Paper - The Terminal Part of the Wolffian Duct
| Embryology - 27 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Frazer JE. The terminal part of the wolffian duct. (1935) J Anat., 69(4): 455–468. PMID 17104551
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Terminal Part of the Wolffian Duct
BY J. Ernest Frazer, London (1935)
It is a matter of common knowledge that the caudal end of the Wolffian duct is concerned in some way with the formation of the bladder, and that in the course of the changes taking place the ureter comes to end separately in that viscus, while the opening of the duct finally is into the prostatic urethra. Nevertheless, although these facts are recognised, there does not seem to be any settled or accepted view concerning the way in which the changes actually take place: in a very general way it is said that the Wolffian duct is “ taken up ” into the bladder, or is “expanded” as far as its ureteric opening, while the apparent change of site of its own opening is usually left without even an attempt at a definite explanation. In this paper I propose to give the result of an enquiry into these matters. A few years ago I indicated the way in which the actual final opening is produced, and the present is a more complete account of observations then shortly recorded, with addition of the earlier changes.
There are actually two parts or stages in the process, that in which the duct disappears as far as the ureteric implantation, and that which brings about the change in level of its aperture. The two stages, although making a continuous whole in their progress in time, are sufficiently distinguished — by their objectives on the one hand, and by the regions of the duct involved on the other hand - to justify the separation in description. The second stage follows immediately on the first. The changes which take place in the Wolflian ducts progress quickly, and individual variations, although quite noticeable at times, are simply the results of temporary differences in the speed at which the changes are effected.
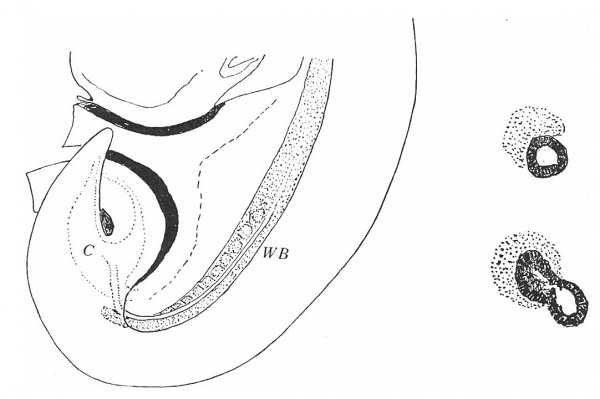
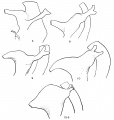
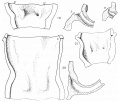
The Wolffian duct, just before its ureteric bud begins its growth, is shown in fig. 1, a semi-schematic drawing from an embryo of 4.9 mm. The duct is seen running down the lateral aspect of the Wolffian body (W B), but, as it nears the caudal part of this ridge, it crosses it obliquely, over its ventral aspect, and then loops forward to reach the cloaca. In doing this the duct lies in close contact with the mesoderm of the ridge, on its ventral side, as is shown in the upper of the two smaller figures. This is from the 4.9 mm. specimen, in which there is no indication of the ureteric bud, but in the lower figure—from a 5 mm specimen—the beginning bud is shown: it arises in actual contact with its metanephric cap, from this part of the duct, springing from its dorsal and somewhat medial aspect.
Evidently, then, it is that part of the duct extending between this ureteric point and the cloaca which disappears to allow the ureter to open into the bladder, while the portion of duct immediately cranial to the point is that which is concerned in making the final opening.
