Paper - The role of the primitive mesothelium in the development of the mammalian spleen (1936)
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Holyoke EA. The role of the primitive mesothelium in the development of the mammalian spleen. (1936) Anat. Rec. 65(3): 333-349.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Role of the Primitive Mesothelium in the Development of the Mammalian Spleen
Edward Agustus Holyoke
Department of Anatomy, College of Medicine, University of Nebraska, Omaha, Nebraska
TWO PLATES (twelve figures)
Introduction
It is generally accepted that the embryonic mammalian spleen can first be recognized as a dense accumulation of cells in the mesenchyme of the dorsal mesogastrium or neighboring rnesentery; The splenie rudiment has been described by Kolliker (1854), Miiller (1870), Minot (1892), Kollmann (’00), Tonkoff (’00), Sabin (’12), Thiel and Downey (’21) and many others. Choronsehitzky (’00) and Danchakoff (’16) have observed a similar anlage in birds. Radford ( ’-08) believed the splenic anlage’ of the frog to be a dense accumulation of lymphocytic cells around the mesenteric artery, While Leon (’32) placed it (in fish) at a definitely predetermined point in the vascular endothelium of the sub—intestinaI vein.
The old controversy concerning the germ layers involved in the developing spleen has apparently been settled, the generally accepted view today being that it is entirely of mesodermal origin. However there still exists some doubt as to which mesodermal elements are directly involved. In a recent paper, Bergel and Gut (’34) have ‘attempted to establish the human spleen as a derivative of mesenchyme alone as has been held for birds and reptiles by Danchakoff (’16) and others, and for Amphibia by Nakajima (’29). On the other hand Kollmann (’00), Tonkofi (’00), Choronschitzky (’00), Thiel and Downey (’21) and Hartmann (’30) have shown that the spleniclrudiment receives cells from the overlying peri toneal layers which are crowded into the mesenchyme during the early stages of development. Toldt (1889), and Janosik ( ’01) were of the opinion that the spleen was exclusively mesothelial in origin.
Recently I have had occasion to examine the splenic region of an extensive series of early human and pig embryos sectioned in various planes and stained by several different methods. Observations of this material have led me to conclusions differing from those of Bergel and Gut.
Inasmuch as most descriptions of the mesenteries before the splenic rudiment appears have been very incomplete, a careful examination of very early material was made. As an exception to such incomplete descriptions stands the work of Thiel and Downey (’21) ; these authors described the dorsal mesogastrium of a 3—mm. gopher embryo, in which stage they found a definite mesentery covered by a loose visceral peritoneum and containing a few formed capillaries. These authors have also described the dorsal mesogastrium of the pig embryo (7.5 mm.) before the appearance of the spleen.
In human embryos of 3 mm I have found no indication whatever of the future splenic rudiment. The region in which the rudiment is to appear can, however, be roughly determined by its relation to the stomach. Examination of the mesentery in that region reveals no characteristic findings. Like all other parts of the mesentery it consists of a comparatively loose mass of mesenchyme covered on its lateral surfaces by dense mantles of irregularly arranged cells which are three or four layers deep. This coelomic epithelium or primitive mesothelium is in a state of active proliferation as evidenced by many mitotic figures. The cells composing these layers bear a striking resemblance to those of the underlying mesenchyme
- Of the human embryos examined some were from the collection of the Department of Histology and Embryology, Cornell University Medical College, Ithaca; some from the collection of the Department of Anatomy, Harvard Medical School; and the remainder from the collection of the Department of Anatomy, University of Nebraska Medical College. The author is most appreciative of the generosity of Dr. B. F. Kingsbury and Dr. J. L. Bremer, who permitted the examination of this material and the use of some of the figures herewith reproduced. Pig embryos examined were locally collected and prepared by the author.
- All measurements of embryos given in this paper refer to crown-rump length.
in that they take an identical cytoplasmic stain and their nuclei are large, containing a few dense masses of chromatin and usually two nucleoli. They are intimately connected with the mesenchyme, protoplasmic continuity being demonstrable in many places. The possibility of .many cells passing from coelomic epithelium to mesenchyme can be readily shown by the intimate contact and irregular boundary between the surface layers and underlying tissue, and by the presence of mitotic spindles lying perpendicular to the surface of the mesentery as described by Thiel and Downey for the 7.5—mm. pig embryo.
