Paper - The early development of the membranous labyrinth in mammalian embryos
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Anson BJ. The early development of the membranous labyrinth in mammalian embryos, with special reference to the endolymphatic duct and the utriculo—endolymphatic duct. (1934) Anat. Rec. 59: 15-25.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Early Development of the Membranous Labyrinth in Mammalian Embryos, with Special Reference to the Endolymphatic Duct and the Utriculo-Endolymphatic Duct
Barry J. Anson
Department of Anatomy, Northwestern University Medical School, Chicago
Thirty Three Figures
- Contribution no. 198 from the Anatomical Laboratory of Northwestern University Medical School. This study constitutes one aspect of an investigation conducted under the auspices of the American Otological Society. Paper read at the New York City meetings of the American Association of Anatomists, February, 1932. (Anat. Rec., vol. 52, pp. 2-9.)
Introduction
Several investigatois have recently drawn attention to the manner in which, in the adult mammalian ear, the apposed epithelial walls of the utricle and of the endolymphatic duct constitute ‘a valve-like’ fold Which, in projecting into the utrieular cavity, guards an elliptical utriculo—endolymphatic communication. The importance of this fold in shaping the embryonic labyrinth has not, however, been appreciated; yet certain other associated folds although less conspicuous and no more essential—-have been pointed to as the determining features in the conversion of the otocyst into a vesicle possessing the subdivisions seen in the adult. It is our present purpose to consider the interrelations of the three folds, as well as the part played by each. In addition, we shall discuss the development of the specialized, and possibly functionally important, folds in the endolymphatic duct.
Material and Methods
The material for the general developmental study of the ear, of which the current report is one phase, consisted of 205 selected series of mammalian embryos from the Harvard Embryological Collection, distributed among twelve species as follows: seventy series of human embryos, from 2.4 to 74 mm. in (CR) length; twenty-six of the cat, 4.6 to 39 mm.; five of the dog, 12.5 to 17 mm.; twenty—four of the rabbit, 3 to 33.8 mm.; twelve of the guinea—pig, 3.5 to 30 mm.; thirteen of the rat, 4.4 to 24.8 mm.; one of the bat, 13.4 mm.; twenty—three of the pig, 3.1 to 48 mm.; three of the calf, 14.2 to 25.2 mm.; seventeen of the sheep, 2.8 to 48.4 mm.; two of the deer, 9.8 to 18.6 mm.; ten of the opossum, 7.5 to 26 mm. The series of infant and of child were added from the collection at Northwestern.
Numerous tracings from these series were then prepared with the Edinger projection apparatus, and the most favorable of these chosen for the figures.
- Online Editor - |Edinger projection apparatus was developed in 1907 by Dr. L. Edinger (director of the Neurologic Institute at Frankfurt on Main). He replaced his drawing and photographing apparatus using either oil or gas light, with one using a small arc lamp. This small arc lamp was worked out and perfected by the optical works of Leitz at Wetzlar.