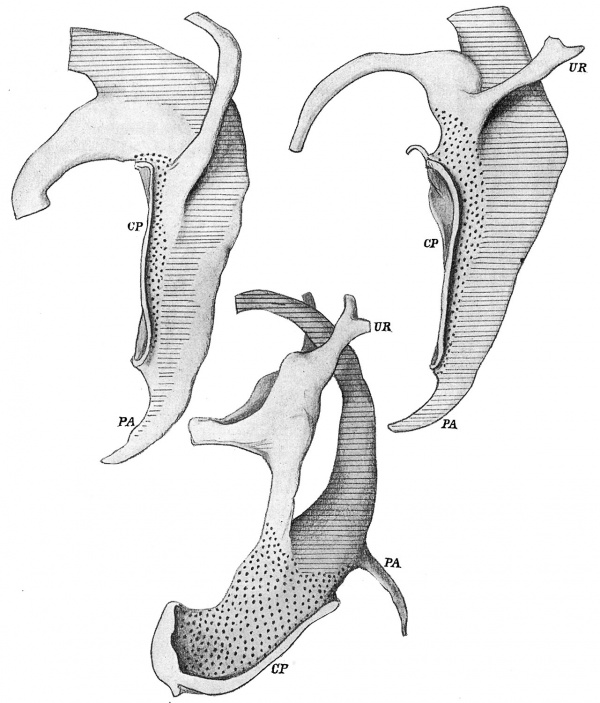
The conditions present in the embryo of 4.9 mm. are shown in fig. 2, from a model of the cloaca and ducts. The length of the duct which will disappear in the first stage of the change is very evident, but there are no such definite indications of the extent of the duct, proximal to the ureter, which will be taken up in completing the second stage.
The Subcloacal Mesoderms
In fig. 2 the various stages figured are represented with indications of mesodermal beds in relation with their walls. Since these mesodermal layers fix the lower part of the cloaca and bear settled relations to the Wolffian duct, and are to be considered in construing the observations made on its changes, they will be dealt with shortly here: it will be seen also that they provide the basis and regional extensions for the growth and changes in the lower parts of the cloaca.
Fig. 1. Wolffian body (WB) and duct projected on to outline of embryo of 4.9 mm. Showing the duct crossing obliquely ventral to the Wolffian body. C, cloaca. The smaller figures are from sections across the duct where it lies ventral to WB, from embryos of 4.9 and 5 mm., as in text.
For present purposes the various mesoderms concerned can be illustrated sufficiently by the drawings in fig. 2. The first of these is from an embryo of less than 5 mm. length. In this the cloacal plate, CP, extends just on to the base of the body stalk, and there is thus no area of the body wall at all which could be called infra-umbilical. A very active mitosis is going on here among the cells immediately adjacent to this anterior end of the membrane, within the base of the body stalk, and this mitotic activity extends caudally from here on both sides of the plate, under the ectoderm. Its position is marked in the drawings by the dotted areas.
Fig. 2. Form of lining walls of the cavities from specimens of 4.9, 5, and 8 mm. length. x 100. Marked to show mesodermal relations, as described in text. CP, cloacal plate; PA, post-anal gut; UR, ureter. From models.
It is to be understood that the models represent only the lining cellular walls of the cavities, so that the mesodermal areas marked on them only show the positions of the layers where they are in contact with these walls. They do not show the whole extent of the layers.
This mesodermal bed, in this young specimen, cannot be followed definitely to the level of the caudal end of the membrane, but in the next drawing, from a specimen slightly older, 5 mm., it now surrounds this structure completely. In this paper the mesodermal layer can be referred to as “central”.
The region marked by horizontal lines is covered by a mesodermal condensation continuous with that surrounding the gut tube: it may conveniently be referred to as “splanchnic”. In the first embryo the layer is not definitely recognisable throughout, and only seems to extend in a broken way on to the post-anal gut, but in the 5 mm. specimen it is complete throughout. This splanchnie mesoderm seems to be the active agent in the formation of the cloacal septum, and builds in its growth a very thick condensation between its enclosed tube and that of the ventral part of the cloaca. The Wolffian duct reaches the cloaca in the angle between these two mesodermal beds.
The duct and the cranial part of the cloaca are left unmarked in these drawings, indicating that they are not covered or enclosed by either of the two mesoderms already mentioned. The mesoderm covering the cloacal portion, continuous with that surrounding the allantoic prolongation within the cord, is a loose non-condensed layer. Although easily distinguished in these earlier stages from the central condensation, it is of course continuous with it in position, and the central bed can be looked on from this point of view as a condensation particularly affecting the ventral portion of the general mesodermal surroundings of this part of the cloaca. Later, the distinction between the two subdivisions of this mesodermal bed becomes less marked, and there is reason to suppose that the contiguous part of the central thickening is “paid into” the other as the contained cavity elongates, thus affording a loose covering for it and permitting its growth.