Essentially the same conditions have been observed in other human embryos up to 6 mm. in length. By the time this stage has been reached, the stomach has become a large conspicuous organ and has started its rotation toward the left. In -rotating it has carried a fold of the mesogastrium with it so that, as seen in transverse section, it makes one acute bend at its root where it joins the body wall and another at its stomach attachment (fig. 1). Near the midpoint of this mesenteric segment there has developed a distinct bulging toward the left.
This bulging of the mesentery is due to a proliferation of mesenchymal cells in that region, but there is no demonstrable condensation. The coelomic epithelium there has retained all of the characteristics described for the earlier embryos. It has remained an irregularly formed mantle of cells continuous With the underlying mesenchyme, and as indicated in the earlier forms, presenting no cytological characteristics which would enable one to distinguish between the two. The only variation of any kind is a slightly deeper’ cytoplasmic stain in the surface cells due, possibly, to crowding.
Elsewhere the general coelomic epithelium has started to differentiate. In most regions it has become a cuboidal or squamous layer sharply cut off from the mesenchyme by a distinct basement. membrane. Examples of this are to be seen in figure 1. It will be noted that the coelomic epithelium on the right side of the mesogastrium has become cuboidal, but that the basement membrane is not yet demonstrable. There remain, however, a few other areas where the coelomic epithelium has retained its earlier form, notably in a series of irregularly located points along the gut. These same conditions are to be found in pig embryos of 7.5 to 8 mm.
The above described bulging of the mesentery probably should not be considered as the splenic anlage for a definite, much less extensive condensation of cells soon appears in this region. In 10- to 12-mm. pig embryos this mass is present in the left part of the mesentery Where it is continuous with both the primitive mesothelium and the mesenchyme (fig. 2). It does not stand out very conspicuously at this stage because of the density of the mesenchyme in general. The cellular mass is not in contact with the mesothelium of the right leaf of the mesentery, but is separated from it by a small area of looser mesenchyme.
A closer study of the region of the anlage in the 10—mm. pig embryo (fig. 2) fails to reveal any line of demarcation between mesothelium and dense mesenchyme. As indicated in the earlier stages of pig and human embryos continuity between the two can be demonstrated and no cytological differences, except as noted, are to be found to separate them. Mitoses are abundant and these occur alike in the deeper cells of the anlage and the free surface of the mesentery A few of the latter figures still present spindles perpendicular to the surface indicating a contribution of cells from this region to the deeper mass. All of these cells have a very similar morphology. The deeper mesenchymal cells are so densely packed that their intercellular bridges are difficult or impossible to distinguish as separate cytoplasmic strands, and their typical stellate form is obscured. These cells, as Well as those near the surface, take a deep basic cytoplasmic stain against which the vesicular nuclei stand out as comparatively pale bodies.. The nuclei are still characterized by a few dense chromatin masses and two nucleoli, as in the earlier embryos. The less dense mesenchyme at the right of the anlage shows nuclei identical to those just described. The cytoplasm of these cells stains less deeply and the typical stellate form is more readily demonstrable. Similar conditions obtain in human embryos of from 8 to 9 mm. in length.
From these findings one is forced to two conclusions. First, there is no morphological basis for believing that the primitive mesothelium is more than a. dense layer of littoral mesenchymal cells which later differentiate to form tl1e adult peritoneal epithelium. Second, both the surface and deeper layers play an important role in the contribution of cells to the splenic mass.
It has been repeatedly shown that primitive mesothelial cells are pleuropotential and that invasion of the deeper tissues by them is of frequent occurrence (Schott, ’O9; Mollier, ’O9; Bremer, ’14; Ilaff, ’14; Scammon, ’15; and others). Emmel (’16) thought that many of the free cellular elements found in the peritoneal cavityof the pig embryo were derived from this same source.