Observations and Discussion
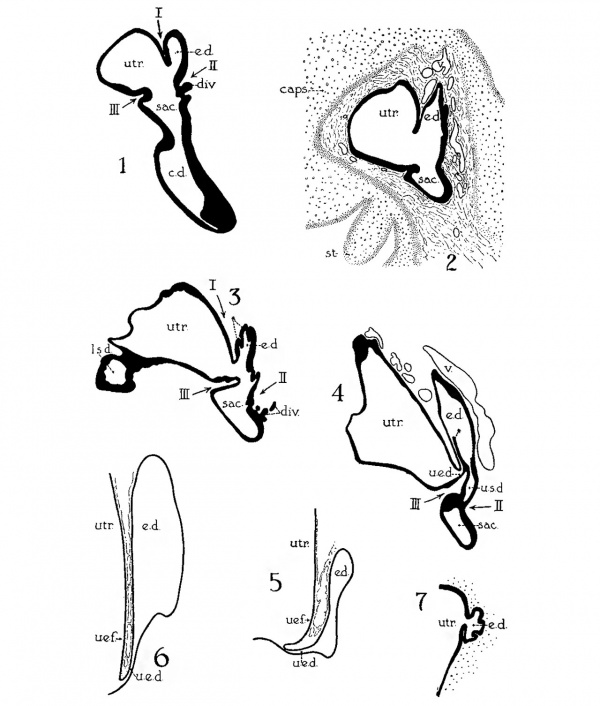
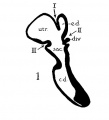
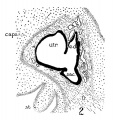
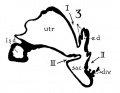
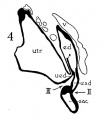
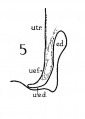
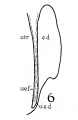
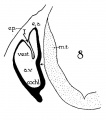
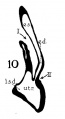
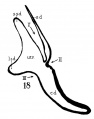
In a recent publication (Anson, ’34) it was pointed out that the otocyst becomes an independent Vesicle, freed from the outer ectoderm, in the embryo of 4 mm. (CR length), although the disrupted stalk of original connection remains as apposed protuberances of otocyst and epidermis, even in the 6.7—mm. embryo (op. cit., fig. 14). In the latter embryo, and representing a further step in a process apparent at 6.3 mm., the Vesicle definitely possesses the dorsomedial projection which is termed the endolymphatic appendage. This appendage is delimited from the vestibular part of the vesicle by a downward directed crease or ledge, the development of which is the first phase of a three-part process which converts the simple otic Vesicle into one consisting of three communicating chambers, namely, endolymphatic duct, utriele, and saccule. The vertical or dorsal crease, which is the primary one in order of appearance, is very evident in the 228mm. human embryo (fig. 1, at arrow I) while the medial one (at arrow II) is not as pronounced; the lateral ledge—which like the preceding is horizontal in plane—is also distinct (arrow III). These folds in the vesicles of 22- and 28—mm. sheep embryos, were recognized by Boettcher (1869 a, figs. 11 and 12, Taf. II), but their further development was not followed. It is to the encroachment of the last-formed, or lateral, ledge that the separation of the saccule and utricle is largely owing, according to the opinions of some investigators; thus, Streeter (’06—’07, p. 165), in describing the subdivision of the primitive vesicle (in embryos between 18 and 20 mm.) describes the ingrowth of a horizontal partition which “ultimately reaches back to the entrance of the endolymphatic duct,” and “divides the orifice of that structure, thus affording it separate openings into the utricle and saccule” (see also Keibel, ’12, p. 267). To the lateral ledge Chatellier (’26) also drew attention, and named it the utriculo—saccular partition (‘cloison inter-utriculo—sacculaire’). We would now point out that initially the two horizontal ledges (lateral and medial) are directed into the vesicle toward each other, and that they are subsequently altered in position so that the medial shelf, even in the 22.8-mm. embryo, is carried caudalward (fig. 1, arrow II) in relation to the lateral one (arrow III)-a change more pronounced in the 40—mm. embryo (figs. 3 and 4, arrows II and III) ; in this migration small saccular diverticula are likewise involved (figs. 1 and 3). The dorsal shelf comes directly downward, at first toward the medialfold (fig. 1, arrows I and II, respectively) ; subsequently (29 mm., fig. 2) it turns somewhat lateralward and overrides the shelf from the lateral wall (figs. 2 and 3, arrows I and III, respectively). Thus, the widely communicating utricle, saccule, and endolymphatic duct of the 22.8—mm. embryo (fig. 1) are brought, at the 40—mm. stage (fig. 4) into the definitive and permanent relationship obtaining in the adult ear—one in which the utricle does not communicate directly with the saccule, but with the latter through two intermediaries, the divergent utriculo-endolymphatic (or utricular) and the utriculo—saccular (or saccular) ducts; both of which appear as limbs of the endolymphatic duct and with it assume the form conventionally described as Y-shaped. The medial ledge or shelf (fig. 4, arrow II), now carried ventralward, marks the line of separation between the ultriculo—saccular duct and the saccule itself. The dorsal fold remains a prominent morphological feature, and in older series of the ear, when sectioned in favorable plane, appears to project into the utricle as an elongate shank (fig. 5, infant; fig. 6, child). To this fold, in the ear of a 183—mm. human fetus, Bast (’28) directed attention, treating it as a particular structure, and regarding it as discoverable only in fortunate series;[1] its position, he believed, indicated that the flap might, in narrowing or closing the orifice of the ultriculo-endolymphatic duct, control sudden changes of pressure within the endolymphatic system. Its form and its constancy of occurrence in man were established by the investigations of Wilson and Anson (’29, two publications; twelve cases), and in mammals generally by those of Hoffman and Bast (’30; eleven species) and of Roberts (’32; one species, sixty series).