The Wolffian duct is surrounded by a mesoderm which becomes continuous with the “cloacal” layer just mentioned, but it is not clearly assignable, at first at any rate, to a particular stratum. It must be remembered in this connection that the Wolffian duct is only a single cellular layer enclosing a lumen, not carrying round it any mesodermal sheath properly and peculiarly belonging to it: if it becomes functional it appears to get its mesodermal coat secondarily and locally. Towards the latter part of the changes we are considering, a definite thickening derived from that of the Wolffian body can be found especially along the concave surface of the duct, but this does not necessarily throw light on the earlier mesodermal value. Fortunately it is not a matter of importance from our present standpoint: it is enough for our purpose to recognise the presence of a more or less loose covering for all this region, including the part passing into the angle between the central and splanchnie strata.
The lower drawing in fig. 2 is from a specimen of 8 mm. It shows how the urogenital sinus is fixed in solid mesodermal condensations at its lower end, leaving the upper part free to expand, thus giving us a fixed region from which we can consider the effect of such expansion and growth, as will be seen later. It must be understood that the interval between the sinus and the rectum is filled, as far up as the peritoneal reflection, by thick splanchnic mesoderm, against which the sinus lies, although these things of course are not seen on the model.
It may be noticed here, incidentally, that the central mesoderm is concerned only, at first, with the making of anterior and lateral walls to the region of the cloacal membrane, and with the swinging round of this region, in -a sagittal plane, so that it comes to face in an opposite direction. Growing at the base of the body stalk, and pushing back from this, it forms a genital tubercle at the front end of the membrane which not only separates this from the stalk, but, as it grows, carries this end of the membrane in a ventral direction. If we realise that, in the younger members of the embryos shown in fig. 2, the cloacal membrane faces actually cranio-dorsally, whereas in the 8 mm. specimen it is already looking ventro-caudally, we can appreciate the rapid change which has been brought about by the downward and caudal swing produced by the anterior growth, aided by the movement of the caudal end in the other direction in association with the atrophy and relative want of growth in this part. Up to this time the central growth adds practically nothing to infra-umbilical depth of belly wall, but is only concerned in reversing the “perineal plane” of the membrane and in building up the external genitals. This is of course combined with great increase in depth of the mesodermal bed surrounding the lower end of the urogenital sinus, with the addition of a new part to its channel—in fact, the lower half or so of the sinus is a new formation not derived from the original cloaca, but built up as an addition enclosed by the great mesodermal formations projecting beyond the original plane of the membrane. As soon as the “ perineal plane ” has been practically attained, we find the infra-umbilical central line slowly coming into being, continuous below with the genitalia, while the ventro-lateral formations of the body wall are beginning to find their way into contact with it.
The upper part of the cloaca, then, can be considered from an early stage as fixed below, itself covered only by a light mesodermal layer, which is no hindrance to its growth and expansion. This increase in its size is already apparent, dorso-laterally, in the 5 mm. specimen (fig. 2), and shortly after this its freedom is rendered greater by the extension of the coelomic angle between it and the rectum.
