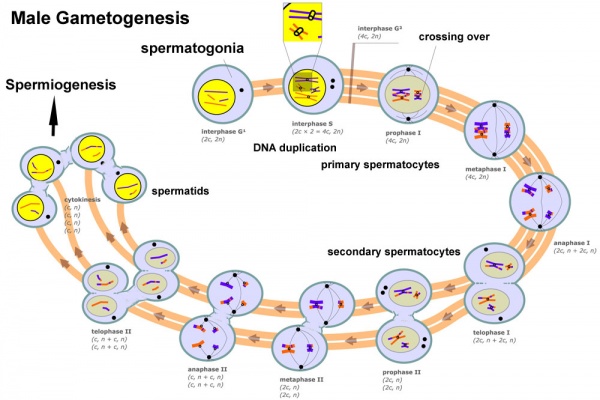
Meiosis
Spermatozoa maturation involves two processes meiosis and spermiogenesis. After puberty, new spermatozoa continue to be generated throughout life from a spermatogonia stem cell (SSC) population in the testis.
Primary Spermatocyte
The first large differentiating diploid (2N) cell before meiosis I, that enters the prophase of the first meiotic division.
- Search PubMed: Primary Spermatocyte
Secondary Spermatocyte
The primary spermatocyte forms two second small cells that complete the second meiotic division to be haploid (N) . Histologically difficult to observe, these cells will complete meiosis, forming next the spermatid stage that is the immature morphological state of the spermatozoa.
- Search PubMed: Secondary Spermatocyte
Differences in Mammalian Meioses
| Female Oogenesis | Male Spermatogenesis | |
| Meiosis initiated | once in a finite population of cells | continuously in mitotically dividing stem cell population |
| Gametes produced | 1 / meiosis | 4 / meiosis |
| Meiosis completed | delayed for months or years | completed in days or weeks |
| Meiosis Arrest | arrest at 1st meiotic prophase | no arrest differentiation proceed continuously |
| Chromosome Equivalence | All chromosomes exhibit equivalent transcription and recombination during meiotic prophase | Sex chromosomes excluded from recombination and transcription during first meiotic prophase |
| Gamete Differentiation | occurs while diploid (in first meiotic prophase) | occurs while haploid (after meiosis ends) |
- Links: meiosis
Spermiogenesis
Spermiogenesis is the final stage of spermatogenesis, morphological changes transform the round spermatids into the mature spermatozoa shape and structure.
- Nuclear compression - chromatin condensation occurs by the replacement of histones with protamines.
- Acrosome formation - located over the anterior part of the spermatid nucleus, cap-like membrane-bound organelle formed through coalescence of the coated vesicles budding from the trans-Golgi network. The acrosome-acroplaxome-manchette complex is a major driver for the shaping of the spermatozoa head.
- Tail development - located over the posterior part of the spermatid nucleus, initially a centriole pair moves, the axoneme develops from the distal centriole. Axoneme consists of a central pair of microtubules surrounded by 9 outer doublet microtubules ("9 × 2 + 2").
- Cytoplasm disposal - cytoplasm transported towards the tail along the manchette and finally its removal.
Other Features
- Nuclear pore redistribution - with packaging of the nuclear pores into the redundant and discarded nuclear envelope. [9]
- Autophagy - a self-digestion process, may also occur regulating cytoskeleton reorganization.[18][19]
Sertoli Cell
The sertoli cell was named after Enrico Sertoli (1842 - 1910) an Italian (Milan) physiologist and histologist. These cells support spermatozoa development and span the wall of the seminiferous tubule.
- sustentacular cells of seminiferous tubules.
- form a “blood-testis” barrier through junctional complexes
- separate the intra-tubular germinal epithelium into two compartments
- basal compartment - cells are exposed to the extra-tubular environment
- luminal compartment - cells are subject to an environment produced by Sertoli cells and germ cells
During infancy and childhood, sertoli cells are the most active cell population in the seminiferous tubule producing AMH from fetal period until mid-puberty.[20]
- Links: sertoli cell | AMH
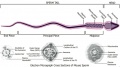
Spermatogenic Cycle
Along the length of the seminiferous tubule spermatozoa develop in a cyclic manner over time progressing through a number of stages, called the spermatogenic cycle, see review.[21] The number of stages appears to differ between species, in mouse there are 12 stages (I – XII) and in the rat 14 stages.
In mouse, one spermatogenic cycle (12 stages) occurs over 8.6 days and four cycles (35 days) are required from spermatogonial stem cell to released spermatozoa.
Human[22]
Gerbil[23]
Spermatozoa Structure
Spermatozoa (mouse) cross-sections of tail (EM) and diagram[14]
Acroplaxome
This structure forms the acrosome plate with intermediate filament bundles of the marginal ring at the leading edge of the acrosome. The acroplaxome site for Golgi-derived proacrosomal vesicles to tether and fuse and anchors the developing acrosome to the elongating spermatid head and may provide a scaffolding for the shaping of the spermatid nucleus.[24]
Acrosome
Derived from the Golgi apparatus in conjunction with transient specialized bundles of microtubules (Template:Manchette), this vesicle releases its contents following progesterone stimulus or zona pellucida binding.