The time of appearance of a definite splenic anlage seems to be relatively constant in many mammalian species. I have found it in human embryos at 7.5 to 8 mm. Tonkoff (’00), Kollmann ( ’OO), Sabin (’12), Ono (’30) and others have described the splenic rudiment in embryos of from 8 to 10 mm. or during the fifth week of development, while Bergel and Gut (’34) found it at 7 mm. In the pig embryo the time of appearance of the spleen may be a little more variable. In some of the series of embryos I have examined, it was poorly developed at 12 mm., while some 10—mm. series showed it distinctly. It is possible that slight errors in measurement and failure to make adequate allowance for shrinkage during preparation may account for this variation. Thiel and Downey (’21) described a definite splenic anlage in the pig embryo at 15 mm., and a well-developed splenic rudiment in the striped gopher at 8 mm. In an 8-mm. cat embryo which I examined, it could be identified clearly.
After the splenie mass has been established, it grows very rapidly causing a more and more marked local bulging of the mesentery which gradually replaces the more diffuse enlargement of the earlier presplenic stages. At the same time the mesentery begins to buckle toward the left at the splenic prominence so that this structure soon lies in the convexity of a definite fold (figs. 4, 5, 7 and 9). Coincidentally, the coelomic epithelium begins to become separated from the splenic anlage as a distinct layer. This is first indicated by the organization of the cells into a single layer and the appearance of a distinct basal zone, free from nuclei and conspicuous because of its light color. Ono (’30) has mentioned this zone in the early human embryo. In my opinion this light zone owes its appearanc solely to segregation of the nuclei in more peripheral portions of the superficial cells. This leads me to the conclusion that the plane of sectioning is not oblique enough in relation to the epithelial surface seriously to distort the picture. These changes just noted are usually demonstrable in 12-mm. pig embryos (fig. 3), although at many points the cellular arrangement is still irregular. and protoplasmic continuity with the mesenchyme is evident. At a, b, and c are shown small groups of cells continuous with the coelomic epithelium, interrupting the light zone and apparently making their way into the splenic mesenchyme. In a few places in this surface layer there are signs of nuclear differentiation, the chromatin breaking up into finer particles than in the mesenchymal nuclei. Ad basement membrane soon appears in several places and begins to extend along the deep surface of the light zone. Between the various segments of this membrane, protoplasmic continuity with the mesenchyme remains and the migration of superficial cells apparently continues. The organization of epithelial cells is complete in human embryos of from 10 to 12 mm. (fig. 4). These cells become more distinctly separated from each other by cell membranes and the general nuclear stain becomes more intense. In pig embryos of 14 to 15 mm. a similar series of changes begins. In a 14-mm. pig embryo the organization of the epithelium into a distinct layer’ is almost complete and the light zone is conspicuous. The basement membrane has not appeared except in a few isolated places and cells are still passing into the underlying mesenchyme (fig. 8).
Soon after the organization and dfferentiation of the coelomic epithelium has begun the basement membrane is almost complete, cutting off a distinct layer of columnar cells. When the basement membrane appears, it is readily demonstrable by ordinary staining methods. In a typical human embryo of 13.5 mm. the membrane stands out conspicuously as a sharp line broken only in a few places by migrating cells (fig. 5). One of these regions is shown in more detail in figure 6. The continuity of the coelomic epithelium with the splenic mesenchyme has remained unbroken at this point, and a cell (mc.) is being added to the splenic anlage. There probably is only an occasional cell added to the mesenchyme in this manner subsequently, for (as can be seen in figs. 5 and 6) the mesothelium is now a distinct layer and is sharply demarcated in most places. A closely graded series of nuclei can now be found in this mesothelial layer; representing various steps between the primitive mesothelium, which is identical with the mesenchyme, and the general mesothelium of this stage of development. A few of these nuclei retain mesenchymal characteristics (fig. 6): Others show a finer distribution of chromatin and the disappearance of one or both nucleoli; and still others are intensely stained and contain finely divided chromatin, in some cases appearing as a delicate network. These are typical of the general mesothelium. There has also been a progressive limitation of the more primitive type of mesothelium to the splenic region alone. In early human embryos (figs. 1 and 4) this primitive non-specific layer extends beyond the locus of the splenic anlage over the surface of the stomach. Going beyond this region in 6- to 7-mm. human embryos, one sees a gradual and continuous transition to the general coelomic epithelium (fig. 1). Later (fig. 5) this transition begins definitely at the margin of the spleen. The cells become progressively more and more flattened as the layer is traced away from the spleen, the light zone narrower and the nuclear stain more intense. The general coelornic epithelium is a low cuboidal or squamous layer and somewhat resembles the adult condition.
