Neural System - Postnatal
| Embryology - 6 Mar 2026 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The human nervous system continues to develop postnatally mostly in glial (white matter) proliferation and in neurons (grey matter) making new connections and remodelling.
In humans, postnatal neurogenesis occurs only in specialised niches within the; rostral sub ventricular zone of lateral ventricles, hippocampal dentate gyrus (subgranular zone), within white matter tracts and external granular layer of the cerebellum.
Some recent studies are now using MRI techniques to measure the differences between preterm and normal term birth neural development based upon cortical folding.[1]
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Postnatal Neural Development |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Cortex Development
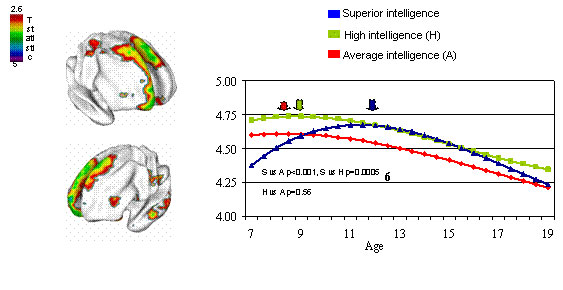
Recent NIH research has looked at the postnatal development of the cortex in children (Cortex Matures Faster in Youth with Highest IQ)
- "The researchers found that the relationship between cortex thickness and IQ varied with age, particularly in the prefrontal cortex, seat of abstract reasoning, planning, and other "executive" functions. .... While the cortex was thinning in all groups by the teen years, the superior group showed the highest rates of change."
The developmental trajectory in cortex thickness differs as the brain matures in different IQ groups. Thickness of the area at the top/front/center, highlighted in MRI brain maps at left, peaks relatively late, at age 12 (blue arrow), in youth with superior intelligence, perhaps reflecting an extended critical period for development of high-level cognitive circuits. (Image and text source: NIMH Child Psychiatry Branch)
Human Tract Development
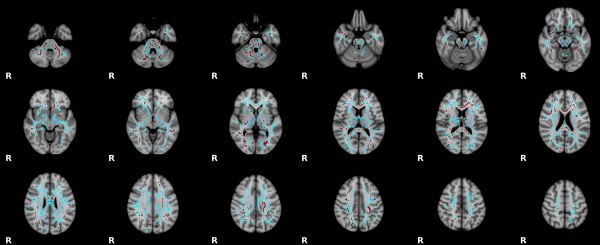
MRI diffusion tensor imaging (DTI) provides information on white matter microstructure, including fractional anisotropy (FA).[8]
Hippocampus - Dentate Gyrus
There are a number of different markers[9] that can be used to identify hippocampal developmental changes:
- proliferative events - PCNA, Ki-67, PH3, MCM2
- early phases of neurogenesis and gliogenesis - nestin, GFAP, Sox2, Pax6
- gliogenesis - vimentin, BLBP, S100beta
- neurogenesis - NeuroD, PSA-NCAM, DCX
Lateral Ventricle - Subventricular Zone
Neurological Assessment
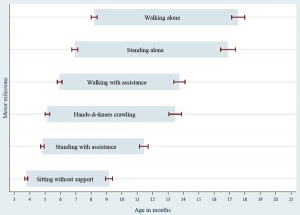
There are many different neurological assessment tests that have been designed over the years using a number of motor and intelligence (comprehension) skill tests. Some of these assessment tests are applicable to specific early neurological development ages. PD Larsen and SS Stensaas from the Utah School of Medicine have also made a series of movies demonstrating normal postnatal neurological development assessment.
Neonatal
- Test of Infant Motor Performance (TIMP) can be used in very early development (from 32 weeks post-conceptional age to 4 months post-term). Involves observation of 28 items and elicitation of 31 items measures behaviours of functional relevance.
- Einstein Neonatal Neurobehavioral Assessment Scale
- Neurobehavioral Assessment of the Preterm Infant
- Bayley Scales of Infant Development (BSID) a postnatal (from 1 to 42 months) neurological assessment scale used in screening and diagnosis of development using 178 item mental scale and the 111 item motor scale, the original BSID was revised in 1993 to version 2 (BSID-II).
- Peabody Developmental Motor Scale II (PDMS-2) tests a child’s motor competence relative to his or her peers. Involves a series of evaluations: reflexes (8 items), stationary/nonlocomotor (30 items), locomotion (89 items), object manipulation (24 items), grasping (26 items) and visual-motor integration (72 items).
Infant
- Alberta Infant Motor Scale (AIMS) birth to 18 months. Identify infants with motor delay (discrimination) and evaluates motor development over time.