Acrosome Reaction
The "acrosome reaction" (AR) a type of specialised exocytosis, or similar to the release of an exosome.[25] This also leads to changes in the spermatozoa membrane. The 26S proteasome[26], has been identified from in vitro studies to be required for zona lysin in many species, including mammals.[27]
- Calcium ion permeability - Ca(2+) release from the acrosome leads to exocytosis of the acrosomal vesicle[28], alkalization appears to be a critical step.[29]
- store-operated Ca(2+) channels and voltage-dependent Ca(2+) channels
- Vesicle membrane - initially holding excreting molecules, remains on the cell surface
- Membrane loss - both outer acrosomal membrane and plasma membrane are lost by forming vesicles during acrosome reaction.
Acrosome reaction has a slow and rapid component:[30]
- Rapid - (seconds) efflux of calcium from intracellular stores, triggers fusion pores opening and the release of hybrid vesicles.
- Slow - (minutes) acrosomal swelling, triggered by activation of an adenylyl cyclase downstream of the opening of store-operated calcium channels. Determines the kinetics of the acrosome reaction.
- Exosomes - extracellular vesicles that are released from cells upon fusion of an intermediate endocytic compartment, the multivesicular body (MVB), with the plasma membrane.
- Proteasomes - protein complexes which typically degrade unneeded or damaged proteins by proteolysis.
Acrosin
- Acrosin is essential for sperm penetration through the zona pellucida in hamsters PNAS February 4, 2020 117 (5) 2513-2518 "Mammalian oocytes are surrounded by the zona pellucida, a glycoprotein coat that protects the oocyte and embryo from mechanical damage during their preimplantation development within the oviduct. Fertilizing spermatozoa must penetrate the zona, but we do not know the exact mechanisms underlying this process. Sperm proteases were thought to work as zona lysins, but gene-knockout studies in mice did not support this assumption. In this study, we generated hamsters without acrosin, the major acrosomal protease, to examine its role in both in vivo and in vitro fertilization. Surprisingly, mutant male hamsters were completely infertile because their spermatozoa were unable to penetrate the zona. We thus demonstrated that, at least in hamsters, acrosin is essential for sperm penetration through the zona."
Human acrosin - 22q13.33
- Links: OMIM - Acrosin
Nucleus
The spermatozoa nucleus undergoes extensive compression, and nuclear DNA chromatin remodelling by tightly packing with spermatozoa-specific protamines.[31]
| It is thought that the lysine-rich protein precursor (H1 histone) has evolved into the arginine-rich protamines.[32] | Three major spermatozoa nuclear basic proteins types:
|
| EM Human Spermatozoa Nucleus | |
|---|---|
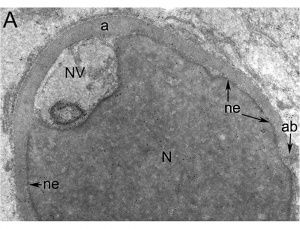
|
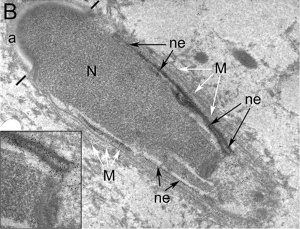
|
| Cap-phase spermatid nucleus[33] | Elongated spermatid nucleus[33] |
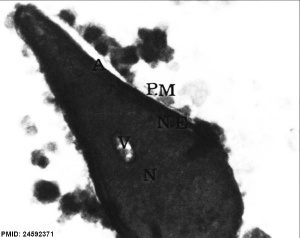
|
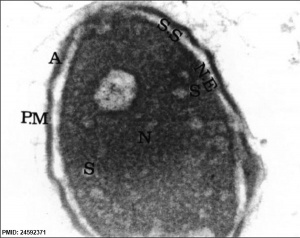
|
| Normal human spermatozoa[34] | Abnormal human spermatozoa[34] |
Axoneme
The stable mature microtubule-containing tail of the sperm.
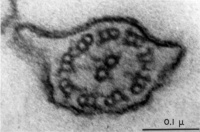
|
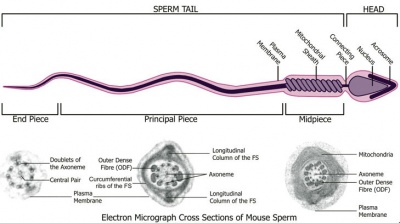
|
| Historic EM spermatozoon tail | Mouse cross-sections of tail[14] |
Centriole
Spermatozoa initially contains 2 centrioles (proximal, distal) and at fertilisation only a single (proximal) is present, which in most mammalian species is contributed to reconstitute the zygotic centrosome. Note that in rodents (rat, mice) both centrioles are lost and only a maternal centrosomal inheritance occurs.
- distal centriole - (perpendicular to membrane) required as the basal body generating the microtubule axoneme and is then lost (disintegration).
- proximal centriole - required after fertilisation for decondensing spermatozoa nucleus allowing development into the male pronucleus.