During the same period the splenic mass becomes more distinct from the surrounding mesentery, the transition from dense to loose mesenchyme becoming more abrupt and the mesenchyme of the mesentery more attenuated. At 11 mm. (fig. 4), the human spleen is not Very clearly marked off, but at 13.5 mm. (fig. 5) the limits of the anlage are very distinct. Similar changes have appeared in the splenic anlage of the 14-mm. pig embryo (fig. 7). The dense mesenchyme here is beginning to show sharp localization. It can be distinctly differentiated from the loose mesenchyme to the right but is not so definitely demarcated on all sides, tending to merge more gradually into the looser surrounding tissue.
As the spleen continues to grow, it expands in the left half of the mesentery and grows around the loose mesenchyme at the right (fig. 9). The large splenic vessels are found here in the concavity of the splenic fold (figs. 4, 5 and 7) and this region becomes established as the hilus. This arrangement is definitely shown by human embryos of from 15 to 16 mm.
From this time on the coelomic epithelium over the spleen diflerentiates rapidly. The light zone becomes narrower as the cells begin to assume a low columnar or cuboidal form, and the basement membrane becomes complete (fig. 9). There remain a few irregularities, in the deep surface of the mesothelium, but it is doubtful whether they could be interpreted as indicating it further exchange of cells. This process is probably complete in human embryos of 14 mm. and in pig embryos of from 15 to 19 mm. The coelomic epithelium of a 22-mm. pig embryo is composed of cells regularly placed upon a definite basement membrane which are sharply separated from one another by distinct cell membranes. By this time they difier from the general mesothelium only in that they are cuboidal rather than squamous in form. This latter condition is not attained in the splenic region of pig embryos before they reach a length of 45 to 50 mm. Figure 11 shows a cuboidal layer over the spleen at 35 mm. In man the adult type of mesothelium appears at a much earlier stage. The mesothelial layer over the spleen at 22 mm. is identical in every way with. the general coelomic epithelium and is approaching the adult condition.
Bergel and Grut (’34) have recently denied any contribution by the eoelomic epithelium to the underlying mesenchymal anlage of the mammalian spleen. Nakajima (’29) ha.s come to similar conclusions in a study of amphibian embryos. From Nakajima’s discussion and figures, I am unable to determine how these conclusions were reached. Bergel and Gut (’34) made an extensive study of human material. In their opinion most investigators have been misled on the mesothelial question, because they have failed to use specific stains, and have studied sections which have not cut the epithelial layers transversely. They state that the basement membrane between the coelomic epithelium and the underlying mesenchyme is demonstrated only by strong cytoplasmic stains, and that the usual technical methods fail to show it. With this statement in mind, I have reexamined all of my material, some of which has been stained with M-al1ory’s connective tissue stain and some by B.ielschowsky’s silver impregnation method. I can find no instance in which these preparations show a complete basement membrane between the eoelomic epithelium and the splenic anlage in pig embryos of less than 15 mm. On the other hand very distinct basement membranes are demonstrable in relation to the general eoelomic epithelium in preparations stained with Delafield’s hematoxylin and azure II eosin, or with Wright’s and Giemsa stains. All of the material used for illustrations of this paper (except fig. 9, where orange G was used with hematoxylin) has been stained by these or similar methods. Basement membranes are conspicuously demonstrated at some point in most of these figures. Figure 1, page 24 of Bergel and Gut ’s paper, they considered a demonstration of a complete basement membrane in the splenic region of an early human embryo (14 mm.). In my opinion this membrane is no more distinct than that shown by other methods in my material. Furthermore the leader pointing out the membrane in their figure could be interpreted as indicating a typical break with the continuity between superficial and underlying cells.
