Notochord
| Embryology - 28 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The notochord (axial mesoderm, notochordal process, chorda dorsalis, Wirbelsäule) is the defining structure forming in all chordate embryos (taxonomic rank: phylum Chordata). It is an early forming midline structure in the trilaminar embryo mesoderm layer initially ventral to the ectoderm, then neural plate and finally neural tube. This is a transient embryonic anatomy structure, not existing in the adult, required for patterning the surrounding tissues. The patterning signal secreted by notochord cells is sonic hedgehog (SHH). This secreted protein binds to receptors on target cells activating a signaling pathway involved in that tissues differentiation and development. This response appears to be concentration dependent, that is the closer to the notochord the higher the SHH concentration.
Thought to have at least 2 early roles in development and later roles in patterning surrounding tissues. 1. Mechanical, influencing the folding of the early embryo; 2. Morphogenic, secreting sonic hedgehog a protein which regulates the development of surrounding tissues (neural plate, somites, endoderm and other organs).
Recent work in chicken[1] suggests that the later patterning of vertebral segmentation is driven by the somite sclerotome, a result which differs from the findings in the zebrafish model.[2]
In humans, the notochord forms in week 3, is eventually lost from vertebral regions and contributes the entire nucleus pulposus[3] of the intervertebral disc during the formation of the vertebral column.
| Notochord Links: notochord | Lecture - Week 3 | sonic hedgehog | Week 3 | stage 7 | stage 8 | epithelial mesenchymal transition | Development Animation - Notochord | neural | axial skeleton | musculoskeletal | gastrulation | Category:Notochord | ||
|
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Notochord Development | Notochord | Notochordal Plate | Notochord Signaling |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Notochord Development
|
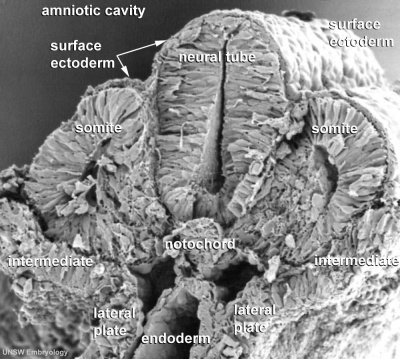
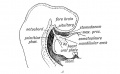
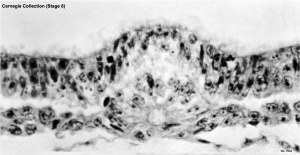
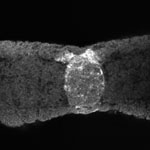
Human Embryo notochordal plate
A scanning electron micrograph (SEM) image of the human embryo (Carnegie stage 8, day 15). The notochordal plate is the initial early transient cellular structure and region lying above the primitive streak, that will later be converted into the notochord. |
| <html5media height="360" width="280">File:Notochord 02.mp4</html5media> | This animation shows the early development of the notochord occurring during week 3 of human development.
This is a dorsal view of the embryonic disc, caudal (tail and connecting stalk end) to the bottom and rostral (head end) to the top. The indentations show the location of the cloacal (bottom) and buccopharyngeal (top) membranes. The raised region in the middle of the embryonic disc is the primitive node (Hensen's node). The right hand side of the gastrulating embryonic disc is removed to the midline to show the the position of the initial axial process (purple). As the animation plays the axial process extends rostrally from the primitive node towards the buccopharyngeal membrane, where it stops. A cross-section view above the primitive node is shown in the second animation below.
|
| <html5media height="200" width="240">File:Notochord 01.mp4</html5media> |
The view is a cross-section showing how the axial process initially is formed, then fused with the endoderm, to finally separate as a midline mesoderm structure.