Manchette
A transient microtubule structure formed in spermatids involved in the process of: assembly of the mammalian sperm tail, mechanical shaping and condensation of the sperm nucleus. These microtubules are also invloved with specific transport, intramanchette transport, which has been likened to intraflagellar transport. This microtubular structure surrounds the nucleus of the developing spermatid and is thought also to assist in both the reshaping of the nucleus and redistribution of spermatid cytoplasm.
Mitochondria
Contained in the initial segment provide the energy for motility and may also enter the egg on fertilization, but are eliminated by a ubiquitin-dependent mechanism.
Perinuclear Theca
Located in the sperm head perinuclear region and contains a cytoskeletal element to maintain the shape of the sperm head and functional molecules leading to oocyte activation during fertilization.
Mature Human Spermatozoa
Features:
Human Spermatazoa Statistics | Development Animation - Spermatozoa |
Human Spermatozoa Statistics
| Spermatozoa | Number/Time |
|---|---|
| Production | |
| produced / day (two testes) | 45 to 207 million |
| compare adult human red blood cell / day | 250,000 million |
| produced / second each day (approx) | 2,000 |
| Storage | |
| stored (epididymal reserves) up to per epididymis | 182 million |
| stored extragonadal | 440 million |
| extragonadal - ductuli deferentia and caudae epididymides per ejaculation | 225 million |
| Transit Times | |
| through the caput | 0.72 day |
| through the corpus | 0.71 days |
| through the cauda epididymidis | 1.76 days |
| Table Data [35] See also WHO human semen reference values(2010).[36] Links: spermatozoa | |
Spermatozoa Morphology
Morphology is a term used to describe the overall appearance of a cell or tissue and is often used to characterise changes in cellular state or activity. Historically, there have been studies comparing the overall appearance of spermatozoa between different species.[37] More recently, there have been several different ways of characterising the morphology of human spermatozoa developed mainly in relation to clinical reproductive technologies.
Integrated Sperm Analysis System (ISAS)
A semi-automated computer-aided system that measures spermatozoa head parameters length (L), width (W), area (A), perimeter (P), acrosomal area (Ac), and the derived values L/W and P/A.[38]
- For each man a homogeneous population of distributions characterized seminal spermatozoa (7,942 cells: median values L 4.4 μm, W 2.8 μm, A 9.8 μm(2), P 12.5 μm, Ac 47.5%, L/W 1.57, P/A 1.27)
- Different men could have spermatozoa of significantly different dimensions.
- Head dimensions for swim-up spermatozoa from different men (4 812 cells) were similar to those in semen, differing only by 2%-5%.
- The values of L, W and L/W fell within the limits given by the World Health Organization (WHO).
- A subpopulation of 404 spermatozoa considered to fit the stringent criteria of WHO 'normal' seminal spermatozoa[36] from both semen and swim-up were characterized by median values (and 95% confidence intervals) of L, 4.3 μm (3.8-4.9), W, 2.9 μm (2.6-3.3), A, 10.2 μm(2) (8.5-12.2), P, 12.4 μm (11.3-13.9), Ac, 49% (36-60), L/W, 1.49 (1.32-1.67) and P/A, 1.22 (1.11-1.35). These median values fall within the 95th centile confidence limits given by WHO, but the confidence intervals for L and W were larger.
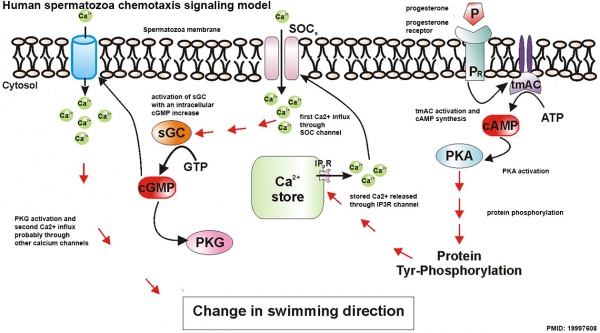
Spermatozoa Chemotaxis
Chemotaxis was first identified in marine species[39], which still remains today as a model system. While the signals may differ, the overall effect is to chemically attack spermatozoa to the oocyte to allow fertilisation to occur.
The following series of 2011 research articles have identified the spermatozoa calcium channel protein (CatSper) as the progesterone activated pathway involved in capacitated spermatozoa chemotaxis.
|
|
Human Spermatozoa Chemotaxis Model (2009)[43]
See also 2008 review.[44]
Sertoli Cell
The sertoli cells are the first cells to be differentiated in development by SRY expression. Post-puberty these are the "support" cells for spermatozoa development and transport from the periphery to lumen of the seminiferous tubule. Sertoli cells form a barrier with cell junctions at the Sertoli cell-cell and Sertoli-germ cell interface.
Sertoli cell postnatal proliferation may be regulated by thyroid status. An animal model study of postnatal transient hypothyroidism has demonstrated Sertoli cell proliferation (6 to 8 fold increase) 2 days after the diet switch and remained elevated the next days.[45]
Histology
Papanicolaou stain (Papanicolaou's stain, Pap stain) a multichromatic (five dyes) staining histological technique developed by George Papanikolaou, used to differentiate cells in smear preparations of various bodily secretions.