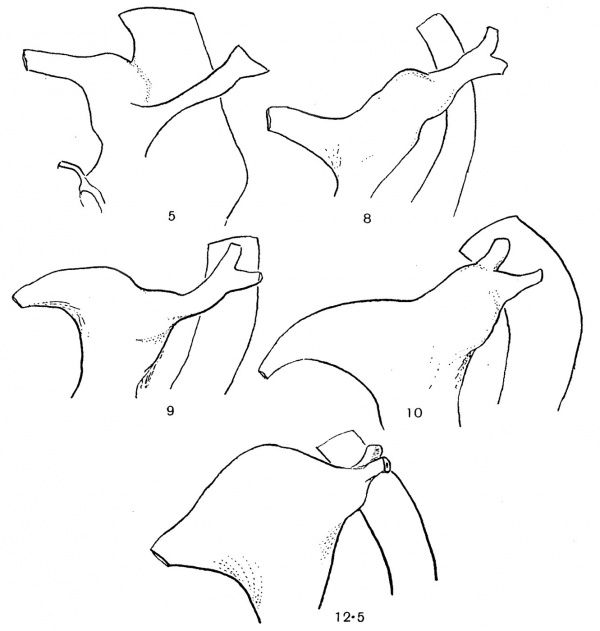
Some stages of this expansion, destined to form the bladder, are given in fig. 3. It can be seen in these that the widening proceeds outwards from the bladder towards the ureter, and is apparently purely a cloacal growth in the course of which the neighbouring portion of the Wolffian duct is lost. The expansion reaches the ureteric point at, or just before, the 12-12-5 mm. stage: the 12-5 mm. stage figured appears to indicate very well the terminal phenomenon of this first stage of the change.
It is necessary now to enquire into the disappearance of the Wolffian duct, with a view to seeing if possible how it is effected. Fortunately the 12-5 mm. specimen affords what seems to be a very satisfactory explanation of the process.
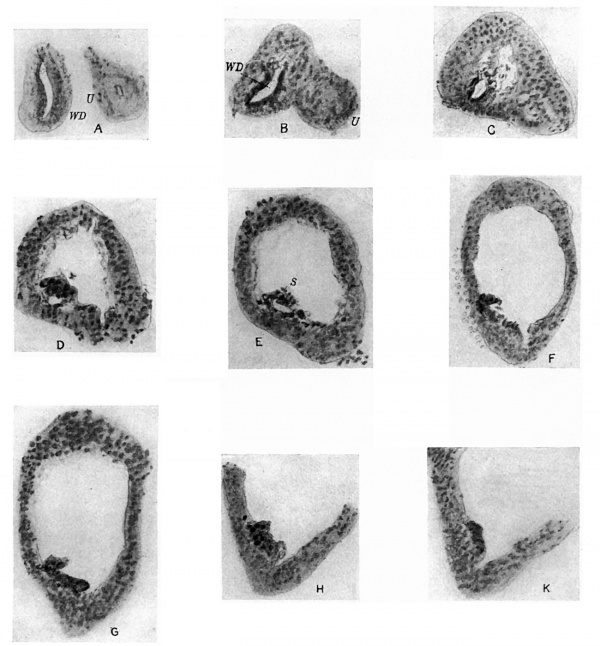
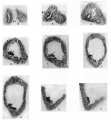
A series of sections of this embryo, passing through the parts concerned, are given in fig. 4: they form practically a continuous series, beginning at the ureteric point and working towards the main cavity.
These sections may almost be said to speak for themselves. The cellular duct wall, which has taken a deeper stain than the cellular layer lining the main cavity, is plainly seen to be carried on, into this latter region, half embedded in its lining layer, and showing no indication of any present or past enlargement. Cells of the main lining are to be found here and there on its superficial aspect, sometimes complete here but more usually fragmented, and in keeping with this we find the tendency for the superficial wall of the embedded duct to break up also, leaving the deep wall in position among the main cells.
Fig. 3. To show the extension of cloacal expansion outwards along the Wolflian duct. Seen from left side: length in mm recorded on each specimen.
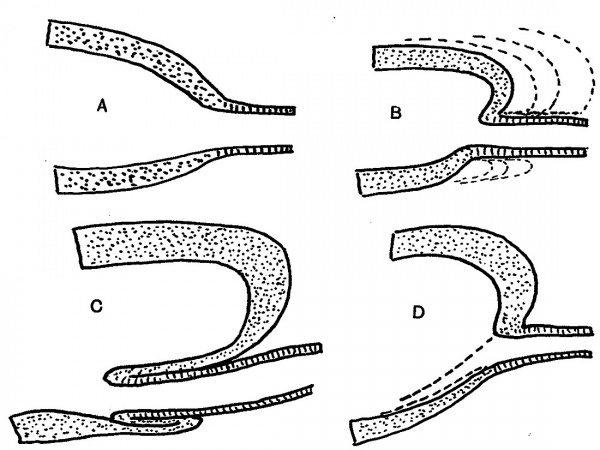
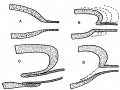
There are certain points in these sections which will be mentioned later, but it can be said at once that study of their appearances, and of other examples, led to formulation of an explanation of the constructions seen, which in its turn was easily put into a diagram. This is shown in fig. 5. The first drawing, A, shows the main lining layer, dotted, meeting that of the duct, lined. In B the main wall is folding itself over the duct, the dotted lines indicating the method of enlargement of the cavity. This leads to the condition in C, while D shows how the disappearance of this intussuscepted part leaves the duct opening in its new position. C really embodies the principle of the process, which is a simple one, to be conceived as easily taking place if we remember that it concerns lining cells only, and not the surrounding mesodermal covering.