Yellow - endoderm | Purple - axial process
Links: MP4 version | Notochord Movie |
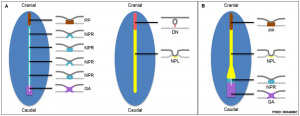
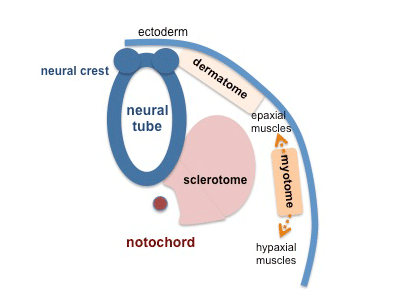
Patterning
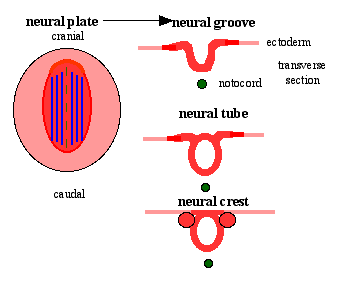
|
|
| Neural tube patterning | Somite patterning |
Development
Week 3
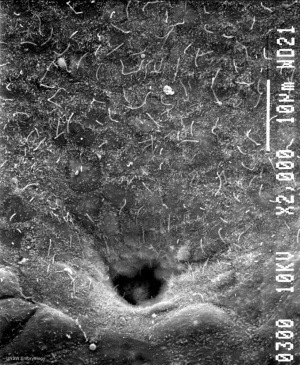
| Neuroenteric Canal | Notochordal Plate |
|---|---|
|

|
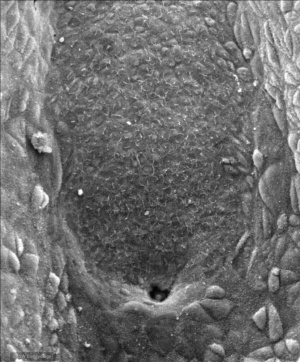
| Human Embryo SEM (Stage 8, day 18) | |
The primitive streak, the caudal eminence and related structures in staged human embryos.[9]
- "The neurenteric canal is an important landmark because rostral to it the neural plate of stages 8, 9, and the main part of the notochord develop, whereas caudal to it the neural plate of stages (10, 11, 12) and the caudal portion of the notochord are formed. All somites at stages 9-11 and probably also at stage 12 arise rostral to the site of the neurenteric canal. (2) A 'chordoneural hinge."
Week 4
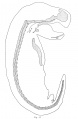
Human embryo 25 days, 19 somite pairs Scanning EM. (Carnegie stage 11)
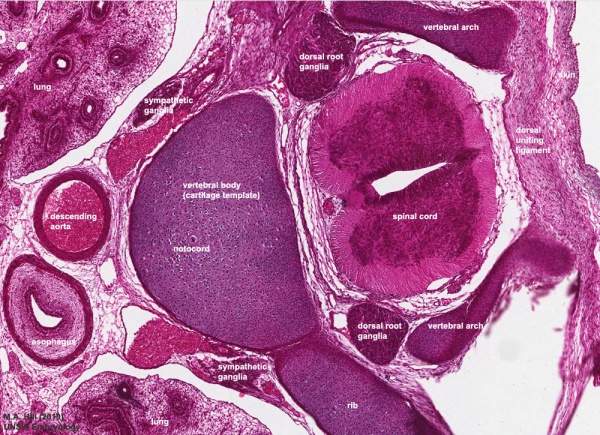
Week 8
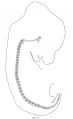
Vertebra and Spinal cord (Carnegie Stage 22)
Nucleus Pulposus
| The notochord is a developmental patterning structure. The only adult anatomy derived from the notochord is the nucleus pulpous of the intervertebral disc.[3]
The embryonic notochordal cells are replaced postnatally by fibrocartilage (about 11 years of age). Composed of a jelly-like material and a loose network of collagen fibres, its physical properties allow the vertebral disc to withstand forces of compression and torsion. |
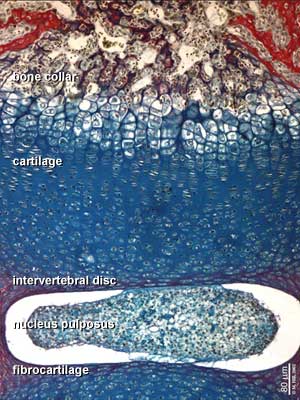
Nucleus pulpous of the intervertebral disc |
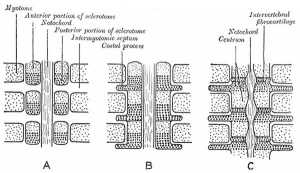
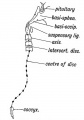
Historic schematic for axial skeleton segmentation |
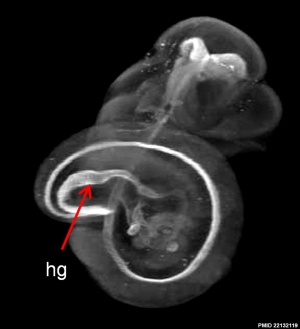
Mouse Notochord
| Mouse (E11) Notochord labeling HNF3beta[10] | ||
|---|---|---|
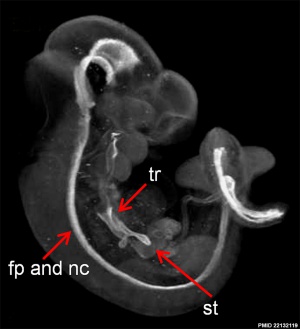
|
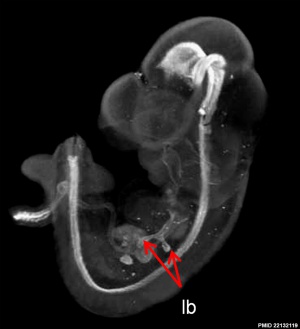
|
|
|
- Links: Image 1 - E11 Notochord | Image 2 - E11 Notochord | Image 3 - E11 Notochord | Image 4 - E11 Notochord | Notochord | Mouse Development | Category:Mouse E11.0 | Image- Mouse embryo E11 and tomography | Image - Mouse embryo E11 tomography | OMIM FORKHEAD BOX A2
Molecular Factors
The notochord secretes both sonic hedgehog (SHH) and Vascular Endothelial Growth Factor (VEGF) as a molecular patterning signals.