- Links: sertoli cell | Testis Histology | Histology Stains | Search PubMed - Sertoli Cell Development
Male Abnormalities
Male Infertility Genes
Examples of known genes resulting in various forms of male infertility.
| Gene abbreviation | Name | Gene Location | Online Mendelian Inheritance in Man (OMIM) |
HUGO Gene Nomenclature Committee (HGNC) |
GeneCards (GCID) | Diagnosis |
|---|---|---|---|---|---|---|
| AURKC | Aurora kinase C | 19q13.43 | 603495 | 11391 | GC19P057230 | Macrozoospermia |
| CATSPER1 | Cation channel sperm-associated 1 | 11q13.1 | 606389 | 17116 | GC11M066034 | Asthenozoospermia |
| CFTR | Cystic fibrosis transmembrane conductance regulator | 7q31.2 | 602421 | 1884 | GC07P117465 | Obstructive azoospermia |
| DNAH1 | Dynein axonemal heavy chain 1 | 3p21.1 | 603332 | 2940 | GC03P052350 | Asthenozoospermia |
| DPY19L2 | Dpy-19-like 2 gene | 12q14.2 | 613893 | 19414 | GC12M063558 | Globozoospermia |
| GALNTL5 | Polypeptide N-acetylgalactosaminyltransferase-like 5 | 7q36.1 | 615133 | 21725 | GC07P151956 | Asthenozoospermia |
| MAGEB4 | MAGE family member B4 | Xp21.2 | 300153 | 6811 | GC0XP030260 | Azoospermia |
| NANOS1 | Nanos C2HC-type zinc finger 1 | 10q26.11 | 608226 | 23044 | GC10P119029 | Azoospermia |
| NR0B1 | Nuclear receptor subfamily 0 group B member 1 | Xp21.2 | 300473 | 7960 | GC0XM030322 | Azoospermia |
| NR5A1 | Nuclear receptor subfamily 5 group A member 1 | 9q33.3 | 184757 | 7983 | GC09M124481 | Azoospermia |
| SOHLH1 | Spermatogenesis and oogenesis-specific basic helix–loop–helix 1 | 9q34.3 | 610224 | 27845 | C09M135693 | Azoospermia |
| vSPATA16 | Spermatogenesis-associated 16 | 3q26.31 | 609856 | 29935 | GC03M172889 | Globozoospermia |
| SYCE1 | Synaptonemal complex central element protein 1 | 10q26.3 | 611486 | 28852 | GC10M133553 | Azoospermia |
| TAF4B | TATA-box binding protein-associated factor 4b | 18q11.2 | 601689 | 11538 | GC18P026225 | Azoospermia |
| TEX11 | Testis expressed 11 | Xq13.1 | 300311 | 11733 | GC0XM070528 | Azoospermia |
| TEX15 | Testis expressed 15, meiosis and synapsis associated | 8p12 | 605795 | 11738 | GC08M030808 | Azoospermia |
| WT1 | Wilms tumour 1 | 8p12 | 607102 | 12796 | GC11M032365 | Azoospermia |
| ZMYND15 | Zinc-finger MYND-type containing 15 | 17p13.2 | 614312 | 20997 | GC17P004740 | Azoospermia |
| Table data source[46] (table 1) Links: fertilization | spermatozoa | testis | Male Infertility Genes | Female Infertility Genes | oocyte | ovary | Genetic Abnormalities | ART Asthenozoospermia - (asthenospermia) term for reduced spermatozoa motility. Azoospermia - term for no spermatozoa located in the ejaculate. Globozoospermia - term for spermatozoa with a round head and no acrosome. | ||||||
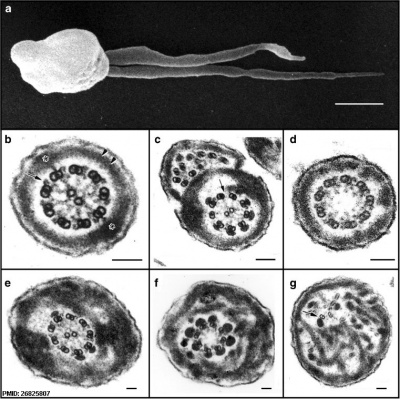
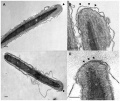
Human sperm pathologies[47] These electron micrographs show a range of tail structural abnormalities including (a) two tails. (b) shows a normal spermatozoa tail cross-section and c to g show a range of abnormal tail structures (open image to see details).