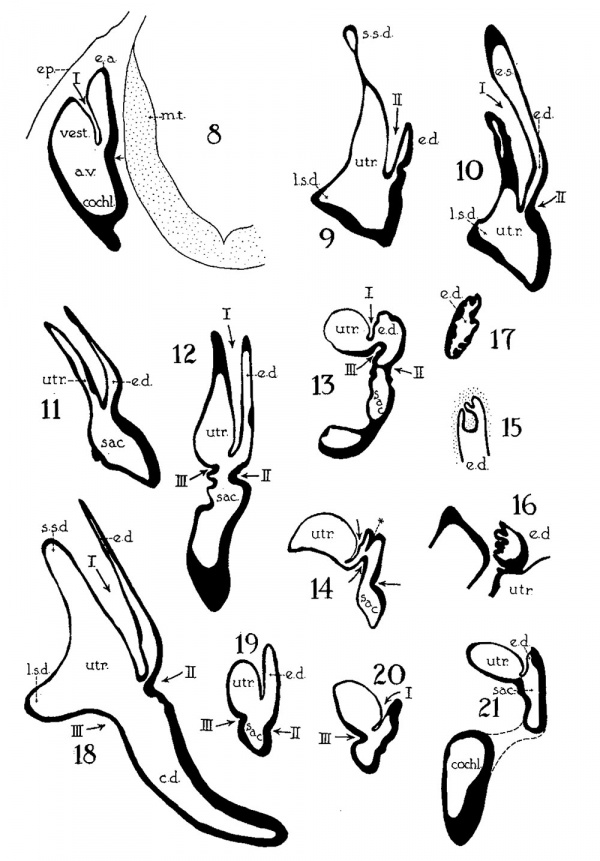
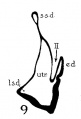
Figs. 1 to 7 Tracings of endolymphatic duct. and adjacent area of membranous labyrinth from human series. figures 1 to 4 and 7, X 37; 5 and 6, X 25. 1, 22.8 mm.; 2, 29 mm.; 34, 40 mm.; 5, infant, 4 months; 6, child, 3 years; 7, 36 mm., a.v., auditory vesicle; caps, cartilaginous otic capsule; c.d., cochlear duct; cocl., cochlear part of vesicle; div., saccular diverticula; e.ap., endolymphatic appendage; e.d., endolynlphatic duct; e.s., endolymphatie sac; ep., epidermis; l.s.<l., lateral semicircular duct; m.t., medullary tube; sae., saccule; s.s.d., superior semicircular duct; st., stapes; utr., utricle; vest, vestibular part of vesicle; u.e.<l., utriculo-endolymphatic duct; u.e.f., utriculur fold (‘valve); v., blood vessels. * Rugae, in tangential section. Numbered arrows designate folds discussed in text.
