Introduction
The uppermost portion of the diencephalon that includes the trigonum habenulae (habenula), the pineal (gland or body), the posterior commissure, and the medullary layers of thalamus. The epithalamus develops from the diencephalon most dorsal part of prosomere 2.
The adult habenular nuclei acts as a neural relay system, connecting the forebrain with the brain stem. The function appears to be to regulate cognitive behaviors by modulating a range of transmitter system (cholinergic, dopaminergic and serotonergic) activities.
|
|
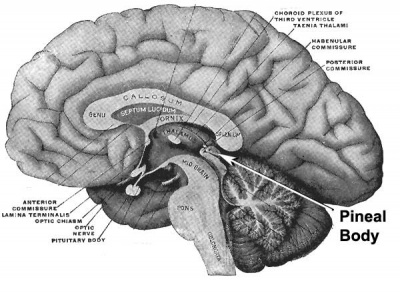
| Adult pineal body (mid-sagittal section view)
|
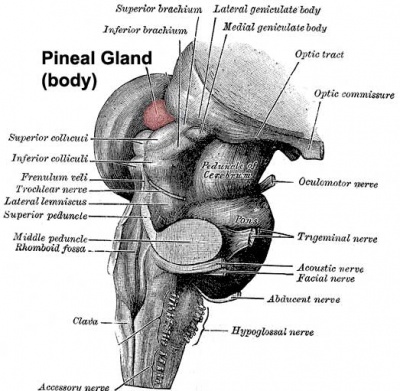
Pineal gland position (dorsolateral view)
|
Some Recent Findings
- Notch signaling restricts FGF pathway activation in parapineal cells to promote their collective migration[1] "Coordinated migration of cell collectives is important during embryonic development and relies on cells integrating multiple mechanical and chemical cues. Recently, we described that focal activation of the FGF pathway promotes the migration of the parapineal in the zebrafish epithalamus. How FGF activity is restricted to leading cells in this system is, however, unclear. Here, we address the role of Notch signaling in modulating FGF activity within the parapineal. While Notch loss-of-function results in an increased number of parapineal cells activating the FGF pathway, global activation of Notch signaling decreases it; both contexts result in defects in parapineal migration and specification. Decreasing or increasing FGF signaling in a Notch loss-of-function context respectively rescues or aggravates parapineal migration defects without affecting parapineal cells specification. We propose that Notch signaling controls the migration of the parapineal through its capacity to restrict FGF pathway activation to a few leading cells."
- Notch signaling restricts FGF pathway activation in parapineal cells to promote their collective migration[1] "Coordinated migration of cell collectives is important during embryonic development and relies on cells integrating multiple mechanical and chemical cues. Recently, we described that focal activation of the FGF pathway promotes the migration of the parapineal in the zebrafish epithalamus. How FGF activity is restricted to leading cells in this system is, however, unclear. Here, we address the role of Notch signaling in modulating FGF activity within the parapineal. While Notch loss-of-function results in an increased number of parapineal cells activating the FGF pathway, global activation of Notch signaling decreases it; both contexts result in defects in parapineal migration and specification. Decreasing or increasing FGF signaling in a Notch loss-of-function context respectively rescues or aggravates parapineal migration defects without affecting parapineal cells specification. We propose that Notch signaling controls the migration of the parapineal through its capacity to restrict FGF pathway activation to a few leading cells."
- Foxg1 deletion impairs the development of the epithalamus[2] "The epithalamus, which is dorsal to the thalamus, consists of the habenula, pineal gland and third ventricle choroid plexus and plays important roles in the stress response and sleep-wake cycle in vertebrates. During development, the epithalamus arises from the most dorsal part of prosomere 2. However, the mechanism underlying epithalamic development remains largely unknown. Foxg1 is critical for the development of the telencephalon, but its role in diencephalic development has been under-investigated. Patients suffering from FOXG1-related disorders exhibit severe anxiety, sleep disturbance and choroid plexus cysts, indicating that Foxg1 likely plays a role in epithalamic development. In this study, we identified the specific expression of Foxg1 in the developing epithalamus. Using a "self-deletion" approach, we found that the habenula significantly expanded and included an increased number of habenular subtype neurons. The innervations, particularly the habenular commissure, were severely impaired. Meanwhile, the Foxg1 mutants exhibited a reduced pineal gland and more branched choroid plexus. After ablation of Foxg1 no obvious changes in Shh and Fgf signalling were observed, suggesting that Foxg1 regulates the development of the epithalamus without the involvement of Shh and Fgfs."
- Pitx2c ensures habenular asymmetry by restricting parapineal cell number[3] "Left-right (L/R) asymmetries in the brain are thought to underlie lateralised cognitive functions. Understanding how neuroanatomical asymmetries are established has been achieved through the study of the zebrafish epithalamus. ...We provide evidence suggesting that antagonism between Nodal and Pitx2c activities sets an upper limit on parapineal cell numbers. We conclude that restricting parapineal cell number is crucial for the correct elaboration of epithalamic asymmetry."
- A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei[4] "Neurons usually migrate and differentiate in one particular encephalic vesicle. We identified a murine population of diencephalic neurons that colonized the telencephalic amygdaloid complex, migrating along a tangential route that crosses a boundary between developing brain vesicles. The diencephalic transcription factor OTP was necessary for this migratory behavior."
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Epithalamus Development | Epithalamus Embryology
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Neural Tube Development
| Neural Tube
|
Primary Vesicles
|
Secondary Vesicles
|
Adult Structures
|
| week 3
|
week 4
|
week 5
|
adult
|
neural plate
neural groove
neural tube
Brain
|
prosencephalon (forebrain)
|
telencephalon
|
Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles
|
| diencephalon
|
epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle
|
| mesencephalon (midbrain)
|
mesencephalon
|
tectum, Cerebral peduncle, cerebral aqueduct, pons
|
| rhombencephalon (hindbrain)
|
metencephalon
|
cerebellum
|
| myelencephalon
|
medulla oblongata, isthmus
|
| spinal cord, pyramidal decussation, central canal
|
References
- ↑ 1.0 1.1 Wei L, Al Oustah A, Blader P & Roussigné M. (2019). Notch signaling restricts FGF pathway activation in parapineal cells to promote their collective migration. Elife , 8, . PMID: 31498774 DOI.
- ↑ Liu B, Zhou K, Wu X & Zhao C. (2018). Foxg1 deletion impairs the development of the epithalamus. Mol Brain , 11, 5. PMID: 29394901 DOI.
- ↑ Garric L, Ronsin B, Roussigné M, Booton S, Gamse JT, Dufourcq P & Blader P. (2014). Pitx2c ensures habenular asymmetry by restricting parapineal cell number. Development , 141, 1572-9. PMID: 24598158 DOI.
- ↑ García-Moreno F, Pedraza M, Di Giovannantonio LG, Di Salvio M, López-Mascaraque L, Simeone A & De Carlos JA. (2010). A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei. Nat. Neurosci. , 13, 680-9. PMID: 20495559 DOI.
Revoews
Articles
Textbooks
- Endocrinology: An Integrated Approach. Nussey, S and Saffron Whitehead, S. Oxford: BIOS Scientific Publishers; 2001. Chapter 7. The pineal gland and melatonin
Search PubmMed
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 28) Embryology Neural - Epithalamus Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Epithalamus_Development
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G