Abnormalities
Abnormalities include remnants of notochord that fail to regress. Locations can be along the embryonic path of the notochord and include: ecchordosis physaliphora, odontoid process of the axis, and in the coccyx. Less common locations are in the nasopharynx (Tornwaldt's cysts).
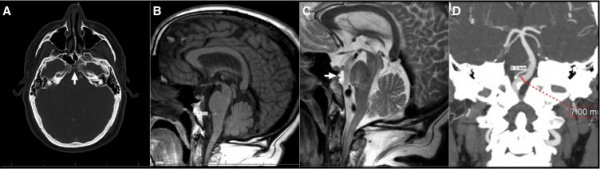
Ecchordosis physaliphora
Benign ectopic nests found along the craniospinal axis forming from notochordal remnants.[11]
Brain radiography showing (A) Axial CTA (bone window); (B) Sagittal T1 MRI; (C) Sagittal T2 MRI showing EP and pontine telangiectasia; (D) CTA showing fenestrated basilar artery.[11]
Tornwaldt's cysts
A rare nasopharyngeal lesion occurring in humans thought to develop from remnants of the embryonic notochord adjacent with the embryonic foregut.[12][13]These cysts are covered by the nasopharynx mucous membrane. Named after Gustav Ludwig Tornwaldt (1843 - 1910) a German physician, the name is also spelled Thornwaldt.
Chordoma
Rare type of bone cancer arising from remnants of the embryonic notochord (for review see{{pmid:26363792|PMID26363792}}) Nearly all chordomas express the T-box transcription factor (TBX) brachyury.
- Links: Tbx | OMIM Chordoma | chordoma foundation
Benign Notochordal Cell Tumour
Benign notochordal cell tumours (BNCTs) may have a relationship with the bones of the base of the skull. The difference between this condition and a chordoma is the absence of extracellular matrix and eosinophil cells and the presence of vacuoles in most tumour cells. Genetically, in contrast to chordoma, chromosome gain or normal copy number was more common while chromosome loss was infrequent in BNCTs. [14]
References
- ↑ 1.0 1.1 Ward L, Pang ASW, Evans SE & Stern CD. (2018). The role of the notochord in amniote vertebral column segmentation. Dev. Biol. , , . PMID: 29654746 DOI.
- ↑ 2.0 2.1 LLeras Forero L, Narayanan R, Huitema LFA, VanBergen M, Apschner A, Peterson-Maduro J, Logister I, Valentin G, Morelli LG, Oates A & Schulte-Merker S. (2018). Segmentation of the zebrafish axial skeleton relies on notochord sheath cells and not on the segmentation clock. Elife , 7, . PMID: 29624170 DOI.
- ↑ 3.0 3.1 3.2 Shapiro IM & Risbud MV. (2010). Transcriptional profiling of the nucleus pulposus: say yes to notochord. Arthritis Res. Ther. , 12, 117. PMID: 20497604 DOI.
- ↑ 4.0 4.1 de Bree K, de Bakker BS & Oostra RJ. (2018). The development of the human notochord. PLoS ONE , 13, e0205752. PMID: 30346967 DOI.
- ↑ Andrews TGR, Pönisch W, Paluch EK, Steventon BJ & Benito-Gutierrez E. (2021). Single-cell morphometrics reveals ancestral principles of notochord development. Development , 148, . PMID: 34343262 DOI.
- ↑ Itoh K, Ossipova O & Sokol SY. (2021). Pinhead antagonizes Admp to promote notochord formation. iScience , 24, 102520. PMID: 34142034 DOI.