In regard to the plane of sectioning of the material, there is no doubt that misleading impressions can be given by sections which are not truly transverse through the layers in question. However, the condition of the coclomic epithelium here represented (except where specially noted) is not to be explained on this basis. Regardless of the plane of sectioning, the condition of the mesothelium over the splenic region (figs. 1 to 9) is a constant finding in these early embryos. On the hilar side of the dorsal mesogastrium in the same sections, the mesothelium is a cuboidal or squamous layer. Further evidence that these sections are nearly transverse was ob tained by tracing the layer through several sections and demonstrating that it shifts only slightly in relation to other structures, surely no more than the comparatively fiat layer on the opposite side of the mesentery. In those stages where several layers of primitive mesothelial cells are found, care was taken to demonstrate that similar conditions were not present on the opposing surface. Also, there are other findings such as cytoplasmic continuity between layers, the presence of transitional cell types and mitotic spindles perpendicular to the surface of the mesentery, which are not to be explained on the basis of plane of section.
Bergel and Gut agree with Hartmann ( ’30) that cords of epithelial cells can be seen growing down into the rudiment. They maintain, however, that these cells are surrounded by a definite basement membrane andlare not incorporated in the mesenchyme. In their figures. one could not definitely determine such a membrane. Finally these two authors state that, because two types of cells are not demonstrable in the splenie anlage, this structure could have been derived from one source only. While it is true that Toldt (1889), Janosik (’01) and others have thought that the free cellular elements of the mammalian spleen were cut off from the coclomic epithelium and entered _a mesenehymal meshwork, and more recently Radford ( ’O8), Leon (’32), and others have found free cellular elements at early stages in lower forms, Thiel and Downey (’21), Danehakoff (’16) and others have shown than in anmiotes the splenic rudiment contains no free cells until a later time, and that such cells are cut off from the mesenchymal syncytium when they do form. This is in accord with my own observations. I am aware of no contention made by recent authors that the coelomic epithelium contributes free elements or any differentiated forms whatever to the spleen. The evidence rather points toward the continuity of mesothelium and mesenchyme. The cells which are crowded into the latter layer are originally a part of the syncytium and remain so. 'In conclusion I should like to point out that the contribution of cells from the coelomic epithelium is important more because it indicates that this layer is essentially mesenchymal in its nature and potentialities than because it indicates a dual source of the splenic rudiment. As the coelomic epithelium is becoming a differentiated layer, this contribution continues sparingly for a time; but. after a certain critical point is reached, it ceases entirely.
Summary
The splenic rudiment appears in the human embryo at 7.5 mm. and in the pig embryo at 10 mm. It is first observed in other mammalian forms at comparable stages.
The anlage develops as a dense mass of mesenchyme in a preformed bulging of the dorsal mesogastrium. It is at first continuous with the overlying coelomic epithelium and the mesenchyme of the mesentery. It gradually becomes more sharply delineated from this latter tissue, which becomes more diffuse as the anlage becomes more condensed.
A small area of loose mesenchyme always separates the anlage from the right leaf of the mesentery. The splenic vessels appear in this area and the spleen grows around it forming the hilus at this point.
Before the splenic rudiment appears the coelomic epithelium is present as a surface condensation of mesenchyme continuous with the underlying tissue.
The spleen forms at the base of the littoral condensation to the left of the dorsal mesogastrium and is derived in part from this and in part from the underlying mesenchyme. The surface layer over the spleen retains its primitive character for a much longer period than coelomic epithelium in general.
Differentiation of the primitive coelomic epithelium in contact With the splenic rudiment can first be recognized by an organization of the component cells into a simple cuboidal layer. This layer later becomes sharply cut off from the underlying tissue by a definite basement membrane. Until this membrane becomes complete some of the cells retain their primitive potentialities as is evidenced by their mesenchymal type of nucleus and by their occasional migration into the splenic mesenchyme.
After the basement membrane becomes complete, all migration ceases. These mesothelial cells then become progressively flattened until the adult squamous form is approached. This condition is attained at a much earlier stage in the human embryo than in the pig.
The primitive mesothelium plays an important role inthe development of the mammalian spleen. This role indicates mesenchymal potentialities in the primitive mesothelium rather than a dual source of the spleen.