Johnsen score
A clinical score (1-10) for assessing spermatogenesis in a human testicular biopsy. Named after the author of the original article .[48]
| Johnsen score |
Description |
|---|---|
| 10 | complete spermatogenesis and perfect tubules |
| 9 | many spermatozoa present but disorganized spermatogenesis |
| 8 | only a few spermatozoa present |
| 7 | no spermatozoa but many spermatids present |
| 6 | only a few spermatids present |
| 5 | no spermatozoa or spermatids present but many spermatocytes present |
| 4 | only a few spermatocytes present |
| 3 | only spermatogonia present |
| 2 | no germ cells present |
| 1 | neither germ cells nor Sertoli cells present |
|
Reference: Johnsen SG. Testicular biopsy score count - a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. (1970) Hormones 1(1): 2-25. PubMed 5527187 | |
| Classification | Count (Millions/mL) |
|---|---|
| Azoospermia | 0 |
| Severe oligozoospermia | less than 1 |
| Moderate oligozoospermia | 1-5 |
| Mild oligozoospermia | 5-20 |
| Normal | greater than 20 |
Oligospermia
(Low Sperm Count) less than 20 million sperm after 72 hour abstinence from sex
Azoospermia
(Absent Sperm) blockage of duct network
Immotile Cilia Syndrome
Lack of sperm motility
Acephalic spermatozoa syndrome
Acephalic spermatozoa syndrome is characterized by the presence of very few intact spermatozoa and tailless sperm heads in the semen and leads to severe male infertility. Sad1 and UNC84 domain-containing 5 (SUN5) is a testis-specific nuclear envelope protein. A recent study has shown that mutations in SUN5 appear to affect the secondary structure of the protein and influence its folding and cellular localization.[49]
- Links: OMIM - SUN5
Infertility - Stem Cells
Recent studies have been able to transplant of own cryostored spermatogonial stem cells (SSCs) is a promising technique for fertility restoration when the SSC pool has been depleted.[50]
Additional Images
Human spermatozoa acrosomal protein SP-10[33]
Human spermatid electron micrograph[33]
Model capacitation-induced acrosome docking to sperm membrane[14]
Mouse spermiogenesis model[14]
Mouse- seminiferous tubule histology[14]
Rat Spermatogenesis figure[51]
Human spermatozoa - phospholipase C zeta localization[52]
Chemotaxis Model[43]
Labeled Chemotaxis Model[43]
Spermatogenesis androgen action[53]
Mouse spermatogenesis stages[54]
References
- ↑ Oliveira JB, Petersen CG, Massaro FC, Baruffi RL, Mauri AL, Silva LF, Ricci J & Franco JG. (2010). Motile sperm organelle morphology examination (MSOME): intervariation study of normal sperm and sperm with large nuclear vacuoles. Reprod. Biol. Endocrinol. , 8, 56. PMID: 20529256 DOI.
- ↑ Khawar MB, Gao H & Li W. (2019). Mechanism of Acrosome Biogenesis in Mammals. Front Cell Dev Biol , 7, 195. PMID: 31620437 DOI.
- ↑ Hao SL, Ni FD & Yang WX. (2019). The dynamics and regulation of chromatin remodeling during spermiogenesis. Gene , 706, 201-210. PMID: 31085275 DOI.
- ↑ Saucedo L, Rumpel R, Sobarzo C, Schreiner D, Brandes G, Lustig L, Vazquez-Levin MH, Grothe C & Marín-Briggiler C. (2018). Deficiency of fibroblast growth factor 2 (FGF-2) leads to abnormal spermatogenesis and altered sperm physiology. J. Cell. Physiol. , , . PMID: 30054911 DOI.
- ↑ Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL & Rando OJ. (2018). Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev. Cell , , . PMID: 30057273 DOI.
- ↑ Steger K & Balhorn R. (2018). Sperm nuclear protamines: A checkpoint to control sperm chromatin quality. Anat Histol Embryol , 47, 273-279. PMID: 29797354 DOI.
- ↑ Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R & Swan SH. (2017). Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum. Reprod. Update , 23, 646-659. PMID: 28981654 DOI.
- ↑ Maree L, du Plessis SS, Menkveld R & van der Horst G. (2010). Morphometric dimensions of the human sperm head depend on the staining method used. Hum. Reprod. , 25, 1369-82. PMID: 20400771 DOI.
- ↑ 9.0 9.1 Ho HC. (2010). Redistribution of nuclear pores during formation of the redundant nuclear envelope in mouse spermatids. J. Anat. , 216, 525-32. PMID: 20136667 DOI.
- ↑ Bastián Y, Roa-Espitia AL, Mújica A & Hernández-González EO. (2010). Calpain modulates capacitation and acrosome reaction through cleavage of the spectrin cytoskeleton. Reproduction , 140, 673-84. PMID: 20716611 DOI.
- ↑ Pellegrini M, Di Siena S, Claps G, Di Cesare S, Dolci S, Rossi P, Geremia R & Grimaldi P. (2010). Microgravity promotes differentiation and meiotic entry of postnatal mouse male germ cells. PLoS ONE , 5, e9064. PMID: 20140225 DOI.
- ↑ Cheng YH, Wong EW & Cheng CY. (2011). Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis , 1, 209-220. PMID: 22319669 DOI.
- ↑ Hunter D, Anand-Ivell R, Danner S & Ivell R. (2012). Models of in vitro spermatogenesis. Spermatogenesis , 2, 32-43. PMID: 22553488 DOI.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 Borg CL, Wolski KM, Gibbs GM & O'Bryan MK. (2010). Phenotyping male infertility in the mouse: how to get the most out of a 'non-performer'. Hum. Reprod. Update , 16, 205-24. PMID: 19758979 DOI.