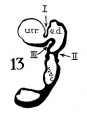
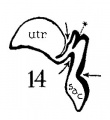
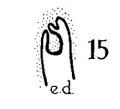
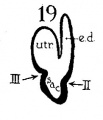
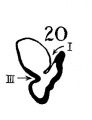
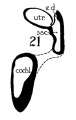
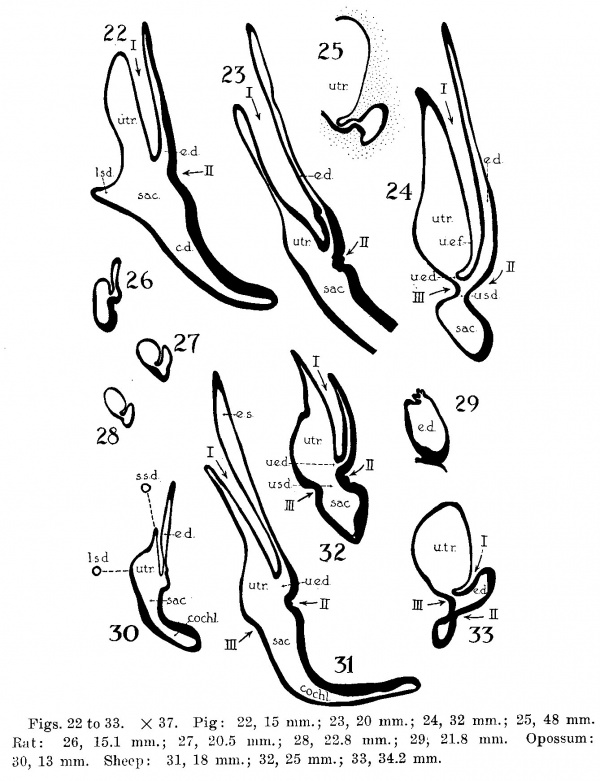
The succession of changes in the development of the membranous labyrinth in other mammals is very similar to that observed in man. In the 7—mm. embryo of the cat the dorsal fold is well developed (fig. 8, arrow I), already marking off the endolymphatic appendage, while the medial ledge is just apparent; the former ledge is pronounced in embryos of 10.6 mm., 14 mm., and 15 mm. (figs. 9 to 11),[2] the medial one much less so. The lateral ledge, evident at 24.1 mm. (fig. 12, arrow III), is approaching the medial fold (arrow II); in extending over its dorsal aspect, and remaining Ventral to the utricular fold (arrow I), it is so situated as to subdivide the opening of the endolymphatic duct into the two portions, the upper one communicating with the utricle, the lower one with the saccnle—a process accomplished in the 32.6-mm. and 39-mm. stages (figs. 13 and 14). The medial ledge finally attains a distinctly ventral position in relation to the lateral one (compare figs. 12 and 14). The three folds are present in the 12.5-mm. dog‘ embryo (fig. 18), a stage developmentally similar to the 14—mm. cat embryo (fig. 10), as they are likewise in the 21-mm. embryo of the rabbit (fig. 19); in two older rabbit embryos, of 25 mm. and 29 mm. (figs. 20 and 21) the manner in which the lateral and dorsal folds approach each other is again illustrated. In pig embryos from 15 to 48 mm. in lengtli the same evolution occurs; at 15 mm. (fig. 22) the dorsal and the medial folds mark off the utriculo-endolym— phatic duct; the second fold at 20 mm. (fig. 23) is deeper, and the proximal extremity of the endolymphatic duet correspondingly more ineurved; it is even more curved in the 32 mm. embryo (fig. 24), while toward it, and somewhat dorsal to it, the lateral fold is pressing toward the orifice of the endolymphatic duct, there to pass beneath, and, in the 48—mm. embryo (fig. 25), to underlie, the medial fold, and thus to convert an originally wide, single, communication between utricle and saccule (fig. 22) into two slender ducts, by which, through the intermediation of a third (the endolymphatic duet) the two main chambers remain in communication. The initial step in the same process is seen in rat embryos between 15.1 and 22.8 mm. (figs. 26, 27, and 28), while the whole succession may be found in sheep embryos of 18, 25, and 34.2 mm. (figs. 31, 32, and 33), to the first of which the 13-mm. opossum (fig. 30) corresponds closely).[3]
Figs. 8 to 21. X 37. Cat: 8, 7 mm.; 9, 10.6 mm.; 10, 14 mm.; 11, 15 mm.; 12, 24.1 mm.; 13, 32.6 mm.; 14-15, 39 mm.; 16, 31 mm. Guinea-pig: 17, 18.5 mm. Dog: 18, 12.5 mm. Rabbit: 19, 21 mm.; 20, 25 mm.; 21, 29 mm.