- Battelle Developmental Inventory Screening Test (BDIST) for children 6 months to 8 years old.
- Brief Assessment of Motor Function (BAMF) is a series of 10-point ordinal scales developed for rapid description of gross motor, fine motor, and oral motor performance.
- Fagan Test of Infant Intelligence (FTII)
- Comprehensive Developmental Inventory for Infants and Toddlers (CDIIT) a developmental test designed in Taiwan.
- Denver-II (CDIIT) a historic test redesigned as a version 2, for 3 and 72 months of age. It has been suggested that the test may require additional revision for better accuracy.
- Bruininks-Oseretsky Test of Motor Proficiency (1978) ages 4.5 to 14.5 years.
- Early Language Milestone Scale-2, Early Intervention Developmental Profile (EIDP), Gross Motor Function Measure (GMFM)
There are also a range of task based tests: Means-End Problem-Solving Task, Operant Discrimination Learning, Mobile/Train Conjugate Reinforcement Tasks, The Transparent Barrier Detour Task, The A-not-B Task
Postnatal Neural Examination
The links below are to a set of postnatal Neural Exam Movies by Paul D. Larsen, M.D., University of Nebraska Medical Center.
Additional postnatal movies are available on the Neural Exam Movies page.
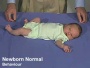
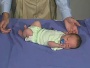
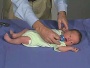
Newborn Normal
|
|
|
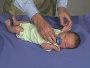
|
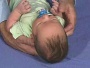
|
|
| behaviour | tone | positions | reflexes | head |
Newborn Abnormal
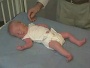
|

|

|
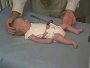
|
|
|
| behaviour | tone | positions | reflexes | head |
Autism
Autism (autism spectrum disorder, ASD) is a behaviourally defined brain disorder in children. Features include: impoverished verbal and non-verbal communication skills, reduced social interactions (bias their attention towards objects rather than the surrounding social situation), behavioural impairments in attention engagement/disengagement, poor emotional discrimination and facial recognition, and fail to response to their own names. There exist many different and unproven claims as to the origins of autism.
Developmentally associated with neural maturation changes in cortical thickness and organization, and particularly affecting pyramidal neurons. A rat model shows structural and behavioural features of autism as a result of altering the trajectory of early postnatal cortical development.[10]
Additional Images
Magnetic Resonance Imaging of Neural Growth
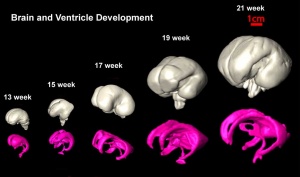
There are a growing number of magnetic resonance imaging (MRI) studies of brain development.
3 months to 30 years - Changes in cerebral grey and white matter volume from infancy to adulthood[12]
- images of 158 normal subjects from infancy to young adulthood were studied (age range 3 months-30 years, 71 males, 87 females).
- volume measures of whole brain, grey matter (GM) and white matter (GM) and gender-specific development
- The resulting growth curve parameter estimates lead to the following observations: total brain volume is demonstrated to undergo an initial rapid spurt. The total GM volume peaks during childhood and decreases thereafter, whereas total WM volume increases up to young adulthood.
- Relative to brain size, GM decreases and WM increases markedly over this age range in a non-linear manner, resulting in an increasing WM-to-GM ratio over much of the observed age range.
- Significant gender differences brain volume and total white and grey matter volume are larger in males than in females, with a time-dependent difference over the age range studied. Over part of the observed age range females tend to have more GM volume relative to brain size and lower WM-to-GM ratio than males.
8 to 30 years - Subcortical brain development[13]
- Brain development during late childhood and adolescence is characterized by decreases in gray matter (GM) and increases in white matter (WM) and ventricular volume.
- developmental trajectories of 16 neuroanatomical volumes in the same sample of children, adolescents, and young adults (n = 171; range, 8-30 years).
- The results revealed substantial heterogeneity in developmental trajectories. GM decreased nonlinearly in the cerebral cortex and linearly in the caudate, putamen, pallidum, accumbens, and cerebellar GM, whereas the amygdala and hippocampus showed slight, nonlinear increases in GM volume. WM increased nonlinearly in both the cerebrum and cerebellum, with an earlier maturation in cerebellar WM.
- Differences between structures within the same regions: among the basal ganglia, the caudate showed a weaker relationship with age than the putamen and pallidum, and in the cerebellum, differences were found between GM and WM development.