- ↑ 15.0 15.1 de Rooij DG. (2017). The nature and dynamics of spermatogonial stem cells. Development , 144, 3022-3030. PMID: 28851723 DOI.
- ↑ Clermont Y. (1966). Spermatogenesis in man. A study of the spermatogonial population. Fertil. Steril. , 17, 705-21. PMID: 5920556
- ↑ Sun F, Xu Q, Zhao D & Degui Chen C. (2015). Id4 Marks Spermatogonial Stem Cells in the Mouse Testis. Sci Rep , 5, 17594. PMID: 26621350 DOI.
- ↑ Shang Y, Wang H, Jia P, Zhao H, Liu C, Liu W, Song Z, Xu Z, Yang L, Wang Y & Li W. (2016). Autophagy regulates spermatid differentiation via degradation of PDLIM1. Autophagy , 12, 1575-92. PMID: 27310465 DOI.
- ↑ Ozturk N, Steger K & Schagdarsurengin U. (2017). The impact of autophagy in spermiogenesis. Asian J. Androl. , 19, 617-618. PMID: 27905325 DOI.
- ↑ Grinspon RP & Rey RA. (2011). New perspectives in the diagnosis of pediatric male hypogonadism: the importance of AMH as a Sertoli cell marker. Arq Bras Endocrinol Metabol , 55, 512-9. PMID: 22218431
- ↑ Clermont Y. (1972). Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. , 52, 198-236. PMID: 4621362 DOI.
- ↑ Chaturvedi PK & Johnson L. (1993). Architectural arrangement of stages of the spermatogenic cycle within human seminiferous tubules is related to efficiency of spermatogenesis. Cell Tissue Res. , 273, 65-70. PMID: 8364962
- ↑ Naylor GJ, McNamee HB & Moody JP. (1970). The plasma control of erythrocyte sodium and potassium metabolism in depressive illness. J Psychosom Res , 14, 179-86. PMID: 5477358
- ↑ Kierszenbaum AL & Tres LL. (2004). The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch. Histol. Cytol. , 67, 271-84. PMID: 15700535
- ↑ Okabe M. (2016). The Acrosome Reaction: A Historical Perspective. Adv Anat Embryol Cell Biol , 220, 1-13. PMID: 27194347 DOI.
- ↑ Bard JAM, Goodall EA, Greene ER, Jonsson E, Dong KC & Martin A. (2018). Structure and Function of the 26S Proteasome. Annu. Rev. Biochem. , 87, 697-724. PMID: 29652515 DOI.
- ↑ Zimmerman SW, Manandhar G, Yi YJ, Gupta SK, Sutovsky M, Odhiambo JF, Powell MD, Miller DJ & Sutovsky P. (2011). Sperm proteasomes degrade sperm receptor on the egg zona pellucida during mammalian fertilization. PLoS ONE , 6, e17256. PMID: 21383844 DOI.
- ↑ Beltrán C, Treviño CL, Mata-Martínez E, Chávez JC, Sánchez-Cárdenas C, Baker M & Darszon A. (2016). Role of Ion Channels in the Sperm Acrosome Reaction. Adv Anat Embryol Cell Biol , 220, 35-69. PMID: 27194349 DOI.
- ↑ Chávez JC, De la Vega-Beltrán JL, José O, Torres P, Nishigaki T, Treviño CL & Darszon A. (2018). Acrosomal alkalization triggers Ca2+ release and acrosome reaction in mammalian spermatozoa. J. Cell. Physiol. , 233, 4735-4747. PMID: 29135027 DOI.
- ↑ Sosa CM, Pavarotti MA, Zanetti MN, Zoppino FC, De Blas GA & Mayorga LS. (2015). Kinetics of human sperm acrosomal exocytosis. Mol. Hum. Reprod. , 21, 244-54. PMID: 25452326 DOI.
- ↑ Rathke C, Baarends WM, Awe S & Renkawitz-Pohl R. (2014). Chromatin dynamics during spermiogenesis. Biochim. Biophys. Acta , 1839, 155-68. PMID: 24091090 DOI.
- ↑ Saperas N & Ausió J. (2013). Sperm nuclear basic proteins of tunicates and the origin of protamines. Biol. Bull. , 224, 127-36. PMID: 23995738 DOI.
- ↑ 33.0 33.1 33.2 33.3 Westbrook VA, Schoppee PD, Vanage GR, Klotz KL, Diekman AB, Flickinger CJ, Coppola MA & Herr JC. (2006). Hominoid-specific SPANXA/D genes demonstrate differential expression in individuals and protein localization to a distinct nuclear envelope domain during spermatid morphogenesis. Mol. Hum. Reprod. , 12, 703-16. PMID: 17012309 DOI.
- ↑ 34.0 34.1 Iranpour FG. (2014). The effects of protamine deficiency on ultrastructure of human sperm nucleus. Adv Biomed Res , 3, 24. PMID: 24592371 DOI.
- ↑ Amann RP & Howards SS. (1980). Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J. Urol. , 124, 211-5. PMID: 6772801
- ↑ 36.0 36.1 Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT & Vogelsong KM. (2010). World Health Organization reference values for human semen characteristics. Hum. Reprod. Update , 16, 231-45. PMID: 19934213 DOI.