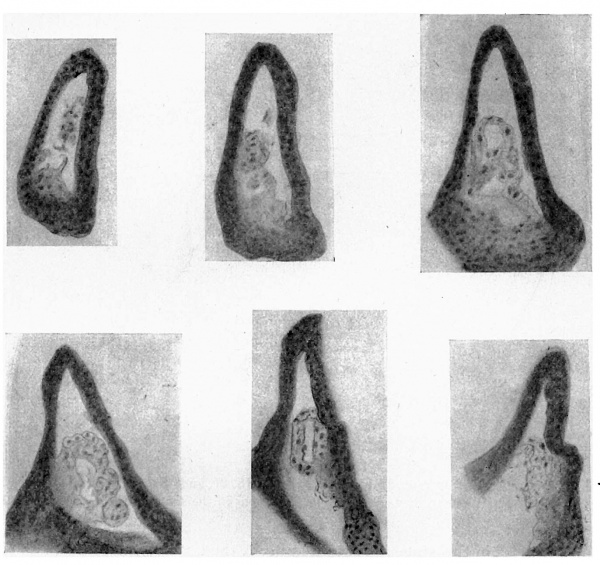
Fig. 4. A series of sections along the lateral projection of the sinus, beginning at the Wolffian duct (WD) and ureter (U), from an embryo of 12-5 mm. The series is complete except for two omitted just before the last. After K no sign of the duct remnant could be found. The lining cells of the sinus envelop the duct and ureter closely in A and B, and irregular degenerating remnants can be found along the duct, as in E at S.
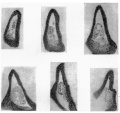
The lining cells folded in in this way do not appear to last very long, and I am inclined to think that the 12-5 mm. conditions are examples of a fortunate state in which their vitality was a little greater than usual. This view depends upon the seemingly rapid disappearance of the included cells in earlier stages, the shortness and broken condition of the remnants in another embryo of the same age, and the fact that in three embryos of 13 and 13-5 mm. there are no definite signs of the remnants to be found at all. All the stages before 12 mm show cellular remnants in this part, but in most cases these are short and near the advancing edge of the dilatation, suggesting that the parts found earlier have broken up. The 8 mm. specimen, however, has a practically complete inclusion which seems to represent—from its extent—the whole invagination so far as it has gone. It is given in the sections in fig. 6. The included cells show more of a delicate tracery than anything else, being presumably on the point of disappearing, but they have been beautifully fixed and preserved, and seem worthy of reproduction. It may be mentioned that the 10 mm. specimen was cut so obliquely that its cellular remnants were not suitable for reproduction, but this very obliquity gave one section which showed well the invaginated continuity between the duct wall and that of the growing bladder.
Fig. 5. Diagrams to show how the end of the Wolffian duct is “taken up” into the advancing cavity of the bladder.
We may sum up the implications of the foregoing statements by saying that the first stage of disappearance of the duct is accomplished as a result of its forming an intussusceptum within the growing bladder. The bladder cavity extends in this region clearly at the expense of the duct, not by opening it up but by destroying it, and the process of destruction involves not only the wall of the duct but also an inturned portion of the lining wall of the bladder. The bladder wall is the active agent, the duct being apparently passive.