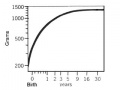
Birth to 2 years - Human brain development[14]
- Ninety-eight children received structural MRI scans: 84 children at 2-4 weeks, 35 at 1 year and 26 at 2 years of age.
- total brain volume increased 101% in the first year, with a 15% increase in the second.
- majority of hemispheric growth was accounted for by gray matter, which increased 149% in the first year
- hemispheric white matter volume increased by only 11%.
- Cerebellum volume increased 240% in the first year.
- Lateral ventricle volume increased 280% in the first year, with a small decrease in the second.
- caudate increased 19% and the hippocampus 13% from age 1 to age 2.
- Cerebellum volume also increased substantially in the first year of life.
- Links: Magnetic Resonance Imaging
References
- ↑ Shimony JS, Smyser CD, Wideman G, Alexopoulos D, Hill J, Harwell J, Dierker D, Van Essen DC, Inder TE & Neil JJ. (2016). Comparison of cortical folding measures for evaluation of developing human brain. Neuroimage , 125, 780-790. PMID: 26550941 DOI.
- ↑ Matsumoto M, Sawada M, García-González D, Herranz-Pérez V, Ogino T, Bang Nguyen H, Quynh Thai T, Narita K, Kumamoto N, Ugawa S, Saito Y, Takeda S, Kaneko N, Khodosevich K, Monyer H, Manuel García-Verdugo J, Ohno N & Sawamoto K. (2019). Dynamic changes in ultrastructure of the primary cilium in migrating neuroblasts in the postnatal brain. J. Neurosci. , , . PMID: 31685650 DOI.
- ↑ Perez EC, Bravo DR, Rodgers SP, Khan AR & Leasure JL. (2019). Shaping the adult brain with exercise during development: Emerging evidence and knowledge gaps. Int. J. Dev. Neurosci. , , . PMID: 31229526 DOI.
- ↑ Conrad MS, Sutton BP, Dilger RN & Johnson RW. (2014). An in vivo three-dimensional magnetic resonance imaging-based averaged brain collection of the neonatal piglet (Sus scrofa). PLoS ONE , 9, e107650. PMID: 25254955 DOI.
- ↑ Whiteus C, Freitas C & Grutzendler J. (2014). Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature , 505, 407-11. PMID: 24305053 DOI.
- ↑ Hou J, Eriksen N & Pakkenberg B. (2011). The temporal pattern of postnatal neurogenesis found in the neocortex of the Göttingen minipig brain. Neuroscience , 195, 176-9. PMID: 21878372 DOI.
- ↑ Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD & Kolodkin AL. (2009). Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature , 462, 1065-9. PMID: 20010807 DOI.
- ↑ Imperati D, Colcombe S, Kelly C, Di Martino A, Zhou J, et al. (2011) Differential Development of Human Brain White Matter Tracts. PLoS ONE 6(8): e23437. doi:10.1371/journal.pone.0023437 PloS One
- ↑ PMID 21647561
- ↑ Chomiak T, Karnik V, Block E, Hu B. Altering the trajectory of early postnatal cortical development can lead to structural and behavioural features of autism. BMC Neurosci. 2010 Aug 19;11:102. PMID: 20723245| BMC Neurosci.
- ↑ <pubmed>20108226</pubmed>
- ↑ <pubmed>20600789</pubmed>
- ↑ <pubmed>19776264</pubmed>
- ↑ <pubmed>19020011</pubmed>
Reviews
Deng W, Aimone JB & Gage FH. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory?. Nat. Rev. Neurosci. , 11, 339-50. PMID: 20354534 DOI.
Pathania M, Yan LD & Bordey A. (2010). A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology , 58, 865-76. PMID: 20097213 DOI.
Ploeger A, Raijmakers ME, van der Maas HL & Galis F. (2010). The association between autism and errors in early embryogenesis: what is the causal mechanism?. Biol. Psychiatry , 67, 602-7. PMID: 19932467 DOI.
Geschwind DH. (2009). Advances in autism. Annu. Rev. Med. , 60, 367-80. PMID: 19630577 DOI.
Muhle R, Trentacoste SV & Rapin I. (2004). The genetics of autism. Pediatrics , 113, e472-86. PMID: 15121991
Articles
Search PubMed
Search Bookshelf postnatal neural development
Search Pubmed postnatal neural development | postnatal Dentate Gyrus | postnatal Subventricular Zone | autism
Terms
- neural maturation index - (NMI) an index using neuroimaging that characterizes brain maturation patterns and identifies those who deviate from this normal trajectory.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2026, March 6) Embryology Neural System - Postnatal. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_System_-_Postnatal
- © Dr Mark Hill 2026, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G