- Fig 8-21 Animal Embryos - Endolymphatic Duct
Figs. 22 to 33. X 37. Pig: 22, 15 mm.; 23, 20 mm.; 24, 32 mm.; 25, 48 mm. Rut: 26, 15.1 mm.; 27, 20.5 mm.; 28, 22.8 mm.; 29, 21.8 mm. Opossum: 30, 13 mm. Sheep: 31, 18 mm.; 32, 25 mm.; 33, 34.2 mm
The endolymphatic duct does not remain a smooth-walled tube as it is found in the 22.8-mm. human embryo (fig. 1); in the slightly older stage, 29 mm., the lining of the wa1.1 of the tube in its proximal portion is rendered irregular by the development of low rugae (fig. 2). In the embryo of 40 mm. (figs. 3 and 4, at asterisks) the rugosities are taller, and are likewise prominent in a slightly earlier stage, the 36-mm. embryo (fig. 7; folds here and in figs. 2, 3, and 4 cut transversely to their long axes[4]); such folds were observed in sixteen additional series of human embryos, in the 39—mm. and the 31—mm. embryos of the cat (figs. 14 and 16, respectively), but not in the other mammals studied.[5]
The wall of the distal portion of the endolymphatic appendage, or, more specifically, of the saccus, is likewise rugose in some of the embyological series studied; in the cat embryo of 39 mm. (fig. 15) the dorsal wall, or roof, of the endolym— phatic sac is rather deeply infolded, as is the corresponding area in the 18.5-mm. embryo of the guinea-pig (fig. 17), and of the 21.8—mm. rat embryo (fig. 29) ;[6] rugae are not yet present in any of the human series examined.[7]
The rugae—proximal and distal—are, in primordial form, the abundant ridges found in the ears of adult mammals. Guild ( ’27 a) described and figured them as seen in sections of the ductus and saccus of the adult guinea—pig where they possess a regionally specialized epithelium and a subjacent vascular stroma; to the Vascularized processes, upon abundant experimental evidence, Guild (’27 b) ascribed the function of resorption of the endolymph. They were, as seen in man, described by Sterzi (’10), and much earlier, with admirable figures, by Boettcher (1869 a, fig. 22, Taf. IV, cat; 1869 b, Taf. viiic, adult cat and human newborn). That the projections in man are actually elongate plaits and not simply villuslike hummocks (as they appear in sections) has been shown recently by means of wax reconstructions (Anson and Wilson, ’30).[8]
Footnotes
- ↑ The structure was figured earlier, but Without being named, by Boettcher (1869 a, fig. 12, Taf. 2), Wittmaaek (’24, Abb. 3), Portmanu (’24, fig. 33), Chatellier (’26, fig. 4), Kolmer (’27, Abb. 50), fischel (’29, Abb. 446), and others.
- ↑ The plane of section is most favorable in the series represented in figure 10; the differing plane in the several frontal series accounts for the apparent discrepancy in size between labyrinths, that in the 32.6~mm. embryo (fig. 13) appearing smaller than that in the 14-mm. (fig. 10).