Literature Cited
BERGEL, A. AND H. GUT 1934 Zur Friihentwicklung der Milz beim Menschen. Zeitschr. f. Anat. u. Entwicklungsgesch, Bd. 103, S. 20-29.
Bremer JL. The earliest blood-vessels in man. (1914) Amer. J Anat. 16(4): 447-475.
CHORONSCHITZKY, B. 1900 Die Entstehung der Milz, Leber, Gallenblase, u. s. w. bei den verschiedenen Abteilungen der Wirbeltiere. Anat. Hefte, Bd. 13, S. 369-614.
DANCHAKOFF, V. 1916 fiber die Entwieklung des Blutes in den Blutbildungsorganen bei Tropidonotus Natrix. Arch. f. mikr. Anat., Bd. 87, S. 497-584.
——1916 The equivalence of difierent hematopoietie anlages by method of stimulation of the different stem cells. Am. J. Anat., vol. 20, pp. 255-327.
EMMEL, V. E. 1916 Concerning certain cellular elements in the coelomic cavities and mesenchyma of the mammalian embryo. Am. J. Anat., vol. ‘ 20, pp. 73-123. MESO'l.‘1LE.LIT7M IN 1'r.u: I)EVELOI’M.EI\TT 05' s1>L1sm: 345
HAI011‘, It. 1914 Bindgewebes und Blutbildungsprozesse in der en1bryo11a1en Leber des I-Iulms. Arch. 1. mikr. Anat., Bd. 84, S. 321-350.
HARTMANN, A. 1930 Die Milz. Ilandbueh den Mikroskopisehen Anatomie des Mensohen. W. V. Mb'1Ie11dor1'.'e. Bd. 6, S. 397-563. Julius Spxingex- Berlin.
JA‘.\Io§IK, J} 1901. Bemerkungen zur der Arbeit Dr. W. Tonkoff, Die Entwicklung den Milz bei den Anmioten. Arch. f. Inikr. A11at., Bd. 57, S. 487-488.
]<{i1.LIKER, A. 1854 Manual of Hum:-m Ilistolegy, vol. 2, pp. 138— L60. Transl. by Brusk and Huxley. Sydenham Society, London.
KOLL1KAR, J. 190() Die Entwieklung der Lympllkniitsehen in dem Blinddzmn und in dem Ptoeessus Vermifnrmes. Die "Entwiekluug der '1‘onsi11e'n und die hlntwieklung der Milz. Arch. f. Anat. u, 'Ph_ysioL Anat. Abt., S. 155-186.
LEON, C. 1932 I-Hstegcmése do 1’ebauche splénique ehez les sa.|mo11'1<l(=,s. Arch. Anat. m'1e1'0s., T. 28, pp. 363-393.
MINOT, C. S. 1892 Human Embryology, pp. 4l5—417, Wm. Wood and C0,, New York.
M()I.LIER, S. 1909 Die Blutbildung in der ombryonale Leber des Me-nsehen und die Wirbeltiere. Arch. 1’. mikr. Anat., Bd. '74, S. 474-524.
ML"L.Lr:R, W. 1870 The Spleen. Stricker’s Manual of Human and Comparafive llistelogy, pp. 349-364. Trans. by Power. Sydenham Soc-iety, Londonl.
'L\'AKAJ1M‘A, A. 1929 'CT1>er die Mm-phngenese der Milz V011 Mega1<.ubu.traeh11s Japonieus. 14‘01ia. Anat. .Tapon., vol. 17, pp. 93-112.
~ 1192b Zur Morphologie der M112 von liynobius Fusells. Folia. Anat. Japun., vol. 17, pp. 305-323.
O.\'o, K. 1930 Untersuehungen iibcr die Entwi‘cklu11g der n1esch11'ehen Milz. Zeitschr. ‘E. Zellfnrseh. 11. mikr. Anat., Bd. 10, S. 573-603.
Rmroxu), M 1908 The development of the spleen. (I. Anat. and Physiel., vol. 49, pp. 289-301.