The expansion of the bladder seemingly includes the terminal bit of the ureter in its spread, as may be seen in the sections in fig. 4. Thus the ureter gains a separate opening into the bladder as a result of the same growth which has destroyed the duct, and there is no question of any duct_value to be given to the intramural part of the ureter. The end of the bladder expansion thus leaves the ureteric and duct openings separated by a definite interval, the last mentioned being on the medial side: we need not consider here the slight change, probably purely mechanical, from the original position of the ureteric bud. The implanted ends of the two structures are surrounded by a thick mass of cells of the lining layer, which may perhaps contain remnants of the included walls (but this is very uncertain), and it is from this arrangement of the structures that the second stage starts.
Fig. 6. A series of sections from the lateral dilatation of the sinus in an embryo of 8 mm. Sections pass towards the duct.
The Second Stage
The second stage of the change begins slowly and gathers speed as it progresses. There is little difference in level of the end of the duct between 13 and 15 mm., but 16, 17, and 18 mm. show increasing amounts of difference.
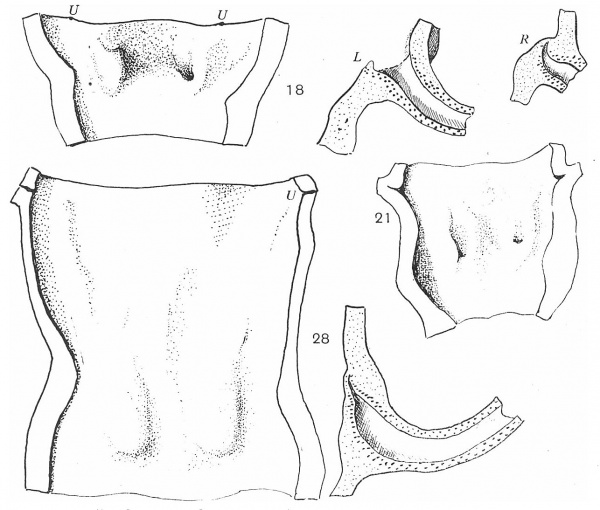
Fig. 7. Dorsal walls of urogenital sinuses, embryos of 18, 21, and 28 mm length. Also sections along lines of Wolffian ducts showing their positions in the sinus walls. L = left; R = right; U = ureter.
Three degrees in this stage are shown in fig. 7, the last of which is from an embryo in which the Mullerian ducts are on the point of joining the urogenital sinus. The figures show the dorsal walls of the sinuses, with the impressions produced on them by the ducts, but linear reconstructions of sections along the lines of the ducts are also given with the corresponding surface views in two of the specimens: in the middle one the section was very much as in the first.
In the 18 mm. example the left duct opens on the surface, but the right one is blocked by a mass of cells, continuous above with rough epithelial debris which can be followed up to the remnant of the epithelial mass already mentioned as occupying the ureteral angle of the bladder. The sections show that the ends of the ducts are turned up and embedded in the lining cells of the cavity, which have not given way to permit an opening on the right side. In the 21 mm. specimen the conditions are somewhat similar, except that both ducts are open here and the track of the right one is seen as a shallow groove on the surface: the distance from the ureter is slightly greater, and epithelial debris is still to be found. In the 28 mm. embryo, however, the distance from the ureters has increased to a very great extent, while the upturned ends of the ducts among the lining cells of the sinus are not open, but are covered by cells belonging partly to themselves and partly to the sinus: there is no epithelial track connecting them with the ureteric region.
The meaning of these observations seems reasonably clear up to a point. Mention was made, when dealing with the mesodermal strata, of a condensation continuous with the Wolffian mesoderm which appeared in association with the duct: this layer has formed before the end of the first stage, and is placed on the upper surface of the duct. Whether due to this or not is hard to say, but the duct appears to be held in position in a mass of mesoderm just behind the sinus, of which mass the condensation just spoken of forms the superficial part. The mesodermal mass has gradually become more pronounced during the progress of the first stage, forming a curved shelf in front of, and ventro-lateral to the peritoneal recess separating it from the rectum. It is continuous centrally with what is left of the older layer in the “angle”, and may be taken to represent it. It forms the beginning and lowest part of the “genital cord” of a later stage.