- ↑ In addition to the series from which sections are shown in the figures (figs. 1 to 6, 8 to 14, 18 to 28, 30 to 33) the valve may be seen out at advantageous plane in the following: man, 32 mm.; cat, 12 mm.; dog, 17 mm.; rabbit, 33.8 mm., rat, 24.8 mm., pig, 10 mm., sheep, 13 mm. and 12 mm. (ser. 648, 404, 2053, 239, 1797, 401, 1345, 1342, respectively).
- ↑ The plaits or rugae, as models prepared from several of the embryological series prove, pass in longitudinal direction within the duct.
- ↑ In addition to the series from which sections are shown in figures 2, 3, 4, 7, 14, and 16, the following series display the longitudinal folds in the proximal portion of the endolymphatic duct: man, 45 mm., 44.3 mm., 42 mm., 37 mm., 31 mm., 30 mm., 29 mm., 27 mm., 25 mm., an(l 24 mm. (series 2128, 1611, 841, 820, 2043, 913, 914, 2248, 2042, 24, respectively). They are less well marked in the following earlier stages: 23 mm. (two series), 22.8 mm. (two series), 22 mm. and 19.3 mm. (ser. 2045, 2046, 737, 871, 851, 1597, respectively); they are wanting in the embryos of 19 mm., 17.5 mm., 16 mm., 15 mm., 14.5 mm., 14.1 mm., 12 mm. (ser. 819, 2155, 2095, 2051, 1003, 2156, 816, respectively) and in other still younger stages.
- ↑ In addition to the series from which sections are shown in the figures (cat, fig. 15; guinea-pig, fig. 17; rat, fig. 29) the following series of the cat display the plicate invaginations in the distal portion of the endolymphatic duct: 39 mm., 32.6 mm., 31 mm. (two series), 24 mm., 23.1 mm. and 19 mm. (series 368, 505, 527, 500, 467, 466, 1985, respectively). Such invaginations are wanting in the following series of the cat: 24.1 mm., 17 mm., 15.6 mm., 15 mm. (two series) (scr. 468, 492, 1983, 436, 438, respectively), and in additional younger stages. They are present in the following series of the rabbit: 33.8 mm., 29 mm., 25 mm., 22 mm., 21 mm. (series 239, 172, 169, 237, 738, respectively); they are inconsiderable in the l2.5—mm. embryo (series 160) and wanting in the 10—mm. (series 155). They occur in the 30—mm. guinea—pig (series 1779), in the rats of 24.4 mm. (series 1708) and 22.8 mm. (series 1941), but are not yet present in the 20.5-mm. embryo (series 1806). Slight distal irregularities are seen in pig embryos of 48 mm., 36 mm., 32 mm., and 20 mm. (series 2041, 2087, 74, 542, respectively). There are none in the 25.2-mm. calf (series 1688); there are slight ones in 48.4—mm. sheep (series 1696), but none in the sheep of 34.2 mm. (series 1692), or in earlier stages. They are present in the 26—mm. and 23.5—mm. opossum em» bryos (series 2077 and 2096, respectively).
- ↑ The remarkable similarity in labyrinthine morphology in mammalian embryos militates, we believe, against Doctor Streeter’s criticism (’27) of the notion of ground-plans in animal structure; resort to the use of schemata has not been necessary in illustrating the developmental stages of the auditory vesicle in mammals, since our actual tracings of sections possess the simplicity of diagrams, and display negligible departures from a form which could readily be selected as ‘archetypal.’ The same opinion was expressed by the present author (Anson, ’27) at the Nashville meeting of the American Association of Anatomists, for which Doctor Streeter’s paper constituted the presidential address; the recurrence of epidermal thickenings about the oral orifice, and of ‘lips,’ among the classes of vertebrates was regarded as an evidence of homology within the limits of Danforth’s definition of that term (Anson, ’29).
- ↑ From unpublished data, by Anson and Wilson, on the 2-year-old child; by Nesselrod and Anson 0n the 3-year-old and the adult.