- ↑ Imuta Y, Koyama H, Shi D, Eiraku M, Fujimori T & Sasaki H. (2014). Mechanical control of notochord morphogenesis by extra-embryonic tissues in mouse embryos. Mech. Dev. , 132, 44-58. PMID: 24509350 DOI.
- ↑ Bressan M, Davis P, Timmer J, Herzlinger D & Mikawa T. (2009). Notochord-derived BMP antagonists inhibit endothelial cell generation and network formation. Dev. Biol. , 326, 101-11. PMID: 19041859 DOI.
- ↑ Müller F & O'Rahilly R. (2004). The primitive streak, the caudal eminence and related structures in staged human embryos. Cells Tissues Organs (Print) , 177, 2-20. PMID: 15237191 DOI.
- ↑ Hajduk P, Sato H, Puri P & Murphy P. (2011). Abnormal notochord branching is associated with foregut malformations in the adriamycin treated mouse model. PLoS ONE , 6, e27635. PMID: 22132119 DOI.
- ↑ 11.0 11.1 Lagman C, Varshneya K, Sarmiento JM, Turtz AR & Chitale RV. (2016). Proposed Diagnostic Criteria, Classification Schema, and Review of Literature of Notochord-Derived Ecchordosis Physaliphora. Cureus , 8, e547. PMID: 27158576 DOI.
- ↑ Miyahara H & Matsunaga T. (1994). Tornwaldt's disease. Acta Otolaryngol Suppl , 517, 36-9. PMID: 7856446
- ↑ Moody MW, Chi DH, Chi DM, Mason JC, Phillips CD, Gross CW & Schlosser RJ. (2007). Tornwaldt's cyst: incidence and a case report. Ear Nose Throat J , 86, 45-7, 52. PMID: 17315835
- ↑ Du J, Xu L, Cui Y, Liu Z, Su Y & Li G. (2018). Benign notochordal cell tumour: clinicopathology and molecular profiling of 13 cases. J. Clin. Pathol. , , . PMID: 30355586 DOI.
Reviews
Harfe BD. (2021). Intervertebral disc repair and regeneration: Insights from the notochord. Semin Cell Dev Biol , , . PMID: 34865989 DOI.
Lawson LY & Harfe BD. (2017). Developmental mechanisms of intervertebral disc and vertebral column formation. Wiley Interdiscip Rev Dev Biol , 6, . PMID: 28719048 DOI.
Risbud MV & Shapiro IM. (2011). Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit. Rev. Eukaryot. Gene Expr. , 21, 29-41. PMID: 21967331
Risbud MV, Schaer TP & Shapiro IM. (2010). Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev. Dyn. , 239, 2141-8. PMID: 20568241 DOI.
Stemple DL. (2005). Structure and function of the notochord: an essential organ for chordate development. Development , 132, 2503-12. PMID: 15890825 DOI.
Articles
de Bree K, de Bakker BS & Oostra RJ. (2018). The development of the human notochord. PLoS ONE , 13, e0205752. PMID: 30346967 DOI.
Imuta Y, Koyama H, Shi D, Eiraku M, Fujimori T & Sasaki H. (2014). Mechanical control of notochord morphogenesis by extra-embryonic tissues in mouse embryos. Mech. Dev. , 132, 44-58. PMID: 24509350 DOI.
Korecki CL, Taboas JM, Tuan RS & Iatridis JC. (2010). Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther , 1, 18. PMID: 20565707 DOI.
Christiansen HE, Lang MR, Pace JM & Parichy DM. (2009). Critical early roles for col27a1a and col27a1b in zebrafish notochord morphogenesis, vertebral mineralization and post-embryonic axial growth. PLoS ONE , 4, e8481. PMID: 20041163 DOI.
Edeling MA, Sanker S, Shima T, Umasankar PK, Höning S, Kim HY, Davidson LA, Watkins SC, Tsang M, Owen DJ & Traub LM. (2009). Structural requirements for PACSIN/Syndapin operation during zebrafish embryonic notochord development. PLoS ONE , 4, e8150. PMID: 19997509 DOI.
Lee JD & Anderson KV. (2008). Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse. Dev. Dyn. , 237, 3464-76. PMID: 18629866 DOI.
Search PubMed
Search NLM Online Textbooks: "Notochord" : Developmental Biology | The Cell- A molecular Approach | Molecular Biology of the Cell | Endocrinology
Search Pubmed: Notochord
Additional Images
Historic
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
External Links
- OMIM SONIC HEDGEHOG; SHH | T BRACHYURY
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Notochord. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Notochord
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G