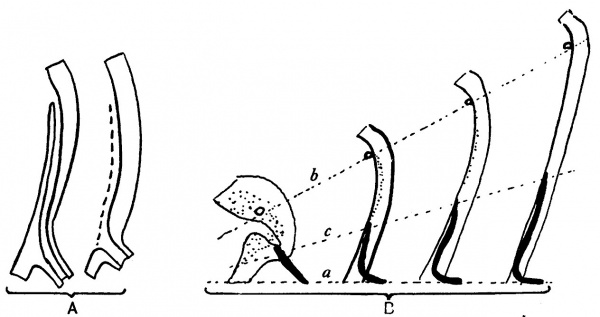
fixed, then, in this mesodermal condensation, the Wolffian duct passes forward to the dorsal wall of the sinus, which it reaches medial to the ureter. Here it is continued into the clump of lining cells, already spoken of as filling this part, and is lost among these. It must be remembered that we are dealing with a tube made by a single layer of cells, not carrying any special wall of mesoderm around them, and that the vesical end of this tube is embedded in the lining cells of the growing bladder: both these lining layers, therefore, are within and are enclosed by the common mesodermal covering of the bladder. Thus, as the vesical structures elongate in a direction away from the fixed lower part of the sinus, the implanted end of the duct is drawn up, making a curve with the fixed part: this upturned end is still enclosed by the common mesodermal coat, and embedded in the thick lining layer of the main cavity. Thus, as the growth goes on, the embedded part is found at relatively lower and lower levels, compared with the ureter, since it is fixed by the fixation of the duct, while the ureteric levels are carried away from it. The superficial wall of the embedded part, breaking down and disappearing in a way reminiscent of the first stage, leaves the final opening at the lowest level. The general idea of the way in which the level of the final opening is attained is embodied in the first scheme in fig. 8.
Although this general idea, diagrammatically shown, is without doubt correct, many details of the process are and must be uncertain. The rapid growth in length of the main sinus, especially in the latter part of the second month, is associated with the rapid growth of the infra-umbilical belly wall, and its fixation below makes the extension effective in an upward direction. A point at issue concerns the Values of the area between the ureters and the openings of the ducts in the prostatic urethra, particularly the lines within that area extending down from the one-time openings of the ducts, medial to, and a little below the ureters, to the prostatic openings. It might be put into the form of a question—Does such a line contain along its length remains of the cellular wall of a duct, or are such remnants to be expected only in its lower part? It may be said at once that careful examination of the wall has failed to reveal anything suggesting cellular remains from the duct, except in the positions indicated in fig. 7, but this negative result does not allow one to assume that such “remains” may not be there, indistinguishable among the cells of the lining layer. Such, in fact, was the way in which I was inclined at first to look at these results, but it was not only an unsatisfactory standpoint in itself, but did not agree with certain minor matters of observation: hence this View of the conditions was given up.
Fig. 8. These diagrams represent only the lining cell layers. A. To show the method by which the actual opening of the duct is gained. The superficial wall of the embedded part breaks down and disappears (interrupted line in second figure), leaving orifice of duct at its low level. B. Diagrams to show the efiect on duct (black) and ureteric level of growth of sinus upwards from the fixed level (a). The ureteric orifice is raised most (b), while the embedded end of the duct is not only turned up but also lengthened (c) to a smaller extent. The epithelial mass (dotted) round openings of ureter and duct in the first figure is hollowed to a small funnel and then drawn out between b and c.
If it is remembered that the level of the duct, behind the sinus, is fixed by surrounding mesoderm, and that in consequence any increase in length of the main cavity, in keeping with infra-umbilical extension, must be an increase only in an upward direction, the nature of the changes becomes clearer. Moreover, it becomes apparent that the ultimate opening is only an expression of the fixed lower level, that it is not in fact an indication of any downward shifting of the orifice of the duct, but of a growth of the ureteric and other levels upwards away from it.