- ↑ Fawcett DW. (1970). A comparative view of sperm ultrastructure. Biol. Reprod. , 2, Suppl 2:90-127. PMID: 5521054
- ↑ Bellastella G, Cooper TG, Battaglia M, Ströse A, Torres I, Hellenkemper B, Soler C & Sinisi AA. (2010). Dimensions of human ejaculated spermatozoa in Papanicolaou-stained seminal and swim-up smears obtained from the Integrated Semen Analysis System (ISAS(®)). Asian J. Androl. , 12, 871-9. PMID: 20852650 DOI.
- ↑ Lillie FR. (1912). THE PRODUCTION OF SPERM ISO-AGGLUTININS BY OVA. Science , 36, 527-30. PMID: 17735765 DOI.
- ↑ Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R & Kaupp UB. (2011). The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature , 471, 382-6. PMID: 21412338 DOI.
- ↑ Johannessen JV. (1992). [Physicians and leadership]. Tidsskr. Nor. Laegeforen. , 112, 2950. PMID: 1412339
- ↑ Armon L & Eisenbach M. (2011). Behavioral mechanism during human sperm chemotaxis: involvement of hyperactivation. PLoS ONE , 6, e28359. PMID: 22163296 DOI.
- ↑ 43.0 43.1 43.2 Teves ME, Guidobaldi HA, Uñates DR, Sanchez R, Miska W, Publicover SJ, Morales Garcia AA & Giojalas LC. (2009). Molecular mechanism for human sperm chemotaxis mediated by progesterone. PLoS ONE , 4, e8211. PMID: 19997608 DOI.
- ↑ Kaupp UB, Kashikar ND & Weyand I. (2008). Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. , 70, 93-117. PMID: 17988206 DOI.
- ↑ Rijntjes E, Gomes MLM, Zupanič N, Swarts HJM, Keijer J & Teerds KJ. (2017). Transient Hypothyroidism: Dual Effect on Adult-Type Leydig Cell and Sertoli Cell Development. Front Physiol , 8, 323. PMID: 28588502 DOI.
- ↑ Harper JC, Aittomäki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I, Lundin K, Mertes H, Morris M, Pennings G, Sermon K, Spits C, Soini S, van Montfoort APA, Veiga A, Vermeesch JR, Viville S & Macek M. (2018). Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur. J. Hum. Genet. , 26, 12-33. PMID: 29199274 DOI.
- ↑ Linck RW, Chemes H & Albertini DF. (2016). The axoneme: the propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility. J. Assist. Reprod. Genet. , 33, 141-56. PMID: 26825807 DOI.
- ↑ Johnsen SG. (1970). Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones , 1, 2-25. PMID: 5527187
- ↑ Shang Y, Yan J, Tang W, Liu C, Xiao S, Guo Y, Yuan L, Chen L, Jiang H, Guo X, Qiao J & Li W. (2018). Mechanistic insights into acephalic spermatozoa syndrome-associated mutations in the human SUN5 gene. J. Biol. Chem. , 293, 2395-2407. PMID: 29298896 DOI.
- ↑ Kanbar M, de Michele F & Wyns C. (2018). Cryostorage of testicular tissue and retransplantation of spermatogonial stem cells in the infertile male. Best Pract. Res. Clin. Endocrinol. Metab. , , . PMID: 30448111 DOI.
- ↑ Winawer SJ, Flehinger BJ, Buchalter J, Herbert E & Shike M. (1990). Declining serum cholesterol levels prior to diagnosis of colon cancer. A time-trend, case-control study. JAMA , 263, 2083-5. PMID: 2319669
- ↑ Aarabi M, Yu Y, Xu W, Tse MY, Pang SC, Yi YJ, Sutovsky P & Oko R. (2012). The testicular and epididymal expression profile of PLCζ in mouse and human does not support its role as a sperm-borne oocyte activating factor. PLoS ONE , 7, e33496. PMID: 22428063 DOI.
- ↑ Verhoeven G, Willems A, Denolet E, Swinnen JV & De Gendt K. (2010). Androgens and spermatogenesis: lessons from transgenic mouse models. Philos. Trans. R. Soc. Lond., B, Biol. Sci. , 365, 1537-56. PMID: 20403868 DOI.
- ↑ Phillips BT, Gassei K & Orwig KE. (2010). Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. Lond., B, Biol. Sci. , 365, 1663-78. PMID: 20403877 DOI.
Journals
- Spermatogenesis | PubMed - Spermatogenesis "Spermatogenesis is a new quarterly, peer-reviewed journal that will publish high-quality articles covering all aspects of spermatogenesis." Note last PubMed entries for this journal in 2016.
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland: World Health Organization; 2010. Online PDF
Reviews
de Rooij DG. (2017). The nature and dynamics of spermatogonial stem cells. Development , 144, 3022-3030. PMID: 28851723 DOI.
Griswold MD. (2016). Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. , 96, 1-17. PMID: 26537427 DOI.