Sabin FR. The Development of the Lymphatic System in Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
SCAMMON, R-. E. 1915 The histogcnesis of the selaehian liver. Am. J. A1mt., vol. 17, pp. 245 -315.
S(711o'1"r, E. 1919 Morphologische uud experimcntelle Untersuo11ungen iiher Bedeutung und Herkunft der Zellcn der serfisen 116111011. Arch. f. mikr. Anan, Bd. 74, S. 14.3—‘>14.
’l.'HIEI., G. AND HAL 1)mvxr.v 1921 The development of the mammalian spleen with speeial refm-euee to its hemopoietit-. activity. Am. J. Annf-., vol. 28, pp. 279-339.
TOLDT, (1. 1889 Zur Anatomic dor Milz. Wiener klin. Wochensch1'., Bd. 2, S. 989.
'I.‘oNK0E‘14*, V". 1900 Die }‘}ntwieklu11g der Milz bei den Amniofen. Arch. 1. mikr. A'r1at., Bd. 56, S. 35)2—<158.
Plates
Plate 1
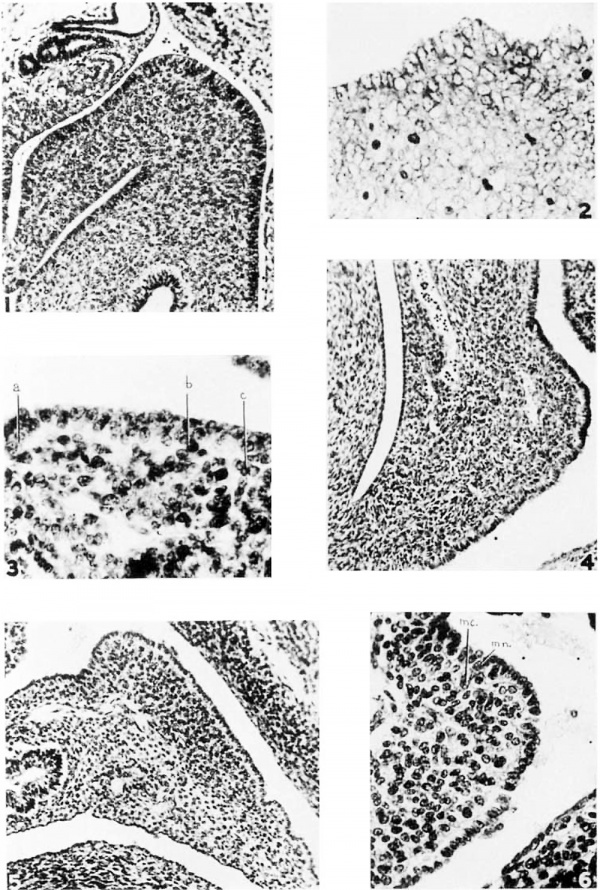
1 The splenic region of a 6—mm. human enibryo showing the relations in the dorsal mesogastrium before the spleen appears. There is a local bulging of the mesentery but no condensation. The primitive ooelomic epithelium is a dense mantle of cells continuous with the underlying meseneiiynial syneytiuin and with the differeiitiaterl mesothelium at the root of the n1esentery_ Ilemaroxylin and eosin. Photo X 200.
2 The splenic anlage of a 10—mm. pig leml)ry0 showing the dense local mass of cells continuous and morphologically similar h0tl1 with the eoelomic epithelium and the adjacent mesenehyme. Wright’s and Giemsa stain. Photo X 550.
3 The splenie anlage of a 12-mm. pig embryo. The cells are a.1'r2111g'e(l in :1 single layer and a ‘ light zone’ is apparent at some points between the peripheral nuclear stratum and the nleseiiehyme. There is no basement membrane and the cells at a, b and e are niigratirig into the mesenchyme. The apparent double nuclear layer at some points inclioates that the section is not exactly transverse. I)elafield’s hematoxylin and azure ll eosin. Photo X 750.