Literature Cited
Anson B. J. 1927 The comparative anatomy of the lips and labial villi. (Abstract.) Nashville meeting, Am. Assoc. Anat., Anat. Rec., vol. 35, pp. 2-3.
1929 The comparative anatomy of the lips and labial villi of vertebrates. J. Morph. and Physiol., vol. 47, pp. 335-413.
Anson BJ. and Black WT. The early relation of the auditory vesicle to the ectoderm in human embryos. (1934) Anat. Rec. 58: 127-137.
ANSON, B. J ., AND J . G. WILSON 1929 The utricular fold in the adult human ear. Anat. Rec., vol. 43, pp. 251-255.
- 1930 The endolymphatic duct system in the 2-year-old child. (Abstract.) Charlottsville meeting, Am. Assoc. Anat.; Anat. Rec., vol. 45, p. 205.
BAST, T. H. 1928 The utriculo-lymphatic valve. Anat. Rec., vol. 40, pp. 61-64.
BOETTCHER, A. 1869 a Ueber Entwickelung und Bau des Gehiirlabyrinths nach Untersuchuugen an Saugethieren. Verh. d. Kais. Leop.-Carol. d. Akad. d. Naturforscher, Bd. 35, S. 1-203.
- 1869 b Ueber den Aquaeductus vestibuli bei Katzen und Menschen. Arch. f. Anat. u. Physiol., Jahrg. 1869, S. 372-380.
CHATELLIER, H. P. 1926 Evolution embryologique de l’appareil endolymphatique et du cloisonnement utriculo-sacculaire chez l’homme. Arch. d’anatomie, d’histo1ogie, et d’embryologie, T. 5, pp. 49-83.
FISCHEL, A. 1929 Lehrbuch der Entwicklung des Menschen. Wien.
GUILD, S. R. 1927 a Observations upon the structure and normal conutents of the ductus and saccus endolymphaticus in the guinea-pig (Cavia cobaya). Am. J. Anat., vol. 39, pp. 1-56.
- 1927b The circulation of the endolymph. Am. J . Anat., vol. 39, pp. 57-81.
HOFFMAN, E. E, AND T. H. BAST 1930 A comparative study of the ‘utriculo endolymphatic valve’ in some of the common mammals. Anat. Rec., vol. 46, pp. 333-347.
Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
KOLMER, W. 1927 Gehiirorgan, in Handbuch d. mikr. Anat. d. Menschen, Bd. 3, T. 1, S. 250-456. Berlin.
PORTMANN, G. 1924 Oreilles, in Travaux scientifique du Dr. Georges Portmann, pp. 16-163. Bordeaux.
ROBERTS, J. T. 1932 On the utriculo-endolymphatic valve in the albino rat. Anat. Rec., vol. 53, pp. 255-264.
STERZI, G. 1910 Il sacco endolinfatieo. Ricerche anatomiche ed embriologiche. Gegenbauer’s morph. Jahrb., Bd. 39, S. 446-496.
STREETER, G. L. 1906-1907 On the development of the membranous labyrinth and the acoustic and facial nerves in the human embryo. Am. J. Anat., Vol. 6, pp. 139-165. 1927 Archetypes and symbolism. Science, N.S., vol. 65, pp. 405-412.
WILSON, J. G., AND B. J. ANsoN 1929 The ‘utriculo-endolymphatic valve’ (Bast) in a 2-year-old child. Anat. Rec., vol. 43, pp. 145-153.
WITTMAACH, K. 1924 Entgegnung zu vorstehenden Bemerkungen Alexanders iiber meine Bespreehung der makroskopischen Anatomie ‘ler netviisen Anteile des Gehiirorgans im Handbuch der Neurologie. Zeitschrift fiir Hals-, N asen-, und Ohrenheilkunde, Bd. 9, S. 80-83.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 28) Embryology Paper - The early development of the membranous labyrinth in mammalian embryos. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_early_development_of_the_membranous_labyrinth_in_mammalian_embryos
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G