Talwar P & Hayatnagarkar S. (2015). Sperm function test. J Hum Reprod Sci , 8, 61-9. PMID: 26157295 DOI.
Yoshida S. (2010). Stem cells in mammalian spermatogenesis. Dev. Growth Differ. , 52, 311-7. PMID: 20388168 DOI.
Hogarth CA & Griswold MD. (2010). The key role of vitamin A in spermatogenesis. J. Clin. Invest. , 120, 956-62. PMID: 20364093 DOI.
Ruwanpura SM, McLachlan RI & Meachem SJ. (2010). Hormonal regulation of male germ cell development. J. Endocrinol. , 205, 117-31. PMID: 20144980 DOI.
Hermo L, Pelletier RM, Cyr DG & Smith CE. (2010). Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc. Res. Tech. , 73, 241-78. PMID: 19941293 DOI.
Hermo L, Pelletier RM, Cyr DG & Smith CE. (2010). Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa. Microsc. Res. Tech. , 73, 279-319. PMID: 19941292 DOI.
Kaupp UB, Kashikar ND & Weyand I. (2008). Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. , 70, 93-117. PMID: 17988206 DOI.
Eddy EM, Toshimori K & O'Brien DA. (2003). Fibrous sheath of mammalian spermatozoa. Microsc. Res. Tech. , 61, 103-15. PMID: 12672126 DOI.
de Rooij DG & Russell LD. (2000). All you wanted to know about spermatogonia but were afraid to ask. J. Androl. , 21, 776-98. PMID: 11105904
Clermont Y. (1972). Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. , 52, 198-236. PMID: 4621362 DOI.
Articles
Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT & Vogelsong KM. (2010). World Health Organization reference values for human semen characteristics. Hum. Reprod. Update , 16, 231-45. PMID: 19934213 DOI.
LEBLOND CP & CLERMONT Y. (1952). Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad. Sci. , 55, 548-73. PMID: 13139144
NCBI Bookshelf
- StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008 Regulation of spermatogonia
MBoC - Sperm | MBoC - Highly simplified drawing of a cross-section of a seminiferous tubule in a mammalian testis | MBoC - Cytoplasmic bridges in developing sperm cells and their precursors
- NCBI Bookshelf spermatozoa | spermatogenesis | spermiogenesis
Search
- Pubmed spermatozoa | spermatogenesis | spermiogenesis
Terms
| Spermatozoa Development (expand to see terms) | ||
|---|---|---|
|
Note there are additional glossaries associated with genital, spermatozoa, oocyte and renal.
See also: Spermatozoa Terms collapse table
|
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- World Health Organization - WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland: World Health Organization; 2010. Online PDF
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 27) Embryology Spermatozoa Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Spermatozoa_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G




![Human spermatozoa acrosomal protein SP-10[33]](/embryology/images/thumb/a/a8/Human_spermatozoa_acrosomal_protein_SP-10.jpg/111px-Human_spermatozoa_acrosomal_protein_SP-10.jpg)
![Human spermatid electron micrograph[33]](/embryology/images/thumb/8/8c/Human_spermatid_electron_micrograph.jpg/81px-Human_spermatid_electron_micrograph.jpg)
![Model capacitation-induced acrosome docking to sperm membrane[14]](/embryology/images/thumb/2/2e/Model_capacitation-induced_acrosome_docking_to_sperm_membrane.jpg/120px-Model_capacitation-induced_acrosome_docking_to_sperm_membrane.jpg)
![Mouse spermiogenesis model[14]](/embryology/images/thumb/4/4f/Mouse_spermiogenesis_model.png/120px-Mouse_spermiogenesis_model.png)
![Mouse- seminiferous tubule histology[14]](/embryology/images/thumb/0/04/Mouse-_seminiferous_tubule_histology.jpg/120px-Mouse-_seminiferous_tubule_histology.jpg)
![Rat Spermatogenesis figure[51]](/embryology/images/thumb/3/3e/Spermatogenesis_cartoon_01.jpg/120px-Spermatogenesis_cartoon_01.jpg)
![Human spermatozoa - phospholipase C zeta localization[52]](/embryology/images/thumb/f/fd/Human_spermatozoa_phospholipase_C_zeta.jpg/120px-Human_spermatozoa_phospholipase_C_zeta.jpg)
![Chemotaxis Model[43]](/embryology/images/thumb/d/da/Human_spermatozoa_chemotaxis_model.jpg/120px-Human_spermatozoa_chemotaxis_model.jpg)
![Labeled Chemotaxis Model[43]](/embryology/images/thumb/8/8a/Human_spermatozoa_chemotaxis_labeled_model.jpg/120px-Human_spermatozoa_chemotaxis_labeled_model.jpg)
![Spermatogenesis androgen action[53]](/embryology/images/thumb/4/49/Spermatogenesis_androgen_action_cartoon.jpg/120px-Spermatogenesis_androgen_action_cartoon.jpg)
![Mouse spermatogenesis stages[54]](/embryology/images/thumb/5/51/Mouse_spermatogenesis_stage_cartoon.jpg/120px-Mouse_spermatogenesis_stage_cartoon.jpg)