4 Human embryo, 11 mm, showing the early buckling of the dorsal meso,<2;ast1'ium toward the left and the appearance of the splenic vessels in the coneavity of the resulting fold. The splenie anlage is indieatecl by the prorninenee at the eoxnexity of the fold as it does not stand out clearly from the adjacent dense mesenchyme. The eoelomie epithelium has become a simple eolumnar layer, but there is no basement membrane. There is a. well-defined euboidal. layer on the concave surface of the mesenteric fold. llematoxylin and eosin. ‘Photo X 200.
5 Human embryo, 13.5 mm. The splenie anlage is a distinct condensation of mesenchyme. The overlying eoelomio epithelium has become a low columnar layer marked off by a. basement membrane except in a few places (fig. 6). This columnar layer is continuous with the cuboidal layers beyond the margins of the anlage. Hematoxylin and eosin. Photo X 200.
6 Detail from preceding figure. The (tell me. is being added to the splenio Inesenchyme. nm. is a typical mesenchymal nueleus in one of the epithelial cells. Photo X 400.
Plate 2
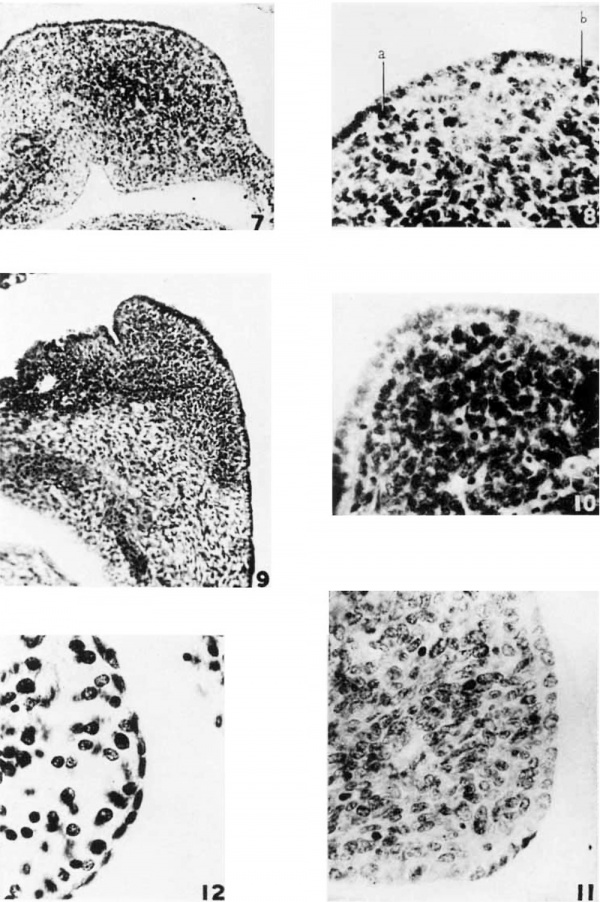
7 Pig embryo, 14 mm., showing the forination of the splenic fold in the dorsal mesogastrium and the bulging due to the growth of the anlage. The splenic condensation is distinct from the surrounding mesenchyme, particularly in the region of the splenic vessels.
8 Detail from preceding figure, showing the cuboidal coelomic epithelium and the early formation of the light zone. a and b indicate cells passing into the mesenchyme. Photo X 550.
9 Human embryo, 15 mm. The spleen is situated in the convexity of a sharp bend in the mesentery. The hilus is located in the loose mesenchyme in the concavity of this bend. The eoelomie epithelium is a columnar layer completely out off from the spleen by a basement nnenibrane. Hematoxylin and orange G. Plmto X 200.
10 Pig embryo, 22 mm. There is 4, distinct euboidal layer of aoeloniic. epithelium sharply marked off by a basement membrane. Delafield’s heamtoxylin and azure II eosin. Photo X 550.
11 Pig embryo, 35 mm., showing the persistence of a cuboidal type of mesotheliuin over the spleen. Delafield’s hematoxylin and azure II eosin. photo X 750.
12 Human embryo, 22 mm. There is a distinct low cuboidal layer of epithelium simulating somewhat the adult, condition. Ilexrmtoxyliii mid eosin. Photo X 750.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The role of the primitive mesothelium in the development of the mammalian spleen (1936). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_role_of_the_primitive_mesothelium_in_the_development_of_the_mammalian_spleen_(1936)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G