The effect on the end of the duct of the beginning of this upward extension is of interest. It was pointed out above that a thick collection of cells occupies the “angle” of the main cavity into which the duct and ureter are inserted at the end of the first stage: it seems to be associated mostly with the end of the duct. The effect of the upgrowth is at once apparent in an upturning of the vesical end of the duct and, connected with this, a decided appearance of drawing downward and backward of the thick cellular collection— in fact, there is a short funnel-shaped recess of the cavity, lined by thickened cell layers, passing backwards and downwards and receiving the end of the duct. Thus, apparently by some stretching of this cellular mass, a small but distinct difference between the duct level and that of the ureter becomes evident at once.
It is possible that this recess formation is in some way a prolongation or extension of the process described in the first stage, now involving the duct only, and complicated by the beginning of upgrowth. The appearance of cell arrangement in some of the sections examined suggested this possibility, but it could not be established certainly. If it were so it would make first and second stages an entirely continuous movement, with somewhat different appearances resulting from it.
This difference of level being established, growth of the walls goes on with increasing rapidity, one of the earlier results being the opening out of the short “ recess ”, so that its thickened lining is now spread between the ureter and the end of the duct. This seems the most likely explanation of the rather rapid disappearance of the recess, and accounts at the same time for the presence of the epithelial debris and thickened patches found (fig. 7) between the ureter and the end of the duct.
The terminal piece of the duct is turned up as a result of the upward growth, and is lying within the mesodermal coat enclosing the sinus, so that, as the recess flattens out, the upturned duct wall blends with the cells lining the sinus. Thus we get the conditions seen in fig. 7. Then, as growth goes on, the lower parts of the wall do not extend so far as the upper part; but there is some extension of it nevertheless, and the upturned end of the duct is elongated accordingly. Thus we find some explanation of its increasing length in the specimens in fig. 7. An attempt to express schematically this view of the mode of growth is given in fig. 8 B.
This conception of the processes of change through which the region passes, in the second stage, avoids the necessity for assuming the presence of duct remnants in the upper part of the line, accounts for the actual lengthening which takes place in the upturned end of the duct, and has the ultimate level of its opening fixed from the beginning, it being necessary only for the covering anterior wall of the embedded portion to degenerate in order that the final orifice may be discovered at its proper level. The remnant line, reaching well into the bladder, is that in which an accessory ureter will open. It is possible that an additional ureteric opening close to the normal one might come from an immediate division of the primary bud, being taken into the bladder by that overlapping of the ureter, by the sinus lining, which we have seen to occur in the 12-5 mm.’ specimen: lower down, however, the embedded end of the duct would be responsible for it as a separate outgrowth.
In this second stage, although the embedded and upturned part of the duct I shows a. relatively fair length, this is only a secondary character, and the actual piece of the duct concerned, if put in terms of the early and complete duct, would probably be exceedingly short.
Summary
The paper deals with two stages in the disappearance of the end of the Wolffian duct: firstly where the part beyond the metanephric bud disappears in the bladder, and secondly Where its opening comes to lie in the prostatic region.
A brief account of subcloacal mesodermal beds is given, to show how the cloaca is fixed below, leaving its upper urogenital part free to expand.
The first stage is effected by extension of the sinus along the duct, up to and just including the ureteric origin, the enclosed duct forming an intussusceptum (with the infolded sinus lining) which rapidly degenerates and disappears.
The second stage, following immediately, is marked by fixation of the duct by mesodermal condensation behind the sinus, with growth of this latter upwards in keeping with the growth of the infra-umbilical belly wall. The implanted end of the duct is thus turned up within the lining layer of the sinus, always at its lowest part, and the breaking down and disappearance of its front wall discovers the opening at the lowest level. In the meantime the upgrowth of the main cavity carries the ureteric levels farther and farther away.
The upturned end of the duct, originally short, is lengthened to some extent in the general lengthening of the wall, thus reaching well into the bladder. This provides a duct “remnant line” in which any structures (such as an accessory ureter) connected with the duct will be placed.
Cite this page: Hill, M.A. (2024, April 27) Embryology Paper - The Terminal Part of the Wolffian Duct. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Terminal_Part_of_the_Wolffian_Duct
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G